Abstract
Parthenolide (PTL) is a sesquiterpene lactone that occurs naturally. It demonstrates a variety of beneficial effects, such as antioxidant, anti-inflammatory, and antiapoptotic properties. The study investigated the potential protective impact of PTL on indomethacin (INDO) induced stomach ulcers in rats. The rats were classified into 5 distinct categories. Group 1 served as the “control” group. Rats in the second group received a single oral dosage of INDO (50 mg kg−1). Rats in Groups three and four received 20 and 40 mg kg−1 oral PTL 1 h before INDO. Omeprazole (30 mg kg−1) was given orally to Group 5 rats 1 h before INDO. Pretreatment with PTL increased stomach pH and decreased gastric volume as well as reduced the morphological and histological changes induced by INDO. Analysis of probable pathways showed that pre-treatment with PTL successfully reduced oxidative, inflammatory, and apoptotic consequences caused by INDO. The ingestion of PTL leads to a notable increase in the levels of glutathione reduced (GSH) and the activities of superoxide dismutase (SOD) and catalase (CAT). Furthermore, PTL decreased the concentration of malondialdehyde (MDA). In contrast, it was shown that PTL increased both cyclooxygenase-1 (COX-1) and prostaglandin E2 (PGE2). PTL shows a significant decrease in the expression of interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), inducible nitric oxide synthase (iNOS), and nuclear factor kappa B (NF-κB). PTL therapy resulted in a decrease in Bcl-2-associated X protein (Bax) levels and an increase in B-cell lymphoma 2 (Bcl2) levels. In conclusion, PTL offers gastroprotection by its antioxidant, anti-inflammatory, and anti-apoptotic qualities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A gastric ulcer (GU) is a common condition that affects the digestive tract and is known for its high clinical prevalence and recurrence rate (Xie et al. 2021). Recent studies have investigated the cause of GU and found an imbalance between its aggressive factors (such as high levels of gastric acid, bile salts, increased pepsin action, ethanol, and drugs) and defensive factors (including cellular regeneration, microcirculation, bicarbonate ion production, mucus secretion, and adequate levels of prostaglandins and nitric oxide) (Ermis et al. 2023; Guzmán-Gómez et al. 2023; Shaik and Eid 2022). The main causes of the condition include Helicobacter pylori infection, stress, and prolonged use of nonsteroidal anti-inflammatory drugs (NSAIDs) (Guzmán-Gómez et al. 2023).
NSAIDs are widely used medications globally, and a significant number of users experience gastrointestinal issues (Tamaddonfard et al. 2019). Indomethacin (INDO) is a commonly used non-selective NSAID for treating arthritis and various inflammatory conditions (Jafari et al. 2022; Ma et al. 2022). Regular use of it causes changes in the integrity of the gastric mucosa, which is a key factor in the progression of gastric ulcers (Drini 2017). INDO has a high ulcerogenic potential compared to other NSAIDs, making it a common choice for inducing ulcers in rodents (Ugan and Un 2020). INDO inhibits the formation of prostaglandin E2 (PGE2) by blocking the gastroprotective enzyme cyclooxygenase-1 (COX-1), which contributes to mucosal injury and the formation of peptic ulcers (Aleid et al. 2021; AlKreathy et al. 2020). Several studies have demonstrated that INDO boosts the production of reactive oxygen species (ROS) and inflammatory cytokines (Danisman et al. 2023; Eraslan et al. 2020; Ito et al. 2016). Excessive generation of ROS impairs the antioxidant defense mechanism, leading to oxidative stress, which contributes to the development of gastric inflammation and peptic ulcers (Mahmoud et al. 2023). Mucosal inflammation is the primary cause of gastric ulceration involving nitric oxide (NO) and inducible nitric oxide synthase (iNOS) (Küçükler et al. 2022; Nathan 1997). Some studies have investigated that ulcerated stomach and duodenal mucosa are linked to increased iNOS mRNA expression (Araki et al. 2002; Mard et al. 2016). Several studies suggest that INDO can also induce apoptosis (Ahmed et al. 2021; Liu et al. 2015b; Ma et al. 2022).
Treating GU conditions can be difficult due to the complex nature of the disease. Gastric ulcers are commonly treated with a variety of treatments such as H2 antagonists, antimicrobials, proton pump inhibitors, and antacids. Prolonged use of the above medications might lead to various adverse effects such as gynecomastia, vitamin B12 deficiency, hypergastrinemia, hypochlorhydria, osteoporotic fractures, depression, and constipation (Benvenutti et al. 2020; Kim et al. 2020a, b; Zhou et al. 2020). Researchers and medical practitioners have utilized natural compounds from plants for many years to heal stomach ulcers due to their therapeutic characteristics. These chemicals are popular because they have few side effects and are highly effective for therapy (Bi et al. 2014). These compounds possess antioxidant, anti-inflammatory, and anti-apoptotic capabilities, which can be effective in alleviating GUs (Ahmed et al. 2021; Barboza et al. 2018; Sabiu et al. 2015; Wang et al. 2014).
Sesquiterpene lactones are bioactive compounds derived from medicinal plants with antioxidant, antimicrobial, anti-migraine, and anti-inflammatory activities. They are used to treat stomach aches, gastric ulcers, cancer, and skin conditions (Shoaib et al. 2017). Parthenolide (PTL) is a sesquiterpene lactone from the germacrene class found in the feverfew plant (Tanacetum parthenium) and is frequently used in traditional medicine (Freund et al. 2020; Pareek et al. 2011; Zhu et al. 2023). Earlier research has shown that PTL has several actions, including antioxidative, anti-inflammatory, and anti-apoptotic effects (Albalawi et al. 2023; Kim et al. 2020a, b; Zhang et al. 2020).
Tournier and coworkers reported that PTL treatment reduces ethanol-induced gastric ulcers in rats (Tournier et al. 2010). However, the precise role of PTL in reducing the occurrence of acute gastric ulcers caused by NSAIDs has not been established. The current study aims to investigate the effect of PTL on gastric injury caused by INDO in rats and to examine its potential mechanisms of gastroprotection.
Materials and methods
Drugs and chemicals
Parthenolide (PTL), indomethacin (INDO), omeprazole (OMEP), and carboxymethyl cellulose sodium (CMC-Na) were purchased from Sigma Aldrich, St. Louis, MO, USA. Formaldehyde, phosphate buffer, and further needed substances were collected and found on the utmost purity mark.
Animals
An overall of 30 male Wistar rats weighing 180 to 200 g had been received by the vivarium (Faculty of Pharmacy (FOP), King Abdulaziz University (KAU), Jeddah, and KSA), and accommodated in a regulated temperature (22 ± 2°C) and humidity (50–55%), under a 12/12 h light/dark cycle. The ethical committee of FOP, KAU reviewed the study procedures and gave its approval (Ref no - PH-1444-40). The food pellets were withdrawn from all the animals 24 h before the study commenced, although they were allowed liberty to access the water.
Experimental protocol
We classified the animals into five clearly defined categories (n = 6). Group 1 (control): This cohort of animals was administered 0.5% CMC-Na via the intragastric route. The rats in the second group were administered INDO orally at a dosage of 50 mg kg−1. Rats in groups 3 and 4 were exclusively administered oral doses of PTL at 20 or 40 mg kg−1, respectively, 1 h prior to the administration of INDO. Group 5 rats were administered a single oral dosage of OMEP (30 mg kg−1), 1 h before the administration of INDO. The PTL doses chosen for this study were based on the proven gastroprotective effectiveness of PTL in a previous study (Tournier et al. 2010). Following a 4-h session of INDO therapy, rats in each experimental group were euthanized under isoflurane anaesthesia (Ermis et al. 2023).
Measurement of gastric juice volume and pH
Following the induction of anesthesia, the animals underwent dissection. The pylorus of the stomach was subsequently ligated, and then the stomach was separated and incised along a larger arc. The stomach contents were promptly collected and transferred into tubes. The tubes were subjected to centrifugation at a speed of 2500× g for a duration of 5 min to separate any solid residues, and the resulting liquid portion was measured. A digital pH meter was used to calculate the pH of the stomach contents (PHscan 40, BANTE, China; Pocket PH tester).
Gastric ulcer area measurement
After detaching the stomach contents, the mucosa was cleansed using a cold saline solution (0.9% w/v NaCl) and then stretched out on filter paper. Subsequently, photographs were taken using a digital camera. Afterwards, the stomachs were checked macroscopically for the appearance of haemorrhagic lesions. The ulcer area was measured in millimeters by using a ruler and ImageJ software (ImageJ, version 1.54h, NIH, Bethesda, MD, USA) (Abdelkader Saidi et al. 2023).
The ulcer index was computed by applying the formula: Ulcer index = 10 ÷ x
x = the total mucosal area/total ulcerated area (Sathish et al. 2011).
Histopathological examinations
The stomach samples were immersed in a liquid of neutral buffered formalin (10%) for a period of 24 h. Subsequently, they underwent dehydration using graded ethanol solutions and were then embedded in paraffin. We obtained sections with a breadth of 4.0 μm and dyed them using hematoxylin and eosin (H&E). An experienced pathologist subsequently evaluated the pathological alterations. The scoring system was applied by the earlier research (AlKreathy et al. 2020): [1] loss of epithelial cell (score: 0–3), [2] hemorrhage (score: 0–4), [3] infiltration of an inflammatory cell (score: 0–2), and [4] mucosal erosions (score: 0–4).
Assessment of oxidative stress markers
The stomach tissues were homogenized by employing a solution composed of 10% ice-cooled phosphate-buffered saline (pH 7.4). The homogenates were centrifuged at 10,000g for 20 min at 4°C. To examine markers of oxidative stress, the supernatant was subjected to centrifugation at 10,000 g for 20 min at 4 °C. The concentration of malondialdehyde (MDA), levels of reduced glutathione (GSH), and activity of superoxide dismutase (SOD) and catalase (CAT) were evaluated using kits. The catalog numbers MD-2529, GR-2511, SD-2521, and CA-2517 refer to products from Bio Diagnostic in Giza, Egypt.
Assessment of COX-1 and PGE2
The assessment of COX-1 concentration and PGE2 levels in stomach tissue was conducted using the kits (Cat. # MBS753621 and Cat # MBS730592, MyBioSource, Inc., San Diego, CA, USA). This assay employed a quantitative competitive approach.
Immunohistochemical assessment of IL-1β, TNF-α, iNOS and NF-κBp65
To conduct immunohistochemical (IHC) studies, the sample sections were subjected to incubation with primary antibodies. The primary antibodies used were, IL-1β, TNFα, iNOS (Cat #. ab283818, ab220210, and ab283655 respectively, Abcam, Cambridge, UK), and NF-κB p65 (Cat. No. 8242, Cell Signaling, Danvers, MA, USA). The incubation was carried out for 12 h at a temperature of 4°C. Following a TBS rinse, the samples were subjected to incubation with a biotinylated secondary antibody (anti-mouse or anti-rabbit), reliant on the reactivity of the prime antibody. The Cell & Tissue Dyeing Kit, with catalog numbers CTS002 and CTS005, was obtained from R&D systems, Minneapolis, MN, USA. The pictures were quantified as optical density (OD) by the method known as the ImageJ software (ImageJ, version 1.54h, NIH, Bethesda, MD, USA) (Schacht and Kern 2015).
Assessment of apoptotic markers
The RNA extraction process utilizing the TRIzol technique was performed on gastric tissues. The confirmation of RNA purity was achieved through the measurement of the A260/A280 proportion. The cDNA synthesis process utilized the Omniscript RT kit (Catalog Number 205113, Qiagen, Maryland, USA). The measurement of mRNA was conducted using qRTPCR with a SYBR Green Master Mix (Catalog Number 180830, Qiagen, Maryland, USA). The nucleotide sequences of the primers are presented in Table 1. The data were subjected to analysis using the ΔΔCT method, with β-actin aiding as the normalization (Livak and Schmittgen 2001).
Statistical analysis
The data is presented in the format of the mean value plus or minus the standard deviation (SD). The histological score data underwent analysis using the Kruskal-Wallis test, followed by Dunn’s multiple comparison test. The remaining data was analyzed using a one-way analysis of variance (ANOVA) approach, accompanied by Tukey’s multiple comparison test, to detect and evaluate any significant differences. A significance level of p < 0.05 was considered to indicate statistical significance. The statistical analysis was performed using GraphPad Prism software version 8, developed by GraphPad Software, Inc. in La Jolla, CA, USA.
Results
Effects of PTL on gastric juice volume and pH
The control group showed a gastric juice volume of 1.01 mL and a pH of 3.63 units. The experimental group, known as INDO, showed a statistically significant rise in the quantity of gastric juice (2.83 mL) as shown in Fig. 1A. In addition, Fig. 1B demonstrates a significant reduction in the acidity of gastric juice (2.08 units) within the same group. Administering PTL at dosages of 20 and 40 mg kg−1 resulted in a significant reduction in the amount of gastric juice (2.11 mL and 1.48 mL, respectively) and a rise in gastric juice pH (2.86 units and 3.53 units, respectively) (Fig. 1A and B). Ingesting OMEP caused a significant reduction in the amount of gastric juice, measuring 0.93 mL, and a notable increase in the pH level of gastric juice, measuring 4.08 units, as shown in Fig. 1A and B.
Effects of PTL on gastric juice volume and pH. (A) The volume of gastric juice in various groups. (B) pH of the stomach in various groups. The data are shown as Mean ± SD (n = 6). *, #, $ and @; statistically significant (p < 0.05) from the control, INDO, PTL (20 mg kg−1) + INDO, and PTL (40 mg kg−1) + INDO, respectively
Effect of PTL pretreatment on stomach morphology and histopathology
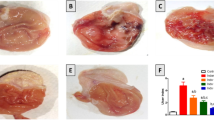
Upon macroscopic inspection, the stomach tissues of the control rats showed a typical peripheral appearance. The stomach mucosa of rats in the control group exhibited no evidence of lesions or redness. The macroscopic examination of the gastric mucosal surface of the rats treated with INDO exhibited bloody streaks, the stomachs of the rats pretreated with PTL (20 mg kg−1) showed mild damage and the rats pretreated with PTL (40 mg kg−1) showed minimal damage with intact mucosa. Pretreatment with OMEP (30 mg kg−1), showed no signs of redness or impairment (Fig. 2).
Macrophotography of the stomachs of rats. (A) Control group showing lesion and redness free gastric mucosa. (B) INDO group showing severe hemorrhagic lesions in the gastric mucosa. (C) PTL (20 mg kg−1) + INDO demonstrating minor wounds. (D) PTL (40 mg kg−1) + INDO showing little damage with healthy mucosa. (E) OMEP (30 mg kg−1) + INDO showing an almost normal mucosa and, exhibiting no redness or damage. (F) Ulcer index. The mean ± SD of the data is displayed. *, #, and $; significantly (p < 0.05) different from control, INDO, and PTL (20 mg kg−1) + INDO respectively
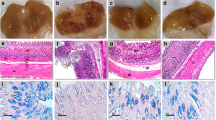
The examination of the stomach mucosa in rats from the control (Fig. 3A) revealed the presence of typical histological characteristics, including outer epithelium, lamina propria encompassing gastric glands, and muscularis mucosa. The nuclei exhibit a vesicular morphology. The analysis of the gastric mucosa in rats belonging to the group with INDO-induced ulcers (Fig. 3B) exhibited significant damage and varying levels of distortion in the gastric mucosa. In certain sections, there were observed instances of partial loss of the gastric mucosa, accompanied by disarray in the organization of the gastric glands and leakage of blood. A multitude of clogged blood vessels of varying dimensions and blood leakage have been detected within the lamina propria and submucosa. The analysis of the stomach mucosa in rats from the PTL (20 mg kg−1) + INDO group (Fig. 3C) demonstrated a modest enhancement in its architecture compared to the INDO group. Specifically, tiny regions of ulceration or erosion were found in the upper sections of gastric glands. The observed phenomenon involved the regeneration of surface epithelial cells, which exhibited discontinuity in certain regions. The evaluation of the gastric mucosa of PTL (40 mg kg−1) + INDO group (Fig. 3D) demonstrated significant enhancement in its structure compared to the INDO group. The observed improvements were comparable to those observed in the control. Additionally, the outer epithelial cells emerged continuously and extended to line the pits of the gastric glands. The arrangement of the stomach glands exhibited regularity. Assessment of the gastric mucosa in the OMEP + INDO group (Fig. 3E) demonstrated general enrichment in the makeup of the gastric mucosa while correlated to the INDO group. While the majority of gastric glands exhibited a normal arrangement, a subset of these glands displayed enlarged lumina accompanied by truncated gastric pits (Table 2).
Photomicrographs of histological examination of the gastric mucosa from rats of different groups: (A) control group indicating the full length of gastric glands (GG) and certain blood vessels in the lamina propria (LP), lumen (L), and muscularis mucosa (Mm). (B) INDO group discloses a tiny loss of the gastric mucosa (
 )with an absence of surface epithelium and superior segments of the gastric glands (GG) and areas of hemorrhage (
)with an absence of surface epithelium and superior segments of the gastric glands (GG) and areas of hemorrhage (
 ). (C) PTL (20 mg kg-1) + INDO group showing small areas of ulceration and erosion of gastric mucosa (
). (C) PTL (20 mg kg-1) + INDO group showing small areas of ulceration and erosion of gastric mucosa (
 ) with the distorted appearance of gastric glands (GG). (D) PTL (40 mg kg-1) + INDO group commonly positioned gastric glands (GG) and spread out on the superficial by narrow gastric pits (gp). (E) OMEP + INDO group showed regularly arranged gastric glands (GG) with some dilated lumina (*)
) with the distorted appearance of gastric glands (GG). (D) PTL (40 mg kg-1) + INDO group commonly positioned gastric glands (GG) and spread out on the superficial by narrow gastric pits (gp). (E) OMEP + INDO group showed regularly arranged gastric glands (GG) with some dilated lumina (*)
Effect of PTL on oxidative stress markers
The evaluation of PTL-induced antioxidant effects was performed by measuring the levels of MDA, content of GSH, and activity of SOD and CAT in the stomach homogenate. According to the data presented in Table 3, exposure to INDO results in a significant increase in MDA levels, a decrease in GSH levels, and a reduction in CAT and SOD activity by 270.22%, 75.50%, 63.39%, and 52.98%, respectively, compared to a control group. Pre-administration of PTL at doses of 20 or 40 mg kg−1, as well as OMEP, resulted in a significant reduction in MDA levels by 34.47%, 58.57%, and 65.84%, respectively, compared to the INDO group. The groups treated with PTL (20 or 40 mg kg−1) as well as OMEP showed a significant rise in stomach GSH levels. The percentage increases were 77.53%, 229.2%, and 258.65%, respectively. When rats were given PTL (at doses of 20 or 40 mg kg−1) and OMEP, there was a considerable increase in SOD activity. The increases were 37.13%, 92.40%, and 116.29%, respectively. In addition, we observed a significant increase in CAT activity in the groups who received pre-treatment with PTL (20 or 40 mg kg−1) and OMEP. The CAT activity in these groups exhibited respective increases of 40.84%, 74.64%, and 92.95% compared to the INDO group.
Effect of PTL pretreatment on COX-1 and PGE2 concentrations
The data presented in Fig. 4A shows that exposure to INDO resulted in a significant decline of 59.53% in the concentration of COX-1 in the stomach tissue compared to the control. Pre-treatment with PTL at 20 and 40 mg kg−1, on the other hand, significantly raised COX-1 levels (46.50%, and 87.36%, respectively) compared to the INDO group. Furthermore, pretreated with OMEP exhibited a substantial rise in COX-1 concentration (118%) compared to the INDO. The results in Fig. 4B demonstrate that INDO ensued a notable reduction (54.48%) in the level of gastroprotective PGE2, compared to the control group. Nevertheless, PTL at (20 and 40 mg kg−1) and OMEP led to a noteworthy upsurge in the PGE2 level, with enhancements of 41.34%, 75.31%, and 94%, respectively, compared to the INDO group (Fig. 4B).
Effect of PTL pretreatment on inflammatory markers
The treatment of INDO produced a significant rise in the IL-1β, TNF-α, iNOS, and total NF-κB p65, expression (210.88%, 126.34%, 292.41%, and 160.41%) related to the control. PTL at 20 mg kg−1 exhibited a considerable reduction of 44%, 25.78%, 36.53%, and 31.74% correspondingly with comparison to the INDO. PTL at 40 mg kg−1 exhibited reductions in their expression levels by 45.89%, 41.98%, 43.18%, and 49.22%, respectively. Furthermore, the OMEP indicated a noteworthy decrease in the expression levels of, IL-1β, TNF-α, iNOS, and total NF-κB p65 by 63.46%, 49.45%, 69.19%, and 55%, respectively, in comparison to the INDO group. Figure 5 illustrates the immunohistochemistry findings.
PTL effect on INDO-induced apoptosis
As depicted in Fig. 6, INDO ensued in the initiation of apoptosis, as evidenced by the notable increase in the expression of the Bax (498%) in comparison with the control. Nevertheless, the administration of PTL at doses of 20 or 40 mg kg−1 and OMEP, in conjunction with INDO, resulted in the mitigation of these alterations. This was achieved through the downregulation of Bax mRNA expression, which exhibited reductions of 27.16%, 45.19%, and 53.81%, respectively when compared to the effects of INDO therapy alone. Additionally, an examination was conducted on the mRNA expression levels of the Bcl-2 in stomach tissue. In comparison to the control, the rats treated with INDO had a notable reduction (61.51%) in Bcl-2 mRNA expression. The administration of PTL at doses of 20 or 40 mg kg−1 and OMEP in combination with INDO caused a substantial upregulation of Bcl-2 expression. The increase in Bcl-2 expression was 40.98%, 91.41%, and 124.58% for the corresponding doses, compared to the group treated with INDO alone.
Discussion
Our study demonstrates the substantial gastroprotective effect of PTL on GU induced by INDO in a rat model. The gastroprotective effects of PTL are likely due to its antioxidative, anti-inflammatory, and antiapoptotic features. An important drawback of NSAIDs that limits their use in clinical settings is the occurrence of GUs (Alp Yildirim et al. 2015). Administering INDO to rats is an experimental method used in research studies to induce GU and study the pathophysiological mechanisms and potential pharmacological treatments for this condition (Chen et al. 2016a, 2016b; Xavier et al. 2018). INDO-induced GU development entails multiple major mechanisms. The mechanisms involve the suppression of protective agents like PGE2, bicarbonate, and mucus release, disturbance of the equilibrium between oxidative and anti-oxidative processes, increased generation of inflammatory and apoptotic markers, and the infiltration of inflammatory cells due to the stimulation of gastric hypermotility (Naito and Yoshikawa 2006; Suleyman et al. 2010; Takeuchi 2012). In this study, it was found that treating rats with INDO led to a significant rise in gastric volume and a decline in pH value. This resulted in diminished protective capabilities of the mucosal membrane, ultimately causing tissue injury. This could be due to the generation of free radicals or the suppression of prostaglandin synthesis. A drop in prostaglandin levels can lead to reduced gastroprotection and increased stomach acid output (Boushra et al. 2019; Sabiu et al. 2016). The quantity and acidity levels of gastric juice reflect the secretion of gastric acid by parietal cells (Schubert 2017). Our study’s findings are consistent with previous studies, indicating that the use of INDO resulted in the formation of stomach ulcers via increasing gastric acidity levels (El-Ashmawy et al. 2016a; Oluwabunmi and Abiola 2015). Our study’s results show the pretreatment of PTL at two distinct doses (20 and 40 mg kg−1) resulted in significant alterations in gastric juice volume and pH induced by INDO.
INDO caused substantial damage to the gastric mucosa, as shown by macroscopic examination. The injury exhibited hemorrhagic streaks and was associated with a surge in the ulcer score. The histological studies in the INDO group showed epithelial injury, inflammatory cell infiltration, hemorrhage, and erosion of gastric mucosa. Gastric mucosal injuries like ulceration, bleeding, and erosion are caused by oxidative damage, which can be accelerated by the production of ROS (Chen et al. 2018; Liu et al. 2017). The significant rise in the macroscopic score following the INDO dose may be attributed to increased ROS levels and reduced prostaglandin production (Sabiu et al. 2015). The PTL at doses of 20 or 40 mg kg−1 reduced the development of gastric lesions, decreased ulcer index, and mitigated histological abnormalities generated by INDO, such as damaged epithelium, inflammatory cell infiltration, hemorrhage, and erosion of gastric mucosa. The effectiveness of its cytoprotective activity is probably due to its antioxidant properties.
A previous study has demonstrated that NSAIDs can cause harmful consequences by inducing oxidation in stomach tissue (Vaananen et al. 1991). INDO leads to an increase in ROS production in the body, resulting in oxidative damage to constitutive macromolecules (Boyacioglu et al. 2016). INDO causes stomach damage in rats by increasing reactive oxygen metabolites and reducing antioxidant parameters, leading to oxidative damage in cellular components (Suleyman et al. 2010; Arifa et al., 2016). Several studies have shown that NSAIDs, like INDO, can trigger lipid peroxidation and cause damage by producing ROS (Danisman et al. 2023; Harakeh et al. 2022).
MDA is a lipid peroxidation byproduct commonly used to measure oxidative stress in cell and tissue damage (Tsikas 2017). The gastrointestinal tract has enzymatic and nonenzymatic defenses that can reduce or prevent stomach tissue damage caused by excessive ROS generation (El-Ashmawy et al. 2016b; Heeba et al. 2009; Mahmoud et al. 2021). Free radicals can modify the cellular antioxidant defense system, which includes GSH, SOD, and CAT. This could result in exacerbated tissue damage during stomach ulceration (Ermis et al. 2023). The current study noted a significant decrease in SOD and CAT activities, as well as a reduction in GSH levels in the stomach tissues of rats given INDO. On the other hand, there is a noticeable rise in MDA levels. Pretreatment with PTL (20 and 40 mg kg−1) or OMEP resulted in increased SOD and CAT activity, higher levels of GSH, and reduced MDA content. These findings are consistent with previous studies that have shown how PTL can boost antioxidant defense systems, decrease lipid peroxidation, and eradicate free radicals generated during oxidative stress (Albalawi et al. 2023; Mao and Zhu 2018; Wang et al. 2020). The current empirical study indicates that PTL may enhance both nonenzymatic and enzymatic antioxidative defense systems, as well as decrease lipid peroxidation. The effects mentioned may play a role in the gastroprotective characteristics properties of PTL.
COX-1 is a crucial enzyme in the synthesis of PGE2 (Adhikary et al. 2011), INDO suppresses COX-1 activity, resulting in reduced levels of circulating PGE2 (Takeuchi 2012). PGE2 significantly affects mucus formation and increases gastric blood flow, contributing to the protective mechanism of the stomach (Musumba et al. 2009). It was noted that the administration of INDO significantly reduced the levels of COX-1 and PGE2. On the other hand, administering PTL resulted in elevated levels of COX-1 and PGE2. PTL treatment is believed to work by increasing the expression of COX-1 and PGE2 by replenishing antioxidant mechanisms, which helps to prevent gastric ulcers.
Oxidative stress often leads to inflammation in gastric tissues when INDO is administered. Oxidative stress causes an increase in ROS, which stimulates the production of NF-κB. We have utilized the IHC approach to evaluate inflammatory markers. IHC offers benefits such as precise antigen localization, user-friendly application, dependability, and adaptability (Schacht and Kern 2015). The study utilized total NF-κB p65 as an inflammatory marker in stomach tissues. NF-κB activation leads to the production of many genes linked to inflammation, leading to the secretion of TNF-α, IL-6, IL-12, and IL-1β. Cytokines play a crucial role in disrupting epithelial cells and contributing to the formation of stomach ulcers (Mahmoud et al. 2023).
Neutrophils and mononuclear cells infiltrating the stomach mucus membrane in stomach ulcers trigger the activation of transcription and the production of IL-1β and TNF-α (Luo et al. 2018). TNF-α and IL-1β contribute to inducing systemic inflammation and initiate the acute inflammatory phase. Moreover, they enhance the generation of superoxide by neutrophils, leading to tissue damage mediated by oxygen radicals (Salem et al. 2018). Conversely, TNF-α significantly contributes to the advancement of gastric injury caused by INDO by reducing blood flow to the mucosa, increasing gastrin expression, and hindering the healing process. It also increases the flow of white blood cells and other cells that cause inflammation in the stomach lining by promoting the production of various adhesion molecules. Moreover, it increases nitrosative stress by activating inducible nitric oxide synthase (iNOS) and enhancing the production of nitric oxide (NO) in the stomach area (Mahmoud et al. 2023; Santucci et al. 1995). Previous studies have shown that TNF-α generation can increase NO production by overexpressing iNOS in gastric ulcers induced by INDO (Nabil et al. 2021). Overproduction of NO by the enzyme iNOS has been shown to significantly contribute to the formation of ulcers (Zaghlool et al. 2019).
The present study indicates that ingesting INDO resulted in an increase in the expression of IL-1β, TNF-α, NF-κB p65, and iNOS in the stomach tissues. This finding aligns with previous studies (Küçükler et al. 2022; Mahmoud et al. 2022; Nabil et al. 2021; Zaghlool et al. 2019). The PTL treatment effectively reduces inflammation by suppressing the expression of IL-1β, TNF-α, NF-κB p65, and iNOS, as anticipated. This finding is consistent with the reported anti-inflammatory effects of PTL, which function via inhibiting IL-1β, TNF-α, NF-κB, and iNOS as reported by several studies (Fiebich et al. 2002; Liu et al. 2015a; Saadane et al. 2007; Sheehan et al. 2002; Zhu et al. 2023).
Research has demonstrated that INDO induces cell death in the stomach lining by apoptosis, necrosis, and autophagy (Gebril et al. 2020; Tomisato et al. 2001). The specific form of cell death appears to be contingent upon factors such as the dose and duration of exposure (Tomisato et al. 2001). Apoptosis has received significant attention for its role in the development and progression of INDO-induced stomach ulcers (Ahmed et al. 2021). NF-κB has a multifaceted impact on the progression of gastric ulcers. It plays a role in controlling inflammation and apoptosis. Activation of NF-ҡB represents an early event in TNF-α signaling and is implicated in mediating the effects of TNF-α, particularly TNF-α-induced cellular injury. (Takeuchi et al. 2002).TNF-α has been demonstrated to induce cell death via necrosis and/or apoptosis across various cell types. Previous investigations have revealed that INDO administration leads to an elevation in TNF-α expression within the gastric mucosa and promotes apoptosis in gastric epithelial cells (Slomiany et al. 1997). ROS and TNF-α are associated with the initiation of the apoptotic signaling pathway. TNF-α interacts with tumor necrosis factor receptor-1 (TNFR-1) to initiate the extrinsic apoptotic pathway (Chen et al. 2016a). TNF-α is crucial in the formation of stomach ulcers by triggering the first inflammatory response, leading to the influx of neutrophils at the site of injury, and activating caspase-3 to induce apoptosis (Hammady 2024). In addition, ROS may initiate the mitochondrial apoptosis pathway (Fleury et al. 2002).
Bcl-2 possesses antioxidant properties and can directly prevent oxidative damage (Grimm et al. 1996). Proapoptotic proteins like Bax promote the initiation of the mitochondrial apoptosis cascade, whereas antiapoptotic proteins like Bcl-2 inhibit this process. Bax can cause the release of cytochrome C and trigger the activation of caspase 3. Bcl-2 can bind to and oppose mitochondrial proapoptotic proteins to regulate apoptosis (Correia et al. 2015). This study shows that INDO treatment induces apoptosis by increasing Bax and decreasing Bcl-2 mRNA expression. Previous studies support the conclusions presented above (Chen et al. 2016b; Ma et al. 2022; Sayed Abdel-Tawab et al. 2020). The dysregulation of apoptosis-related proteins was significantly improved by the administration of PTL, which successfully suppressed Bax and increased Bcl-2 mRNA expression in the stomach tissue. Previous research has shown that PTL can decrease Bax levels and increase Bcl-2 activity (Albalawi et al. 2023; Yao et al. 2007; Zhang et al. 2004). It is reasonable to suggest that the anti-apoptotic effect may have contributed to the preventive action of PTL against gastric damage caused by INDO.
Conclusion
The study highlights the significant gastroprotective properties of PTL in preventing INDO-induced gastric ulcers. PTL has the potential as a therapeutic agent for treating gastrointestinal ulcers due to its strong antioxidant, anti-inflammatory, and apoptosis-modulating properties. The findings of this study not only provide insight into the complex mechanisms that contribute to the protective effects of parthenolide but also emphasize its potential clinical importance in reducing gastric ulcers. Therefore, additional investigation and clinical studies are necessary to fully utilize the therapeutic capabilities of parthenolide in the treatment of gastric ulcer disease.
Data availability
No datasets were generated or analysed during the current study.
References
Abdelkader Saidi S, Al-Shaikh TM, Hamden K (2023) Evaluation of gastroprotective effect of Betalain-rich ethanol extract from Opuntia stricta var. Dillenii employing an in vivo rat model. J Food Qual 2023:2215454. https://doi.org/10.1155/2023/2215454
Adhikary B, Yadav SK, Roy K, Bandyopadhyay SK, Chattopadhyay S (2011) Black tea and theaflavins assist healing of indomethacin-induced gastric ulceration in mice by antioxidative action. Evidence-Based Complement Altern Med 2011:1–11. https://doi.org/10.1155/2011/546560
Ahmed MAE, Mohanad M, Ahmed AAE, Aboulhoda BE, El-Awdan SA (2021) Mechanistic insights into the protective effects of chlorogenic acid against indomethacin-induced gastric ulcer in rats: modulation of the cross talk between autophagy and apoptosis signaling. Life Sci 275:119370. https://doi.org/10.1016/j.lfs.2021.119370
Albalawi RS, Binmahfouz LS, Hareeri RH, Shaik RA, Bagher AM (2023) Parthenolide phytosomes attenuated gentamicin-induced nephrotoxicity in rats via activation of Sirt-1, Nrf2, OH-1, and NQO1 axis. Molecules 28:2741. https://doi.org/10.3390/molecules28062741
Aleid IS, Alfheeaid HA, Aljutaily T, Alhomaid RM, Alharbi HF, Althwab SA, Abdel-Rahman HA, Algeffari MA, Barakat H (2021) Gastroprotective effects of spirulina platensis, golden kiwifruit flesh, and golden kiwifruit peel extracts individually or in combination against indomethacin-induced gastric ulcer in rats. Nutrients 13:3499. https://doi.org/10.3390/nu13103499
AlKreathy HM, Alghamdi MK, Esmat A (2020) Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm J 28:916–926. https://doi.org/10.1016/j.jsps.2020.06.012
Alp Yildirim FI, Uyanik Ö, Özyoğurtçu H, Gürel A, Atukeren P, Gümüştaş K, Özdemir O, Uydeş-Doğan S (2015) Aggravating effect of atorvastatin on indomethacin-induced gastric injury: focus on PGE2, TNF-α, neutrophils and iNOS. Prostaglandins Other Lipid Mediat 121:53–62. https://doi.org/10.1016/j.prostaglandins.2015.07.002
Araki H, Komoike Y, Matsumoto M, Tanaka A, Takeuchi K (2002) Healing of duodenal ulcers is not impaired by indomethacin or rofecoxib, the selective COX-2 inhibitor, in Rats. Digestion 66:145–153. https://doi.org/10.1159/000066759
Barboza KRM, Coco LZ, Alves GM, Peters B, Vasquez EC, Pereira TMC, Meyrelles SS, Campagnaro BP (2018) Gastroprotective effect of oral kefir on indomethacin-induced acute gastric lesions in mice: impact on oxidative stress. Life Sci 209:370–376. https://doi.org/10.1016/j.lfs.2018.08.035
Benvenutti RC, Dalla Vecchia CA, Locateli G, Serpa PZ, Lutinski JA, Rodrigues Junior SA, Corralo V, Gutiérrez MV, Vilegas W, Somensi LB, Longo B, Knihs JF, Mota da Silva L, de Andrade SF, Roman Junior WA (2020) Gastroprotective activity of hydroalcoholic extract of the leaves of Urera baccifera in rodents. J Ethnopharmacol 250:112473. https://doi.org/10.1016/j.jep.2019.112473
Bi WP, Man HB, Man MQ (2014) Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol. https://doi.org/10.3748/wjg.v20.i45.17020
Boushra AF, Elsayed AM, Ibrahim NA, Abdelwahed MK, Ahmed EI (2019) A comparative study on the possible protective effect of esomeprazole, spirulina, wheatgrass on indomethacin-induced gastric ulcer in male albino rats. Mol Biol Rep 46:4843–4860. https://doi.org/10.1007/s11033-019-04933-1
Boyacioglu M, Kum C, Sekkin S, Yalinkilinc HS, Avci H, Epikmen ET, Karademir U (2016) The effects of lycopene on DNA damage and oxidative stress on indomethacin-induced gastric ulcer in rats. Clin Nutr 35:428–435. https://doi.org/10.1016/j.clnu.2015.03.006
Chen N, Wei F, Wang L, Cui S, Wan Y, Liu S (2016a) Tumor necrosis factor alpha induces neural stem cell apoptosis through activating p38 MAPK pathway. Neurochem Res 41:3052–3062. https://doi.org/10.1007/s11064-016-2024-8
Chen X-Y, Chen H-M, Liu Y-H, Zhang Z-B, Zheng Y-F, Su Z-Q, Zhang X, Xie J-H, Liang Y-Z, Fu L-D, Lai X-P, Su Z-R, Huang X-Q (2016b) The gastroprotective effect of pogostone from Pogostemonis Herba against indomethacin-induced gastric ulcer in rats. Exp Biol Med 241:193–204. https://doi.org/10.1177/1535370215600099
Chen X, Liu R, Liu X, Xu C, Wang X (2018) L-ascorbic acid-2-glucoside inhibits Helicobacter pylori-induced apoptosis through mitochondrial pathway in gastric epithelial cells. Biomed Pharmacother 97:75–81. https://doi.org/10.1016/j.biopha.2017.10.030
Correia C, Lee S-H, Meng XW, Vincelette ND, Knorr KLB, Ding H, Nowakowski GS, Dai H, Kaufmann SH (2015) Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment Biochimica et Biophysica Acta (BBA). Mol Cell Res 1853:1658–1671. https://doi.org/10.1016/j.bbamcr.2015.03.012
Danisman B, Cicek B, Yildirim S, Bolat I, Kantar D, Golokhvast KS, Nikitovic D, Tsatsakis A, Taghizadehghalehjoughi A (2023) Carnosic acid ameliorates indomethacin-induced gastric ulceration in rats by alleviating oxidative stress and inflammation. Biomedicines 11:829. https://doi.org/10.3390/biomedicines11030829
Drini M (2017) Peptic ulcer disease and non-steroidal anti-inflammatory drugs. Aust Prescr 40:91–93. https://doi.org/10.18773/austprescr.2017.037
El-Ashmawy NE, Khedr EG, El-Bahrawy HA, Selim HM (2016) Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition 32:849–854. https://doi.org/10.1016/j.nut.2016.01.010
El-Ashmawy NE, Khedr EG, El-Bahrawy HA, Selim HM (2016) Nebivolol prevents indomethacin-induced gastric ulcer in rats. J Immunotoxicol 13:580–589. https://doi.org/10.3109/1547691X.2016.1142488
Eraslan E, Tanyeli A, Güler MC, Kurt N, Yetim Z (2020) Agomelatine prevents indomethacin-induced gastric ulcer in rats. Pharmacol Rep 72:984–991. https://doi.org/10.1007/s43440-019-00049-2
Ermis A, Aritici Colak G, Acikel-Elmas M, Arbak S, Kolgazi M (2023) Ferulic acid treats gastric ulcer via suppressing oxidative stress and inflammation. Life 13:388. https://doi.org/10.3390/life13020388
Fiebich BL, Lieb K, Engels S, Heinrich M (2002) Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol 132:18–24. https://doi.org/10.1016/S0165-5728(02)00279-5
Fleury C, Mignotte B, Vayssière J-L (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141. https://doi.org/10.1016/S0300-9084(02)01369-X
Freund RRA, Gobrecht P, Fischer D, Arndt HD (2020) Advances in chemistry and bioactivity of parthenolide. Nat Prod Rep. https://doi.org/10.1039/c9np00049f
Gebril SM, Ito Y, Abu-Dief EE, Hussein MRA, Elsayed HM, Mohammad AN, Abdelaal UM, Higuchi K (2020) Ultra-structural study of the indomethacin-induced apoptosis and autophagy in rat gastric parietal cells. Ultrastruct Pathol 44:300–313. https://doi.org/10.1080/01913123.2020.1772429
Grimm S, Bauer MK, Baeuerle PA, Schulze-Osthoff K (1996) Bcl-2 down-regulates the activity of transcription factor NF-kappaB induced upon apoptosis. J Cell Biol 134:13–23. https://doi.org/10.1083/jcb.134.1.13
Guzmán-Gómez O, García-Rodríguez RV, Pérez-Gutierrez S, Rivero-Ramírez NL, García-Martínez Y, Pablo-Pérez SS, Pérez-Pastén-Borja R, Cristóbal-Luna JM, Chamorro-Cevallos G (2023) Protective effect of the phycobiliproteins from Arthrospira maxima on indomethacin-induced gastric ulcer in a rat model. Plants 12:1586. https://doi.org/10.3390/plants12081586
Hammady M (2024) Application of immunohistochemistry in rat models of erosive gastritis. Egypt J Veterin Sci 55:1021–1036
Harakeh S, Saber SH, Akefe IO, Shaker S, Barkaat Hussain M, Saad Almasaudi A, Saleh SMM, Almasaudi S (2022) Saudi honey alleviates indomethacin-induced gastric ulcer via improving antioxidant and anti-inflammatory responses in male albino rats. Saudi J Biol Sci 29:3040–3050. https://doi.org/10.1016/j.sjbs.2022.01.031
Heeba GH, Hassan MKA, Amin RS (2009) Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: role of nitric oxide and prostaglandins. Eur J Pharmacol 607:188–193. https://doi.org/10.1016/j.ejphar.2009.02.008
Ito H, Matsui H, Hirayama A, Indo HP, Majima HJ, Hyodo I (2016) Reactive oxygen species induced by nonsteroidal antiinflammatory drugs enhance the effects of photodynamic therapy in gastric cancer cells. J Clin Biochem Nutr 58:180–185. https://doi.org/10.3164/jcbn.155124
Jafari A, Andishfar N, Esmaeilzadeh Z, Khezri MR, Ghasemnejad-Berenji M (2022) Gastroprotective effect of topiramate on indomethacin-induced peptic ulcer in rats: biochemical and histological analyses. Basic Clin Pharmacol Toxicol 130:559–568. https://doi.org/10.1111/bcpt.13718
Kim CY, Kang B, Hong J, Choi HS (2020) Parthenolide inhibits lipid accumulation via activation of Nrf2/Keap1 signaling during adipocyte differentiation. Food Sci Biotechnol 29:431–440. https://doi.org/10.1007/s10068-019-00672-y
Kim YS, Lee JH, Song J, Kim H (2020) Gastroprotective effects of inulae flos on hcl/ethanol-induced gastric ulcers in rats. Molecules 25:1586. https://doi.org/10.3390/molecules25235623
Küçükler S, Kandemir FM, Yıldırım S (2022) Protective effect of chrysin on indomethacin induced gastric ulcer in rats: role of multi-pathway regulation. Biotechnic Histochem 97:490–503. https://doi.org/10.1080/10520295.2021.2014569
Liu Q, Zhao J, Tan R, Zhou H, Lin Z, Zheng M, Romas E, Xu J, Sims N (2015a) Parthenolide inhibits pro-inflammatory cytokine production and exhibits protective effects on progression of collagen-induced arthritis in a rat model. Scand J Rheumatol 44:182–191. https://doi.org/10.3109/03009742.2014.938113
Liu Y-H, Zhang Z-B, Zheng Y-F, Chen H-M, Yu X-T, Chen X-Y, Zhang X, Xie J-H, Su Z-Q, Feng X-X, Zeng H-F, Su Z-R (2015b) Gastroprotective effect of andrographolide sodium bisulfite against indomethacin-induced gastric ulceration in rats. Int Immunopharmacol 26:384–391. https://doi.org/10.1016/j.intimp.2015.04.025
Liu W, Shang P, Liu T, Xu H, Ren D, Zhou W, Wen A, Ding Y (2017) Gastroprotective effects of chebulagic acid against ethanol-induced gastric injury in rats. Chem Biol Interact 278:1–8. https://doi.org/10.1016/j.cbi.2017.09.019
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Luo C, Chen H, Wang Y, Lin G, Li C, Tan L, Su Z, Lai X, Xie J, Zeng H (2018) Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: characterization of potential molecular mechanisms. Life Sci 193:47–56. https://doi.org/10.1016/j.lfs.2017.12.004
Ma N, Sun Y, Yi J, Zhou L, Cai S (2022) Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J Ethnopharmacol 284:114752. https://doi.org/10.1016/j.jep.2021.114752
Mahmoud MF, Nabil M, Abdo W, Abdelfattah MAO, El-Shazly AM, El Kharrassi Y, Sobeh M (2021) Syzygium samarangense leaf extract mitigates indomethacin-induced gastropathy via the NF-κB signaling pathway in rats. Biomed Pharmacother 139:111675. https://doi.org/10.1016/j.biopha.2021.111675
Mahmoud MF, Nabil M, Hasan RA, El-Shazly AM, El-Ansari MA, Sobeh M (2022) Pentagalloyl glucose, a major compound in mango seed kernel, exhibits distinct gastroprotective effects in indomethacin-induced gastropathy in rats via modulating the NO/eNOS/iNOS signaling pathway. Front Pharmacol 13:800986. https://doi.org/10.3389/fphar.2022.800986
Mahmoud MF, Abdo W, Nabil M, Drissi B, El-Shazly AM, Abdelfattah MAO, Sobeh M (2023) Apple (Malus domestica Borkh) leaves attenuate indomethacin-induced gastric ulcer in rats. Biomed Pharmacother 160:114331. https://doi.org/10.1016/j.biopha.2023.114331
Mao W, Zhu Z (2018) Parthenolide inhibits hydrogen peroxide-induced osteoblast apoptosis. Mol Med Rep. https://doi.org/10.3892/mmr.2018.8908
Mard SA, Pipelzadeh MH, Teimoori A, Neisi N, Mojahedin S, Khani MZS, Ahmadi I (2016) Protective activity of crocin against indomethacin-induced gastric lesions in rats. J Nat Med 70:62–74. https://doi.org/10.1007/s11418-015-0938-0
Musumba C, Prichard DM, Pirmohamed M (2009) Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther 30:517–531. https://doi.org/10.1111/j.1365-2036.2009.04086.x
Nabil M, El Raey MA, Abdo W, Abdelfattah MAO, El-Shazly AM, Sobeh M, Mahmoud MF (2021) Gastro-protective effects of Albizia anthelmintica leaf extract on indomethacin-induced gastric ulcer in Wistar rats: in silico and in vivo studies. Antioxidants 10:176. https://doi.org/10.3390/antiox10020176
Naito Y, Yoshikawa T (2006) Oxidative stress involvement and gene expression in indomethacin-induced gastropathy. Redox Rep 11:243–253. https://doi.org/10.1179/135100006X155021
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make? J Clin Invest. https://doi.org/10.1172/JCI119782
Oluwabunmi I, Abiola T (2015) Gastroprotective effect of methanolic extract of Gomphrena celosioides on indomethacin induced gastric ulcer in Wistar albino rats. Int J Appl Basic Med Res 5:41. https://doi.org/10.4103/2229-516X.149238
Pareek A, Suthar M, Rathore GS, Bansal V (2011) Feverfew (Tanacetum parthenium L.): a systematic review. In: Pharmacognosy reviews, vol. 5, issue 9. pp 103–110. https://doi.org/10.4103/0973-7847.79105
Saadane A, Masters S, DiDonato J, Li J, Berger M (2007) Parthenolide inhibits IκB kinase, NF-κB activation, and inflammatory response in cystic fibrosis cells and mice. Am J Respir Cell Mol Biol 36:728–736. https://doi.org/10.1165/rcmb.2006-0323OC
Sabiu S, Garuba T, Sunmonu T, Ajani E, Sulyman A, Nurain I, Balogun A (2015) Indomethacin-induced gastric ulceration in rats: protective roles of Spondias mombin and Ficus exasperata. Toxicol Rep 2:261–267. https://doi.org/10.1016/j.toxrep.2015.01.002
Sabiu S, Garuba T, Sunmonu TO, Sulyman AO, Ismail NO (2016) Indomethacin-induced gastric ulceration in rats: ameliorative roles of Spondias mombin and Ficus exasperata. Pharm Biol 54:180–186. https://doi.org/10.3109/13880209.2015.1029050
Salem NA, Wahba MA, Eisa WH, El-Shamarka M, Khalil W (2018) Silver oxide nanoparticles alleviate indomethacin-induced gastric injury: a novel antiulcer agent. Inflammopharmacology 26:1025–1035. https://doi.org/10.1007/s10787-017-0424-2
Santucci L, Fiorucci S, Di Matteo FM, Morelli A (1995) Role of tumor necrosis factor α release and leukocyte margination in indomethacin-induced gastric injury in rats. Gastroenterology 108:393–401. https://doi.org/10.1016/0016-5085(95)90065-9
Sathish R, Vyawahare B, Natarajan K (2011) Antiulcerogenic activity of Lantana camara leaves on gastric and duodenal ulcers in experimental rats. J Ethnopharmacol 134:195–197. https://doi.org/10.1016/j.jep.2010.11.049
Sayed Abdel-Tawab M, Mostafa Tork O, Mostafa-Hedeab G, Ewaiss Hassan M (2020) Protective effects of quercetin and melatonin on indomethacin induced gastric ulcers in rats. Rep Biochem Mol Biol 9:278–290. https://doi.org/10.29252/rbmb.9.3.278
Schacht V, Kern JS (2015) Basics of immunohistochemistry. J Invest Dermatol 135:1–4. https://doi.org/10.1038/jid.2014.541
Schubert ML (2017) Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr Opin Gastroenterol 33:430–438. https://doi.org/10.1097/MOG.0000000000000392
Shaik RA, Eid BG (2022) Piceatannol affects gastric ulcers induced by indomethacin: association of antioxidant, anti-inflammatory, and angiogenesis mechanisms in rats. Life 12:356. https://doi.org/10.3390/life12030356
Sheehan M, Wong HR, Hake PW, Malhotra V, O’Connor M, Zingarelli B (2002) Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol 61:953–963. https://doi.org/10.1124/mol.61.5.953
Shoaib M, Shah I, Ali N, Adhikari A, Tahir MN, Shah SWA, Ishtiaq S, Khan J, Khan S, Umer MN (2017) Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement Altern Med 17:27. https://doi.org/10.1186/s12906-016-1517-y
Slomiany BL, Piotrowski J, Slomiany A (1997) Induction of tumor necrosis factor-α and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand J Gastroenterol 32:638–642. https://doi.org/10.3109/00365529708996511
Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z (2010) Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 33:224–234. https://doi.org/10.1007/s10753-009-9176-5
Takeuchi K (2012) Pathogenesis of NSAID-induced gastric damage: importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol 18:2147. https://doi.org/10.3748/wjg.v18.i18.2147
Takeuchi T, Miura S, Wang L, Uehara K, Mizumori M, Kishikawa H, Hokari R, Higuchi H, Adachi M, Nakamizo H, Ishii H (2002) Nuclear factor-kappaB and TNF-alpha mediate gastric ulceration induced by phorbol myristate acetate. Dig Dis Sci 47:2070–8. https://doi.org/10.1023/a:1019633114854
Tamaddonfard E, Erfanparast A, Farshid AA, Imani M, Mirzakhani N, Salighedar R, Tamaddonfard S (2019) Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sci 224:88–94. https://doi.org/10.1016/j.lfs.2019.03.054
Tomisato W, Tsutsumi S, Rokutan K, Tsuchiya T, Mizushima T (2001) NSAIDs induce both necrosis and apoptosis in guinea pig gastric mucosal cells in primary culture. Am J Physiol Gastrointest Liver Physiol 281:G1092-100. https://doi.org/10.1152/ajpgi.2001.281.4.G1092
Tournier H, Schinella G, De Balsa EM, Buschiazzo H, Mañez S, De Buschiazzo PM (2010) Effect of the chloroform extract of Tanacetum vulgare and one of its active principles, parthenolide, on experimental gastric ulcer in rats. J Pharm Pharmacol 51:215–219. https://doi.org/10.1211/0022357991772169
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30. https://doi.org/10.1016/j.ab.2016.10.021
Ugan RA, Un H (2020) The protective roles of butein on indomethacin induced gastric ulcer in mice. Eurasian J Med 52:265–270. https://doi.org/10.5152/eurasianjmed.2020.20022
Vaananen PM, Meddings JB, Wallace JL (1991) Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J of Physiol-Gastrointest Liver Physiol 261:G470–G475. https://doi.org/10.1152/ajpgi.1991.261.3.G470
Wang T, Zhao S, Wang Y, Yang Y, Yao L, Chu L, Du H, Fu F (2014) Protective effects of escin against indomethacin-induced gastric ulcer in mice. Toxicol Mech Methods 24:560–566. https://doi.org/10.3109/15376516.2014.951815
Wang J, Tong M, Zhao B, Zhu G, Xi D, Yang J (2020) Parthenolide ameliorates intracerebral hemorrhage-induced brain injury in rats. Phytother Res 34:153–160. https://doi.org/10.1002/ptr.6510
Xavier S, Magalhães J, Cotter J (2018) Proton pump inhibitors: are they a real threat to the patient? GE Port J Gastroenterol 25:243–252. https://doi.org/10.1159/000487154
Xie C, Liu L, Zhu S, Wei M (2021) Effectiveness and safety of Chinese medicine combined with omeprazole in the treatment of gastric ulcer: a protocol for systematic review and meta-analysis. Medicine (United States) 100:E25744. https://doi.org/10.1097/MD.0000000000025744
Yao H, Tang X, Shao X, Feng L, Wu N, Yao K (2007) Parthenolide protects human lens epithelial cells from oxidative stress-induced apoptosis via inhibition of activation of caspase-3 and caspase-9. Cell Res 17:565–571. https://doi.org/10.1038/cr.2007.6
Zaghlool SS, Abo-Seif AA, Rabeh MA, Abdelmohsen UR, Messiha BAS (2019) Gastro-protective and antioxidant potential of Althaea officinalis and Solanum nigrum on pyloric ligation/indomethacin-induced ulceration in rats. Antioxidants 8:512. https://doi.org/10.3390/antiox8110512
Zhang S, Ong C-N, Shen H-M (2004) Involvement of proapoptotic Bcl-2 family members in parthenolide-induced mitochondrial dysfunction and apoptosis. Cancer Lett 211:175–188. https://doi.org/10.1016/j.canlet.2004.03.033
Zhang Y, Huang Q, Chen Y, Peng X, Wang Y, Li S, Wu J, Luo C, Gong W, Yin B, Xiao J, Zhou W, Peng F, Long H (2020) Parthenolide, an NF-κB inhibitor, alleviates peritoneal fibrosis by suppressing the TGF-β/Smad pathway. Int Immunopharmacol 78:106064. https://doi.org/10.1016/j.intimp.2019.106064
Zhou D, Yang Q, Tian T, Chang Y, Li Y, Duan LR, Li H, Wang SW (2020) Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed Pharmacother 126:110075. https://doi.org/10.1016/j.biopha.2020.110075
Zhu S, Sun P, Bennett S, Charlesworth O, Tan R, Peng X, Gu Q, Kujan O, Xu J (2023) The therapeutic effect and mechanism of parthenolide in skeletal disease, cancers, and cytokine storm. Front Pharmacol 14:1111218. https://doi.org/10.3389/fphar.2023.1111218
Acknowledgements
The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Funding
This research work was funded by the Institutional Fund Projects under grant no. (IFPIP:1356-166-1443).
Author information
Authors and Affiliations
Contributions
Author reviewed the manuscript. The author declares that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted by the Declaration of Helsinki, and approved by the Institutional Ethics Committee of FOP, KAU (Ref no - PH-1444-40).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shaik, R.A. Parthenolide alleviates indomethacin-induced gastric ulcer in rats via antioxidant, anti-inflammatory, and antiapoptotic activities. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03110-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03110-x