Abstract
Rationale
Neurokinin-1 receptor (NK1R) signaling modulates behaviors associated with psychostimulants and opioids. Psychostimulants, such as amphetamine (AMPH) and cocaine, bind to monoamine transporters and alter their functions. Both dopamine and norepinephrine transporters are regulated by NK1R activation suggesting a role for NK1R mediated catecholamine transporter regulation in psychostimulant-mediated behaviors.
Objectives
The effect of in vivo administration of aprepitant (10 mg/kg) on the expression of AMPH (0.5 and 2 mg/kg) and cocaine (5 and 20 mg/kg)-induced conditioned place preference (CPP) as well as locomotor activation was examined in C57BL/6J mice. The effect of aprepitant on morphine (1 and 5 mg/kg)-induced CPP was also examined to identify the specific actions of aprepitant on psychostimulant versus opioid-induced behaviors.
Results
Aprepitant administration significantly attenuated the CPP expression and locomotor activation produced by AMPH and cocaine. In contrast, aprepitant significantly enhanced the expression of CPP produced by morphine while significantly suppressing the locomotor activity of the mice conditioned with morphine. Aprepitant by itself did not induce significant CPP or conditioned place aversion or locomotor activation or suppression.
Conclusions
Attenuation of AMPH or cocaine-induced CPP and locomotor activation by aprepitant suggests a role for NK1R signaling in psychostimulant-mediated behaviors. Stimulation of morphine-induced CPP expression and suppression of locomotor activity of morphine-conditioned mice suggest differential effects of NK1R antagonism on conditioned psychostimulant versus opioid reward. Collectively, these findings indicate that clinically used NK1R antagonist, aprepitant may serve as a potential therapeutic agent in the treatment of psychostimulant abuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Substance P, the endogenous neurokinin-1 receptor (NK1R) agonist, and its receptor NK1R are implicated in modulating behaviors associated with addiction including drug seeking (Commons 2010; Kraft et al. 2001; Lindefors et al. 1989; Noailles and Angulo 2002; Placenza et al. 2006; Placenza et al. 2005; Van den Bos et al. 1989). Substance P is released in the ventral striatum following AMPH administration and enhances its stimulant effects (Van den Bos et al. 1990). Site-specific infusion of substance P analog into ventral tegmental area or ICV infusion of an NK1R agonist induces reinstatement of cocaine-seeking behavior (Placenza et al. 2005). However, subsequent studies showed that ICV infusion of an NK1R antagonist (GR82334) suppresses opiate-induced locomotor activation and self-administration, but not cocaine-induced locomotor activation and self-administration (Placenza et al. 2006). It has also been shown that site-specific infusion of substance P into globus pallidus induces conditioned place preference (CPP), which is blocked by NK1R antagonist WIN 51,708 (Kertes et al. 2010). This study also showed that the NK1R antagonist WIN 51,708 alone does not induce CPP or conditioned place aversion. Interestingly, NK1R knockout mice exhibit lack of AMPH or morphine-induced CPP (Gadd et al. 2003; Murtra et al. 2000; Yan et al. 2009). While observations are conflicting with regard to NK1R antagonism on morphine reward (Commons 2010; Jones et al. 2013; Murtra et al. 2000; Ripley et al. 2002; Robinson et al. 2012; Walsh et al. 2013), NK1R antagonism is shown to attenuate AMPH-induced locomotor activation (Gonzalez-Nicolini and McGinty 2002). Studies from genetic and pharmacological blockade of NK1R show modulation of psychostimulant-induced release of NE and DA, indicating a role for NK1R in psychostimulant-mediated behaviors as well as a close relationship between neurokinin and catecholaminergic systems (Fisher et al. 2007; Yan et al. 2009).
Psychostimulants target monoamine transporters including the catecholamine transporters, norepinephrine transporter (NET), and dopamine transporter (DAT), and enhance monoaminergic signaling, and this effect is known to mediate important aspects of drug reinforcement (Gainetdinov and Caron 2003; Hall et al. 2009; Rocha et al. 1998; Salahpour et al. 2008; Sotnikova et al. 2006; Xu et al. 2000). Studies by us and other investigators have demonstrated that both NET and DAT are regulated by signaling mechanisms downstream of receptor activation including NK1R as well as by psychostimulants via phosphorylation-dependent and independent mechanisms (Foster et al. 2006; Jayanthi and Ramamoorthy 2005; Kahlig and Galli 2003; Kristensen et al. 2011; Ramamoorthy et al. 2011; Rudnick et al. 2014). Importantly, we showed that a common trafficking motif is required for both AMPH and NK1R-mediated NET regulation (Annamalai et al. 2010; Jayanthi et al. 2006) and demonstrated a regulated interaction between NET and NK1R (Arapulisamy et al. 2013). We also demonstrated cocaine-induced p38 MAPK-mediated phosphorylation-dependent NET regulation and its role in cocaine-elicited behaviors (Mannangatti et al. 2011; Mannangatti et al. 2015). Several protein kinases including PKC, ERK/MAPK, and Akt are linked to NK1R signaling (Amadoro et al. 2007; Chu et al. 2011; Lallemend et al. 2003; Monastyrskaya et al. 2005; Nakamura et al. 2014), thus suggesting a close relationship between NK1R signaling and psychostimulant-induced behaviors via catecholamine transport regulation. We postulated that NK1R antagonism might block psychostimulant elicited behaviors and examined the effect of aprepitant, a clinically used NK1R antagonist, on conditioned psychostimulant reward. While AMPH and cocaine directly target NET and DAT to modulate their functions, morphine is not known to target NET or DAT directly. Therefore, we also hypothesized that aprepitant will not affect opioid-mediated behaviors and investigated the effect of aprepitant on conditioned morphine reward.
Methods
Subjects
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) of 8–9 weeks age and weighing around 25 g were used for the experiments. A total of 115 mice were used in our experiments: 11 animals for conditioning with intraperitoneal (i.p.) saline as control for AMPH or cocaine; 11 animals each for conditioning with 0.5 mg/kg and 2 mg/kg AMPH (given i.p.); 11 animals each for conditioning with 5 mg/kg and 20 mg/kg cocaine (given i.p.); 19 animals for conditioning with subcutaneous (s.c.) saline (11 + 8 in two sets) as control for morphine and 8 animals for conditioning with 1 mg/kg morphine (given s.c.) and 11 animals for conditioning with 5 mg/kg morphine (given s.c.); 11 animals for conditioning with i.p. vehicle as control for aprepitant and 11 animals for conditioning with aprepitant (given i.p.). Mice were housed in groups of 4–5 in polypropylene cages with corn-cob bedding and had free access to food (Harlan Teklad) and tap water. They were maintained on a 12-h light/12-h dark cycle at an ambient temperature of 22 °C and 42% humidity. All animal procedures were in accordance with the National Institutes of Health guide for the Care and Use of Laboratory animals. The protocols of this study were approved by Virginia Commonwealth University Institutional Animal Care and Use Committee.
Drug administrations
Amphetamine (D-Amphetamine hemisulfate) (AMPH) or cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in injectable grade isotonic saline solution (0.9% NaCl). Injections of i.p. saline or AMPH (0.5 or 2 mg/kg) or cocaine (5 or 20 mg/kg) were given in a volume of 10 μl/g body weight. Saline or morphine (1 or 5 mg/kg) was given s.c. in a volume of 10 μl/g body weight. Vehicle or aprepitant (10 mg/kg, i.p.) was administered in a volume of 10 μl/g body weight. Aprepitant, (5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3- (4-fluorophenyl)morpholin-4-yl]methyl]-1,2-dihydro-1,2,4-triazol-3-one) (Merck & Co., Kenilworth, NJ) was dissolved in dimethyl sulfoxide (DMSO) and diluted with saline so that the final DMSO concentration is 0.002% when injected. Vehicle control contained 0.002% DMSO.
Conditioned place preference
An unbiased mouse CPP paradigm was utilized as described by us previously (Mannangatti et al. 2015). In brief, mice were placed in enriched environment and handled for 3 days prior to initiation of CPP testing. The CPP apparatus (Med-Associates, St. Albans, VT, ENV3013) consisted of white and black chambers (20 × 20 × 20 cm each), which differed in floor texture (white mesh and black rod: Med-Associates, ENV-3013WM and ENV-3013BR) to help the mice further differentiate between the two environments. Place conditioning chambers were separated by a smaller intermediate gray compartment with a smooth PVC floor and partitions that allowed access to the black and white chambers. On day 1, mice were introduced into the chamber and their baseline preference for each chamber recorded for 15 min after 5 min acclimation time. After testing for initial chamber preference on day 1 (preconditioning), for conditioning, mice received i.p. saline in one chamber in the AM and saline or 0.5 or 2.0 mg/kg AMPH or 5 or 20 mg/kg cocaine in the opposite chamber in the PM once a day for 3 days (days 2–4) having chambers counterbalanced across treatments. Following this conditioning period, on day 5, CPP test (postconditioning) was conducted in the AM following an injection of vehicle (saline containing 0.002% DMSO given 15 min prior to CPP testing) and in the PM following an injection of aprepitant (10 mg/kg i.p. given 15 min prior to CPP testing). We designed this type of postconditioning testing protocol to achieve within the subject controls. Other groups of mice were conditioned with subcutaneous (s.c.) injections of saline or 1 or 5 mg/kg morphine for 3 days and on postconditioning day, CPP was tested in the AM following an injection of vehicle and in the PM following an injection of aprepitant. To examine whether aprepitant alone induces CPP, mice were conditioned with vehicle or aprepitant (10 mg/kg i.p.) for 3 days and tested for CPP on postconditioning day following a vehicle injection. The doses of AMPH, cocaine, morphine, and aprepitant are chosen based on previous studies (Lim et al. 2008; Mannangatti et al. 2015; Ramsey et al. 2008; Stuart et al. 2013; Utsumi et al. 2016). Preference scores measured in seconds reflect the time the mice spent in the drug-paired side during postconditioning day, subtracted from the time spent in the drug-paired side preconditioning, when baseline scores are taken. A positive number indicated a preference for the drug-paired side, whereas a negative number implied an aversion to the drug-paired side. A number of zero or near zero indicated no preference for either side. The distance traveled was also recorded simultaneously for further analysis of the ambulatory (movement) counts.
Locomotor activity
The locomotor activity of the mice were recorded as movement counts during CPP testing on postconditioning day following vehicle injection in the AM as well as following aprepitant injection in the PM. The movements of the mice were tracked using 16 evenly spaced infra-red (I/R) sources and sensors juxtaposed around the periphery of the four sides of the chamber. Total activity is calculated by adding the movement counts from both the compartments. Counts from middle gray compartment were not included in the total counts.
Statistical analyses
Statistical analyses of the data were performed using Prism software (GraphPad, San Diego, CA). Values are expressed as mean ± S.E.M. Two-way ANOVA with Tukey’s multiple comparisons test was used for examining the effect of conditioning drug as well as the effect of aprepitant treatment on CPP induced by AMPH, cocaine, or morphine. Similar analysis was used for examining the effects of conditioning drug and aprepitant treatment on locomotor activity. Tukey’s post hoc test compares each group with every other group in all possible ways. Two-tailed unpaired Student’s t test analysis was performed for comparisons between two groups, vehicle and aprepitant. A value of p ≤ 0.05 was considered statistically significant.
Results
Aprepitant attenuates AMPH-induced CPP and locomotor activity
Mice conditioned with either 0.5 or 2 mg/kg AMPH exhibited a significant CPP expression compared to saline-conditioned mice (Fig. 1a, b). Mice conditioned with 0.5 mg/kg AMPH exhibited significantly higher CPP expression following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.005) or aprepitant (p < 0.005) (Fig. 1a). Following aprepitant, the CPP expression observed in mice conditioned with 0.5 mg/kg AMPH did not differ significantly from that observed in saline-conditioned mice (Fig. 1a). When compared to vehicle, aprepitant significantly reduced the CPP expression induced by 0.5 mg/kg AMPH conditioning (p < 0.05) (Fig. 1a). With regard to CPP expression, tabular results from two-way ANOVA showed significant effect by AMPH conditioning using 0.5 mg/kg dose (F 1 ,40 = 9.98, p = 0.003) and by aprepitant treatment (F 1,40 = 4.47, p = 0.041). There was no significant interaction between AMPH conditioning and aprepitant treatment (F 1,40 = 3.07, p = 0.09). Mice conditioned with 2 mg/kg AMPH exhibited significantly higher CPP expression following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0001) or aprepitant (p < 0.0001) (Fig. 1b). Mice conditioned with 2 mg/kg AMPH exhibited significantly higher CPP expression following aprepitant, when compared to saline-conditioned mice following vehicle (p < 0.005) or aprepitant (p < 0.005) (Fig. 1b). Nonetheless, when compared to vehicle, aprepitant significantly reduced the CPP expression induced by 2 mg/kg AMPH (p < 0.05) (Fig. 1b). Saline-conditioned mice exhibited very low CPP expression following vehicle, and this CPP expression did not differ significantly from that following aprepitant (Fig. 1a, b). With regard to CPP expression, tabular results from two-way ANOVA showed significant effect by AMPH conditioning using 2 mg/kg dose (F 1,40 = 58.06, p < 0.0001) and by aprepitant treatment (F 1,40 = 5.42, p = 0.025). There was no significant interaction between AMPH conditioning and aprepitant treatment (F 1,40 = 3.50, p = 0.07). Locomotor activity measured as movement counts during CPP testing showed significantly higher locomotor activityin AMPH-conditioned mice compared to saline-conditioned mice (Fig. 1a, b). Mice conditioned with 0.5 mg/kg AMPH showed significantly higher locomotor activity following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0001) or aprepitant (p < 0.0001) (Fig. 1c). Mice conditioned with 0.5 mg/kg AMPH exhibited significantly higher locomotor activity following aprepitant, only when compared to saline-conditioned mice following aprepitant (p < 0.005) (Fig. 1c). Nonetheless, when compared to vehicle, aprepitant significantly reduced the locomotor activation observed in mice conditioned with 0.5 mg/kg AMPH (p < 0.05) (Fig. 1c). With regard to locomotor activity, tabular results from two-way ANOVA showed significant effect by AMPH conditioning using 0.5 mg/kg dose (F 1,40 = 40.75, p < 0.0001) and by aprepitant treatment (F 1,40 = 9.01, p = 0.005). There was no significant interaction between AMPH conditioning and aprepitant treatment (F 1,40 = 1.13, p = 0.29). Mice conditioned with 2 mg/kg AMPH exhibited significantly higher locomotor activity following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0003) or aprepitant (p < 0.0001) (Fig. 1d).
CPP scores recorded during postconditioning testing, given as means ± SEM, show significant CPP in mice conditioned with AMPH a) 0.5 mg/kg (n = 11) or b) 2 mg/kg (n = 11) compared to mice conditioned with saline (n = 11). Treatment with aprepitant (10 mg/kg) significantly reduced AMPH CPP. ** and **** indicate significant effect by AMPH (p < 0.005 and p < 0.0001, respectively) and ^ indicates significant effect by aprepitant treatment in the AMPH group (p < 0.05). Movement counts recorded simultaneously during postconditioning testing, given as means ± SEM, show significant locomotor activation in mice conditioned with AMPH c) 0.5 mg/kg or d) 2 mg/kg compared to mice conditioned with saline. Treatment with aprepitant (10 mg/kg) significantly reduced AMPH-induced locomotor activity. **, ***, and **** indicate significant effect by AMPH (p < 0.005, p < 0.0003, p < 0.0001) and ^ indicates significant effect by aprepitant treatment in the AMPH group (p < 0.05)
Following aprepitant, the locomotor activity of mice conditioned with 2 mg/kg AMPH did not differ significantly from that of saline-conditioned mice (Fig. 1d). When compared to vehicle administration, aprepitant administration significantly reduced the locomotor activation seen in the mice conditioned with 2 mg/kg AMPH (p < 0.05) (Fig. 1d). In saline-conditioned mice, although we observed a slightly reduced locomotor activity following aprepitant administration, it did not reach statistical significance when compared to vehicle administration (Fig. 1c, d). With regard to locomotor activity, tabular results from two-way ANOVA showed significant effect by AMPH conditioning using 2 mg/kg dose (F 1,40 = 20.33, p < 0.0001) and by aprepitant treatment (F 1,40 = 10.50, p = 0.002). There was no significant interaction between AMPH conditioning and aprepitant treatment (F 1,40 = 1.85, p = 0.18).
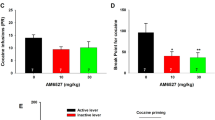
Aprepitant attenuates cocaine-induced CPP and locomotor activity
Above results suggested a role for NK1R in AMPH-induced CPP expression as evidenced by attenuation by NK1R antagonist, aprepitant. Therefore, next, we examined whether this effect is specific only to AMPH or to other psychostimulants in general. Similar to our published study (Mannangatti et al. 2015), both 5 and 20 mg/kg cocaine conditioning produced significant CPP expression compared to saline control group (Fig. 2a, b). Mice conditioned with 5 mg/kg cocaine exhibited significantly higher CPP expression following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0001) or aprepitant (p < 0.0001) (Fig. 2a). Following aprepitant, the CPP expression observed in mice conditioned with 5 mg/kg cocaine did not differ significantly from that observed in saline-conditioned mice (Fig. 2a). When compared to vehicle administration, aprepitant administration significantly reduced the CPP expression induced by 5 mg/kg cocaine conditioning (p < 0.05) (Fig. 2a). With regard to CPP expression, tabular results from two-way ANOVA showed significant effect by cocaine conditioning using 5 mg/kg dose (F 1,40 = 26.47, p < 0.0001) and by aprepitant treatment (F 1,40 = 4.27, p = 0.045). There was no significant interaction between cocaine conditioning and aprepitant treatment (F 1,40 = 3.37, p = 0.074). Mice conditioned with 20 mg/kg cocaine exhibited significantly higher CPP expression following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0001) or aprepitant (p < 0.0001) (Fig. 2b). Mice conditioned with 20 mg/kg cocaine exhibited significantly higher CPP expression following aprepitant, only when compared to saline-conditioned mice following aprepitant (p < 0.05) (Fig. 2b). Nonetheless, when compared to vehicle administration, aprepitant administration significantly reduced the CPP expression induced by 20 mg/kg cocaine (p < 0.05) (Fig. 2b). With regard to CPP expression, tabular results from two-way ANOVA showed significant effect by cocaine conditioning using 20 mg/kg dose (F 1,40 = 32.90, p < 0.0001) and by aprepitant treatment (F 1,40 = 4.49, p = 0.040). There was no significant interaction between cocaine conditioning and aprepitant treatment (F 1,40 = 3.42, p = 0.072). Saline-conditioned mice exhibited very low CPP expression following vehicle, and this CPP expression did not differ significantly from that following aprepitant (Fig. 2a, b). Locomotor activity, measured as movement counts during CPP testing, showed that mice conditioned with 5 mg/kg cocaine did not show locomotor activation following vehicle when compared to saline-conditioned mice following vehicle (Fig. 2c). However, when compared to saline conditioned mice following aprepitant, mice conditioned with 5 mg/kg cocaine showed slight but significant locomotor activation following vehicle (p < 0.005) as well as following aprepitant (p < 0.05) (Fig. 2c). With regard to locomotor activity, tabular results from two-way ANOVA showed significant effect by cocaine conditioning using 5 mg/kg dose (F 1,40 = 11.79, p = 0.0014) and no significant effect by aprepitant treatment (F 1,40 = 2.17, p = 0.15). There was no significant interaction between cocaine conditioning and aprepitant treatment (F 1,40 = 0.30, p = 0.59). Mice conditioned with 20 mg/kg cocaine exhibited significantly higher locomotor activity following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0003) and following aprepitant (p < 0.0001) (Fig. 2d). Mice conditioned with 20 mg/kg cocaine showed significantly higher locomotor activity following aprepitant, only when compared to saline-conditioned mice following aprepitant (p < 0.05) (Fig. 2d). Nonetheless, when compared to vehicle, aprepitant significantly reduced the locomotor activation seen in the mice conditioned with 20 mg/kg cocaine (p < 0.005) (Fig. 2d). With regard to locomotor activity, tabular results from two-way ANOVA showed significant effect by cocaine conditioning using 20 mg/kg dose (F 1,40 = 25.48, p < 0.0001) and by aprepitant treatment (F 1,40 = 15.4, p = 0.0003). There was no significant interaction between cocaine conditioning and aprepitant treatment (F 1,40 = 0.72, p = 0.40). In saline-conditioned mice, although we observed a slightly reduced locomotor activity following aprepitant administration, it did not reach statistical significance when compared to vehicle administration (Fig. 2c, d).
CPP scores recorded during postconditioning testing, given as means ± SEM, show significant CPP in mice conditioned with cocaine a) 5 mg/kg (n = 11) or b) 20 mg/kg (n = 11) compared to mice conditioned with saline (n = 11). Treatment with aprepitant (10 mg/kg) significantly reduced cocaine CPP. * and **** indicate significant effect by cocaine (p < 0.05 and p < 0.0001, respectively) and ^ and ^^ indicate significant effect by aprepitant treatment in the cocaine group (p < 0.005 and p < 0.05, respectively). Movement counts recorded simultaneously during postconditioning testing, given as means ± SEM, show significant locomotor activation in mice conditioned with cocaine c) 5 mg/kg (n = 11) or d) 20 mg/kg (n = 11) compared to mice conditioned with saline (n = 11). Treatment with aprepitant (10 mg/kg) significantly reduced cocaine-induced locomotor activity. *, **, ***, and **** indicate significant effect by cocaine (p < 0.05, p < 0.005, p < 0.0003, and p < 0.0001) and ^^ indicates significant effect by aprepitant treatment in the cocaine group (p < 0.005)
Aprepitant enhances the CPP induced by high-dose morphine and reduces the locomotor activity of morphine-conditioned mice
Having known that aprepitant could effectively blunt the expression of CPP induced by psychostimulants, we next examined its effect on the expression of opioid-induced CPP. Compared to saline conditioning, morphine conditioning produced significant CPP expression (Fig. 3a, b). Mice conditioned with 1 mg/kg morphine exhibited significantly higher CPP expression following vehicle or aprepitant, when compared to saline-conditioned mice following vehicle (p < 0.0001) or aprepitant (p < 0.0001) (Fig. 3a). There was a slight enhancement in the CPP expression following aprepitant, but it did not reach statistical significance (Fig. 3a). With regard to CPP expression, tabular results from two-way ANOVA showed significant effect by morphine conditioning using 1 mg/kg dose (F 1,50 = 84.16, p < 0.0001) and no significant effect by aprepitant treatment (F 1,50 = 1.51, p = 0.225). There was no significant interaction between morphine conditioning and aprepitant treatment (F 1,50 = 0.367, p = 0.548). Mice conditioned with 5 mg/kg morphine exhibited significantly higher CPP expression following vehicle, when compared to saline-conditioned mice following vehicle (p < 0.0003) or aprepitant (p < 0.005) (Fig. 3b). The CPP expression in 5 mg/kg significantly enhanced the CPP expression induced by 5 mg/kg morphine (p < 0.005) (Fig. 3b). With regard to CPP expression, tabular results from two-way ANOVA showed significant effect by morphine conditioning using 5 mg/kg dose (F 1,56 = 67.22, p < 0.0001) and by aprepitant treatment (F 1,56 = 8.15, p = 0.006). There was a significant interaction between morphine conditioning and aprepitant treatment (F 1,56 = 5.66) (p = 0.021). The locomotor activity of morphine and saline-conditioned mice remained same following vehicle (Fig. 3c, d). However, morphine-conditioned mice exhibited significantly lower locomotor activity following aprepitant, when compared to saline, or morphine-conditioned mice following vehicle (p < 0.005 and p < 0.05, respectively) (Fig. 3c, d). With regard to locomotor activity, tabular results from two-way ANOVA showed no significant effect by morphine conditioning (F 1,50 = 1.05, p = 0.31 and F 1,50 = 1.16, p = 0.30 at 1 and 5 mg/kg doses, respectively). However, there was a significant effect by aprepitant treatment (F 1,50 = 14.06, p = 0.0005 and F 1,56 = 12.73, p = 0.0005 for 1 and 5 mg/kg morphine, respectively). There was no significant interaction between morphine conditioning and aprepitant treatment (F 1,50 = 1.31, p = 0.258 and F 1,56 = 1.95, p = 0.168 for 1 and 5 mg/kg morphine, respectively). There was a small non-significant reduction in the locomotor activity of saline-conditioned mice following aprepitant treatment compared to vehicle treatment (Fig. 3c, d).
CPP scores recorded during postconditioning testing, given as means ± SEM, show significant CPP in mice conditioned with morphine a) 1 mg/kg (n = 8) or b) 5 mg/kg (n = 11) compared to mice conditioned with saline (n = 19). Treatment with aprepitant (10 mg/kg) significantly reduced morphine CPP. ** *** and *** * indicate significant effect by morphine (p < 0.005, p < 0.0003, and p < 0.0001, respectively) and ^^ indicates significant effect by aprepitant treatment in the morphine group (p < 0.005). Movement counts recorded simultaneously during postconditioning testing, given as means ± SEM, show significant locomotor activation in mice conditioned with morphine c) 1 mg/kg (n = 8) or d) 5 mg/kg (n = 11) compared to mice conditioned with saline (n = 19). Treatment with aprepitant (10 mg/kg) significantly reduced locomotor activity of morphine-conditioned mice. ** indicates significant effect by morphine (p < 0.005) and ^ indicates significant effect by aprepitant treatment in the morphine group (p < 0.05)
Aprepitant conditioning does not induce CPP or locomotor activation
Mice conditioned with aprepitant (10 mg/kg given i.p. for 3 days) when tested for CPP did not show either enhanced or decreased preference to drug-conditioned compartment as compared to saline-conditioned group (Fig. 4a). Unpaired Student’s t test showed a p value of 0.80120. There was also no significant change in the locomotor activity of the mice conditioned with aprepitant compared to that of saline-conditioned mice (Fig. 4b). Unpaired Student’s t test showed a p value of 0.9903. These results suggest that aprepitant does not induce CPP or conditioned place aversion, and it does not affect locomotor activity.
a) CPP scores recorded during postconditioning testing shows no CPP in mice conditioned with Aprepitant (10 mg/kg i.p. daily for 3 days) compared to vehicle conditioned mice. Data represent means ± SEM. b) Locomotor activity of mice recorded simultaneously as movement counts during postconditioning testing shows no significant difference between vehicle-conditioned mice and aprepitant-conditioned mice. Data represent means ± SEM
Discussion
Here, we demonstrate that a clinically used NK1R antagonist, aprepitant attenuates the expression of CPP induced by psychostimulants, AMPH, and cocaine while enhancing morphine-induced CPP. Substance P, a neuropeptide is highly expressed in brain stem monoaminergic nuclei, the brain regions involved in cognition, mood, and motor control and also in regions such as prefrontal cortex, amygdala and striatum where these monoaminergic nuclei project (Caberlotto et al. 2003; Chen et al. 2000; Commons 2010; Ebner et al. 2009; Griffante et al. 2006; Hargreaves 2002). Substance P infusions into these monoaminergic nuclei produce increased motor activity and stereotypy (Kelley and Iversen 1978; Stinus et al. 1978). Genetic deletion of NK1R renders ADHD-like phenotype, and there is a 2–4-fold increase in extracellular NE levels in the prefrontal cortex of NK1R knockout mice (Yan et al. 2009). Interestingly, NK1R exists in a physical complex with NET in the prefrontal cortex and also in the nucleus accumbens as shown by our previous study (Arapulisamy et al. 2013). All of these studies indicate a substantial interaction between NK1R signaling and NE transmission. While NK1R antagonism is shown to attenuate acute AMPH-induced locomotor activity by a previous study (Gonzalez-Nicolini and McGinty 2002), our current study for the first time demonstrates that NK1R antagonism attenuates both AMPH and cocaine-induced CPP and locomotor activation.
The current study demonstrates that psychostimulant-induced behaviors are sensitive to NK1R antagonism in that a single injection of aprepitant prior to postconditioning test significantly attenuated CPP expression and motor activity induced by AMPH and cocaine. Since, CPP across multiple drug classes appears to follow inverted u-shaped dose-response (Uhl et al. 2014), we tested both a low and a high dose for each of the drugs we tested. When tested on the postconditioning day (no drug on board at the time of testing), we observed robust CPP expression induced by both AMPH and cocaine at the doses tested. In our experimental conditions, while both 0.5 and 2 mg/kg AMPH conditioning produced enhanced locomotor activity, only 20 mg/kg cocaine conditioning enhanced locomotor activity. Aprepitant given just 15 min prior to CPP testing effectively attenuated both the expression of CPP and locomotor activation produced by these two psychostimulants. The use of within the subject controls further substantiates our finding that aprepitant effectively attenuates AMPH and cocaine-mediated behaviors. Furthermore, aprepitant did not affect the CPP expression induced by 1 mg/kg morphine conditioning. Moreover, in contrast to its attenuating effects on AMPH and cocaine CPP, aprepitant treatment enhanced the expression of CPP induced by 5 mg/kg morphine. These results indicate differential effect of NK1R antagonism on psychostimulant versus opioid reinforcing behaviors. Furthermore, the opposite results of NK1 antagonism on the expression of psychostimulant versus opioid CPP suggest that while endogenous SP/NK1 signaling may in part be necessary for the expression of conditioned psychostimulant reward, it may limit the expression of conditioned opioid seeking behavior.
Morphine-induced CPP expression was similar at both 1 and 5 mg/kg doses. It is known that morphine produces CPP to the same extent at doses 1 and 2.5 mg/kg, and only slightly more at 10 mg/kg dose (Leite-Morris et al. 2014). Although not statistically significant, aprepitant increased the CPP expression induced by 1 mg/kg morphine. However, aprepitant significantly enhanced the CPP expression induced by 5 mg/kg morphine. Enhanced morphine CPP expression following aprepitant is not surprising because in human studies, aprepitant indeed enhanced craving for opioid use (Jones et al. 2013; Walsh et al. 2013). There was no significant interaction between AMPH or cocaine conditioning and aprepitant treatment, suggesting a direct effect of aprepitant on psychostimulant-mediated behaviors. Interestingly, there was a significant interaction between morphine conditioning (at 5 mg/kg dose) and aprepitant treatment, which suggests neurokinin signaling may influence morphine-induced behaviors. While both AMPH and cocaine target NET and DAT, morphine is not known to target NET or DAT. Although psychostimulants and opiates share some of the mechanisms involving catecholamine signaling, there are distinct differences in the neurobiological mechanisms underlying psychostimulant versus opioid addiction (Badiani et al. 2011).
Although not statistically significant, aprepitant slightly reduced the locomotor activity of saline-conditioned mice and significantly reduced the locomotor activity of morphine-conditioned mice. While we cannot rule out the possibility that aprepitant at 10 mg/kg dose may have sedative effects contributing to reduced locomotor activity, NK1R antagonism appears to exacerbate sedative effects of morphine. Evidence exists in support of functional cross-talk between NK1 and opioid receptors in that both of these systems physiologically interact with respect to pain and analgesia (Bowman et al. 2015; Pfeiffer et al. 2003). Similarly, there is significant interaction between NE and opioid signals. In this regard, NET-KO mice exhibit enhanced morphine-induced analgesia via α2-adrenergic receptor activation suggesting a role for NET and NE homeostasis in morphine-induced analgesia (Bohn et al. 2000). Moreover, noradrenergic neuronal activity in the prefrontal cortex and locus coeruleus nuclei, as well as α1 and α2-adrenergic receptors, have been implicated in locomotor and rewarding effects of morphine (Drouin et al. 2002; Van Bockstaele and Valentino 2013; Ventura et al. 2005). A close anatomical and physiological association exists between substance P and NE systems in the brain (Chen et al. 2000; Fisher et al. 2007). A possible interaction between NE, opioid, and NK1 signals might be contributing to enhanced morphine CPP expression and reduced locomotor activity following aprepitant treatment.
Aprepitant by itself is not reinforcing as evidenced by its failure to induce CPP, but blunted both AMPH and cocaine CPP while enhancing morphine CPP. These results indicate that aprepitant effectively reaches brain regions following systemic administration and elicits its effects. Substance P in several neuronal pathways have been linked to stress and addiction, which are interconnected (Commons 2010). Thus, the utility of NK1 directed therapeutics in the clinic is an area of interest in addiction field. Animal studies indicate therapeutic benefits of NK1R antagonists in treating stimulant abuse, depression, and cancer (Gabrielian et al. 2013; Gonzalez-Nicolini and McGinty 2002; Kramer et al. 2004; Lewis et al. 2013). Species differences exist with respect to response to non-peptide NK1R antagonists, and rats and mice have amino acid residue changes at antagonist binding sites relative to humans and guinea pigs (Olive 2015; Saria 1999). However, aprepitant has been used in studies using both rats and mice as an effective NK1R antagonist (Ruzza et al. 2014; Utsumi et al. 2016; Yamamoto et al. 2014). In addition, aprepitant is already in clinical use as an antiemetic in cancer patients undergoing chemotherapy (Hargreaves et al. 2011; Patel and Lindley 2003). Recently, NK1R antagonism is found to decrease alcohol craving in humans (George et al. 2008). Another study demonstrated that NK1R antagonist L822429 suppresses stress-induced reinstatement of alcohol and cocaine seeking in rats (Schank et al. 2014). Thus, current study adds further insights into the potential therapeutic benefits of NK1R antagonists in the treatment of drug abuse and addiction.
References
Amadoro G, Pieri M, Ciotti MT, Carunchio I, Canu N, Calissano P, Zona C, Severini C (2007) Substance P provides neuroprotection in cerebellar granule cells through Akt and MAPK/Erk activation: evidence for the involvement of the delayed rectifier potassium current. Neuropharmacology 52:1366–1377
Annamalai B, Mannangatti P, Arapulisamy O, Ramamoorthy S, Jayanthi LD (2010) Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis. J Neurochem 115:23–35
Arapulisamy O, Mannangatti P, Jayanthi LD (2013) Regulated norepinephrine transporter interaction with the neurokinin-1 receptor establishes transporter subcellular localization. J Biol Chem 288:28599–28610
Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011) Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12:685–700
Bohn LM, Xu F, Gainetdinov RR, Caron MG (2000) Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci 20:9040–9045
Bowman SL, Soohoo AL, Shiwarski DJ, Schulz S, Pradhan AA, Puthenveedu MA (2015) Cell-autonomous regulation of Mu-opioid receptor recycling by substance P. Cell Rep 10:1925–1936
Caberlotto L, Hurd YL, Murdock P, Wahlin JP, Melotto S, Corsi M, Carletti R (2003) Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci 17:1736–1746
Chen LW, Wei LC, Liu HL, Rao ZR (2000) Noradrenergic neurons expressing substance P receptor (NK1) in the locus coeruleus complex: a double immunofluorescence study in the rat. Brain Res 873:155–159
Chu JM, Chen LW, Chan YS, Yung KK (2011) Neuroprotective effects of neurokinin receptor one in dopaminergic neurons are mediated through Akt/PKB cell signaling pathway. Neuropharmacology 61:1389–1398
Commons KG (2010) Neuronal pathways linking substance P to drug addiction and stress. Brainresearch 1314:175–182
Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP (2002) Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. The Journal of neuroscience: the official journal of the Society for Neuroscience 22:2873–2884
Ebner K, Sartori SB, Singewald N (2009) Tachykinin receptors as therapeutic targets in stress-related disorders. Curr Pharm Des 15:1647–1674
Fisher AS, Stewart RJ, Yan T, Hunt SP, Stanford SC (2007) Disruption of noradrenergic transmission and the behavioural response to a novel environment in NK1R-/- mice. Eur J Neurosci 25:1195–1204
Foster JD, Cervinski MA, Gorentla BK, Vaughan RA (2006) Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol:197–214
Gabrielian L, Helps SC, Thornton E, Turner RJ, Leonard AV, Vink R (2013) Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir Suppl 118:201–204
Gadd CA, Murtra P, De Felipe C, Hunt SP (2003) Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:8271–8280
Gainetdinov RR, Caron MG (2003) Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol 43:261–284
George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M (2008) Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319:1536–1539
Gonzalez-Nicolini V, McGinty JF (2002) NK-1 receptor blockade decreases amphetamine-induced behavior and neuropeptide mRNA expression in the striatum. Brain Res 931:41–49
Griffante C, Carletti R, Andreetta F, Corsi M (2006) [3H]GR205171 displays similar NK1 receptor binding profile in gerbil and human brain. Br J Pharmacol 148:39–45
Hall FS, Li XF, Randall-Thompson J, Sora I, Murphy DL, Lesch KP, Caron M, Uhl GR (2009) Cocaine-conditioned locomotion in dopamine transporter, norepinephrine transporter and 5-HT transporter knockout mice. Neuroscience 162:870–880
Hargreaves R (2002) Imaging substance P receptors (NK1) in the living human brain using positron emission tomography. The Journal of clinical psychiatry 63(Suppl 11):18–24
Hargreaves R, Ferreira JC, Hughes D, Brands J, Hale J, Mattson B, Mills S (2011) Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann N Y Acad Sci 1222:40–48
Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S (2006) Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem 281:23326–23340
Jayanthi LD, Ramamoorthy S (2005) Regulation of monoamine transporters: influence of psychostimulants and therapeutic antidepressants. AAPS J 7:E728–E738
Jones JD, Speer T, Comer SD, Ross S, Rotrosen J, Reid MS (2013) Opioid-like effects of the neurokinin 1 antagonist aprepitant in patients maintained on and briefly withdrawn from methadone. The American journal of drug and alcohol abuse 39:86–91
Kahlig KM, Galli A (2003) Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol 479:153–158
Kelley AE, Iversen SD (1978) Behavioural response to bilateral injections of substance P into the substantia nigra of the rat. Brain Res 158:474–478
Kertes E, Laszlo K, Berta B, Lenard L (2010) Positive reinforcing effects of substance P in the rat globus pallidus revealed by conditioned place preference. Behav Brain Res 215:152–155
Kraft M, Ahluwahlia S, Angulo JA (2001) Neurokinin-1 receptor antagonists block acute cocaine-induced horizontal locomotion. Ann N Y Acad Sci 937:132–139
Kramer MS, Winokur A, Kelsey J, Preskorn SH, Rothschild AJ, Snavely D, Ghosh K, Ball WA, Reines SA, Munjack D, Apter JT, Cunningham L, Kling M, Bari M, Getson A, Lee Y (2004) Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 29:385–392
Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63:585–640
Lallemend F, Lefebvre PP, Hans G, Rigo JM, Van de Water TR, Moonen G, Malgrange B (2003) Substance P protects spiral ganglion neurons from apoptosis via PKC-Ca2+-MAPK/ERK pathways. J Neurochem 87:508–521
Leite-Morris KA, Kobrin KL, Guy MD, Young AJ, Heinrichs SC, Kaplan GB (2014) Extinction of opiate reward reduces dendritic arborization and c-Fos expression in the nucleus accumbens core. Behav Brain Res 263:51–59
Lewis KM, Harford-Wright E, Vink R, Ghabriel MN (2013) NK1 receptor antagonists and dexamethasone as anticancer agents in vitro and in a model of brain tumours secondary to breast cancer. Anti-Cancer Drugs 24:344–354
Lim R, Morrill JM, Prushik SG, Reed KL, Gower AC, Leeman SE, Stucchi AF, Becker JM (2008) An FDA approved neurokinin-1 receptor antagonist is effective in reducing intraabdominal adhesions when administered intraperitoneally, but not orally. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract 12:1754–1761
Lindefors N, Brodin E, Ungerstedt U (1989) Amphetamine facilitates the in vivo release of neurokinin A in the nucleus accumbens of the rat. Eur J Pharmacol 160:417–420
Mannangatti P, Arapulisamy O, Shippenberg TS, Ramamoorthy S, Jayanthi LD (2011) Cocaine up-regulation of the norepinephrine transporter requires threonine 30 phosphorylation by p38 mitogen-activated protein kinase. J Biol Chem 286:20239–20250
Mannangatti P, Narasimha Naidu K, Damaj MI, Ramamoorthy S, Jayanthi LD (2015) A role for p38 mitogen-activated protein kinase mediated threonine 30 dependent norepinephrine transporter regulation in cocaine sensitization and conditioned place preference. J Biol Chem 290:10814–10827
Monastyrskaya K, Hostettler A, Buergi S, Draeger A (2005) The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J Biol Chem 280:7135–7146
Murtra P, Sheasby AM, Hunt SP, De Felipe C (2000) Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature 405:180–183
Nakamura Y, Izumi H, Fukushige R, Shimizu T, Watanabe K, Morioka N, Hama A, Takamatsu H, Nakata Y (2014) Continuous infusion of substance P into rat striatum alleviates nociceptive behavior via phosphorylation of extracellular signal-regulated kinase 1/2. J Neurochem 131:755–766
Noailles PA, Angulo JA (2002) Neurokinin receptors modulate the neurochemical actions of cocaine. Ann N Y Acad Sci 965:267–273
Olive MF (2015) Neurokinin-1 (NK(1)) receptor antagonists as possible therapeutics for psychostimulant use disorders. CNS & neurological disorders drug targets 14:700–706
Patel L, Lindley C (2003) Aprepitant—a novel NK1-receptor antagonist. Expert Opin Pharmacother 4:2279–2296
Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schroder H, Hollt V, Schulz S (2003) Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem 278:51630–51637
Placenza FM, Fletcher PJ, Vaccarino FJ, Erb S (2006) Effects of central neurokinin-1 receptor antagonism on cocaine- and opiate-induced locomotor activity and self-administration behaviour in rats. Pharmacol Biochem Behav 84:94–101
Placenza FM, Vaccarino FJ, Fletcher PJ, Erb S (2005) Activation of central neurokinin-1 receptors induces reinstatement of cocaine-seeking behavior. Neurosci Lett 390:42–47
Ramamoorthy S, Shippenberg TS, Jayanthi LD (2011) Regulation of monoamine transporters: role of transporter phosphorylation. Pharmacol Ther 129:220–238
Ramsey AJ, Laakso A, Cyr M, Sotnikova TD, Salahpour A, Medvedev IO, Dykstra LA, Gainetdinov RR, Caron MG (2008) Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 33:2701–2714
Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN (2002) Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology 43:1258–1268
Robinson JE, Fish EW, Krouse MC, Thorsell A, Heilig M, Malanga CJ (2012) Potentiation of brain stimulation reward by morphine: effects of neurokinin-1 receptor antagonism. Psychopharmacology 220:215–224
Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG (1998) Cocaine self-administration in dopamine-transporter knockout mice. Nature Neurosci 1:132–137
Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F (2014) The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Archiv: European journal of physiology 466:25–42
Ruzza C, Rizzi A, Malfacini D, Cerlesi MC, Ferrari F, Marzola E, Ambrosio C, Gro C, Severo S, Costa T, Calo G, Guerrini R (2014) Pharmacological characterization of tachykinin tetrabranched derivatives. Br J Pharmacol 171:4125–4137
Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci U S A 105:4405–4410
Saria A (1999) The tachykinin NK1 receptor in the brain: pharmacology and putative functions. Eur J Pharmacol 375:51–60
Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M, Weinshenker D, Schroeder JP (2014) The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39:1093–1101
Sotnikova TD, Beaulieu JM, Gainetdinov RR, Caron MG (2006) Molecular biology, pharmacology and functional role of the plasma membrane dopamine transporter. CNS & neurological disorders drug targets 5:45–56
Stinus L, Kelley AE, Iversen SD (1978) Increased spontaneous activity following substance Pinfusion into A10 dopaminergic area. Nature 276:616–618
Stuart SA, Butler P, Munafo MR, Nutt DJ, Robinson ES (2013) A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38:1625–1635
Uhl GR, Drgonova J, Hall FS (2014) Curious cases: altered dose-response relationships in addiction genetics. Pharmacol Ther 141:335–346
Utsumi D, Matsumoto K, Amagase K, Horie S, Kato S (2016) 5-HT3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Br J Pharmacol 173:1835–1849
Van Bockstaele EJ, Valentino RJ (2013) Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses. Adv Pharmacol 68:405–420
Van den Bos R, Cools AR, Ogren SO (1989) Neurokinin A enhances the stimulatory effects of d-amphetamine on motor activity in the nucleus accumbens of the rat. Acta Physiol Scand 137:547–548
Van den Bos R, Cools AR, Ogren SO (1990) Neurokinin A enhances the stimulatory effects of d-amphetamine on motor activity in the nucleus accumbens of the rat. Acta Physiol Scand 138:423–424
Ventura R, Alcaro A, Puglisi-Allegra S (2005) Prefrontal cortical norepinephrine release is critical for morphine-induced reward, reinstatement and dopamine release in the nucleus accumbens. Cereb Cortex 15:1877–1886
Walsh SL, Heilig M, Nuzzo PA, Henderson P, Lofwall MR (2013) Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addict Biol 18:332–343
Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG (2000) Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci 3:465–471
Yamamoto K, Asano K, Tasaka A, Ogura Y, Kim S, Ito Y, Yamatodani A (2014) Involvement of substance P in the development of cisplatin-induced acute and delayed pica in rats. Br J Pharmacol 171:2888–2899
Yan TC, Hunt SP, Stanford SC (2009) Behavioural and neurochemical abnormalities in mice lacking functional tachykinin-1 (NK1) receptors: a model of attention deficit hyperactivity disorder. Neuropharmacology 57:627–635
Acknowledgements
Dr. Jayanthi and Dr. Ramamoorthy declare that the funding for this research was provided in part by NIH grants DA039451 and MH083928 as well as by Virginia Commonwealth University. Dr. Mannangatti gratefully acknowledges post-doctoral support from the department of Pharmacology and Toxicology at Virginia Commonwealth University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal procedures were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guide (NIH) guidelines published in the National Research Council (2011) Guide for the Care and Use of Laboratory animals, 8th edition, National Academies Press, Washington, DC.
Rights and permissions
About this article
Cite this article
Mannangatti, P., Sundaramurthy, S., Ramamoorthy, S. et al. Differential effects of aprepitant, a clinically used neurokinin-1 receptor antagonist on the expression of conditioned psychostimulant versus opioid reward. Psychopharmacology 234, 695–705 (2017). https://doi.org/10.1007/s00213-016-4504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4504-6