Abstract
The present study was designed to elucidate the mechanism(s) of the gastro-protective effect of crocin against indomethacin-induced gastric lesions. Crocin or pantoprazole was administered to rats 30 min before indomethacin. Five hours later, the animals were killed and their stomachs were removed and examined macroscopically. Samples of gastric mucosa were collected for microscopic evaluation, mRNA expression of caspase-3, inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 was quantified by RT-PCR, and protein levels of COX-1, COX-2, iNOS and caspase-3 were assessed by Western blotting. The pH, volume of gastric effluent and antioxidant activity were measured in 5 separate groups of rats following pylorus ligation. Indomethacin induced significant increases in mRNA and protein expression of iNOS and caspase-3 and increased MDA levels, and reduced the pH of the gastric effluent and protein and mRNA expression of COX-2 and protein expression of COX-1 and mucus content associated with gastric ulceration. Crocin and pantoprazole significantly inhibited mRNA and protein expression of iNOS, caspase-3 and MDA, and reduced mucus content induced by indomethacin. However, unlike pantoprazole, crocin failed to increase COX-1 and pH, but had variable increasing effects on mRNA and protein expression of COX-2. Macroscopic and microscopic observations showed that mucosal erosions induced by indomethacin were significantly inhibited by pantoprazole and crocin. These findings suggest that crocin exerts its gastro-protective effects mainly by inhibition of MDA, reduction in iNOS and caspase-3, and inhibition of the reduction in mucus content induced by indomethacin. Crocin is a novel agent that has potential in the prevention of ulceration induced by NSAIDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are perhaps the most widely used drugs worldwide. The most common side effect of these analgesic drugs is their gastrointestinal and renal damage, which is largely believed to be mediated by cyclooxygenase (COX)-dependent and COX-independent mechanisms [1]. Inhibition of COX enzymes (COX-1 and COX-2) leads to reduction in the production of cytoprotective prostaglandins (PGs). PGs are believed to protect the stomach lining from the secreted acid, maintain blood flow in gastric mucosa, and produce bicarbonate [2]. Other mechanisms that mediate NSAID-induced gastric ulceration include: increase in membrane permeabilization resulting in a direct cytotoxic effect on gastric mucosal cells, causing lesions and injury [3], induction of necrosis and apoptosis in gastric mucosal cells leading to enhancement of release of proinflammatory mediators such as tumor necrosis factors [4], causing further occlusion of gastric microvessels and reduction in gastric blood flow and enhancement of free radical induction of inducible nitric oxide synthase (iNOS) [5].
In order to reduce gastric erosion induced by NSAIDs, administration of selective COX-2 inhibitors has been recommended. However, the initial enthusiasm for this class of drugs has faded after reports of side effects on the cardiovascular system, notably among patients who are under aspirin treatment [6]. Furthermore, in order to produce protection against NSAID-induced gastric damage, recent clinical recommendations suggest concurrent administration of H2 blockers, proton pump inhibitors (PPIs) or prostaglandin analogues [7]. PPIs have proved to be most effective, both for prophylaxis and treatment of ulcers associated with NSAIDs [8].
A previous study demonstrated that 2 h after intra-gastric administration of indomethacin (60 mg/kg) extensive multiple hemorrhagic lesions were produced, with a marked enhancement in caspase-3 activity (a marker for apoptosis) and significant induction of iNOS (a marker for inflammation) expression. The mucosal expression of iNOS showed a positive correlation with the extent of increase in caspase-3 activity [9]. In addition, pantoprazole reversed the effects of NSAIDs on mucosal contents of myeloperoxidase, malonyldialdehyde and glutathione (GSH), without interfering with the decrease in PGE2 levels or indomethacin-induced COX-2 expression [10].
Crocus sativus Linn. (saffron) belongs to the Iridaceae family. The four major pharmacologically active constituents of saffron stigma are crocin, crocetin, picrocrocin and safranal [11]. Crocin is the most important and abundant antioxidative constituent of C. sativus stigma [12], and is a hydrophilic glycoside with protective activities on the nervous [13], cardiac [14] and renal systems [15]. Moreover, previous studies showed that crocin has antioxidant [16] and anti-inflammatory properties, reducting edema in the acetic acid rat paw model [17]. Moreover, saffron and its active constituents, crocin and safranal, have been reported to attenuate the development of gastric mucosal lesions in different models of experimentally induced gastric ulcer including water immersion restraint stress, histamine-induced, NSAID-induced, and pylorus-ligation-induced gastric ulcers in rat [18–21]. The mechanism of this protection was proposed to be mediated by a decrease in gastric non-protein sulfhydryl contents [21]. In addition, crocin and safranal produced their gastro-protective effects against indomethacin, in both diabetic and non-diabetic rats, by increasing glutathione levels and diminishing lipid peroxidation. These gastro-protective effects were comparable with omeprazole [20].
Proton pump inhibitors, such as pantoprazole and esomeprazole, in addition to their inhibitory action on acid secretion, may confer their gastro-protective effect by increasing the expression of COX-2 mRNA, decreasing caspase-3 expression [10, 22], inhibiting the expression of iNOS [23] and antioxidant activity [24]. However, whether crocin also provides its ulcer protective effect via these mechanisms is not clear. Therefore, the aim of the present study was to evaluate the prophylactic efficacy of crocin on gastric mucosal lesions induced by indomethacin in rat by evaluating the changes in MDA (as a marker for lipid peroxidation), mucus content, pH of gastric effluent, mRNA expression and protein levels of caspase-3, iNOS, COX-1 and COX-2. For comparison, pantoprazole was employed as a standard agent.
Methods
Animals
Male Wistar rats (body weight 150–200 g) were purchased from the animal house of Ahvaz Jundishapur University of Medical Sciences. The animals were fed on a conventional diet and had free access to tap water. They were maintained under standard conditions of humidity, temperature (22 ± 2 °C) and 12 h light/dark cycle. The animals were deprived of food but not water overnight before intervention. All experiments were carried out in accordance with the regulations set by the ethics committee of Ahvaz Jundishapur University of Medical Sciences (research project PRC106).
Animal grouping and experimental procedures for employment of indomethacin-induced gastric erosion model
The rats were randomly assigned to seven groups (n = 8), treated with control indomethacin (Sigma, USA) (40 mg/kg, p.o.), crocin (Sigma, USA) (7.5, 15 or 30 mg/kg, i.p.) and pantoprazole (Nycomed GmbH, Germany) (50 mg/kg, i.p.). The sixth (crocin alone) and seventh (normal intact control) groups received, respectively, the effective dose of crocin (15 mg/kg, i.p.) and normal saline that did not induce gastric ulcer. The first 5 groups received the allocated intervention 30 min prior to the induction of gastric ulcer by indomethacin (40 mg/kg. p.o.) [20]. Five hours later the animals were killed by cardiac exsanguination.
Evaluation of macroscopic and microscopic mucosal damage
In order to measure the gastric mucosal lesions, the stomachs were removed, opened along the greater curvature, rinsed with physiological saline and pinned out in ice-cold saline. To calculate the degree of gastric lesions, the length (mm) and width (mm) of mucosal ulcers were measured. The ulcer index (UI) was defined as the total area of the lesions (in mm2), which was calculated using the following relationship [25]:
Immediately after the measurement of the surface area of gastric lesions, 100 mg of gastric mucosal tissue, including the ulcer area and the surrounding ulcer margin, were quickly excised, snap-frozen in liquid nitrogen and stored at −80 °C until use.
For histological evaluation, stomachs from normal intact control, indomethacin control and crocin- and pantoprazole-treated animals were fixed in 10 % formalin, dehydrated in graded ethanol and embedded in paraffin. Thereafter, 5 µm sections of tissue were cut, stained with hematoxylin and eosin and assessed microscopically (Olympus IX50).
RNA extraction and cDNA synthesis
The total RNA was extracted from the frozen tissue samples using TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN, USA). The concentration and purity of the total RNA was determined spectrophotometrically at 260 and 280 nm wavelength (Bio-Photometer Plus, Eppendorf, Germany). To remove the genomic DNA, 2 µg of the extracted RNA was added to a mixture containing DNase I reaction buffer (10×) and 1 U DNase I (Cinnagen, Iran). The mixture was incubated at 37 °C for 30 min, then 1 µl of EDTA (25 mM) was added, and the mixture was incubated at 65 °C for 5 min to stop the reaction. Complementary DNA (cDNA) was synthesized from 1 µg of the total RNA using the AccuPower CycleScript RT PreMix kit (Bioneer, Daejoun, South Korea) according to the manufacturer’s instruction.
Measurement of mRNA expression of iNOS, caspase-3, COX-2 and GAPDH
All PCR amplifications were performed in a final volume of 25 µl containing 1 µg cDNA, 50 nm of specific primers, 2.5 µl of 10× PCR buffer, 1 U DNA Taq polymerase and 50 nm of dNTP. The mRNA levels of iNOS, caspase-3, COX-2 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were measured by RT-PCR using MasterCycler Personal (Eppendorf AG, Hamburg, Germany). The specific primers (Bioneer) used in this study are listed in Table 1. The thermal cycling conditions for the amplification of GAPDH, iNOS, and COX-2 genes were as follows: initial denaturation for 5 min at 94 °C followed by 40 cycles of 1 min at 94 °C, 45 s at 53 °C and 45 s at 72 °C, with a final elongation cycle for 5 min at 72 °C. The same cycle was employed for the amplification of caspase-3 mRNA but the annealing temperature was set at 49 °C. The PCR products were analyzed on a 2 % agarose gel and the density of each band was measured with ImageJ software. The levels of the studied genes––iNOS, COX-2 and caspase-3––were determined by calculating the density ratio of each studied mRNA to GAPDH mRNA.
Protein extraction
TriPure Isolation Reagent was used to extract the total proteins from gastric mucosal tissue, according to the manufacturer’s instructions. To analyze the protein fraction, protein pellets obtained using the TriPure Isolation Reagent from gastric mucosal samples were resuspended in 1 % SDS. The total recovery and integrity of these fractions were determined by Bradford assay and SDS-polyacrylamide gel electrophoresis.
Western blot analysis
Mucosal proteins were separated by SDS-PAGE on 10 % acrylamide gels and were transferred onto a nitrocellulose membrane. The membranes were blocked with 5 % non-fat skim milk dissolved in Tris-buffered saline with 0.1 % Tween 20 (TBST, pH 7.6) for 6 h and then incubated overnight at 4 °C with anti-iNOS antibody (rabbit polyclonal, dilution 1:200; Abcam [ab95441], USA), anti-caspase-3 antibody (rabbit polyclonal, dilution 1:1000; Abcam [ab90437]), anti-COX-1/COX-1 antibody (mouse monoclonal, dilution 1:1000; Abcam [ab695]), anti-COX-2/COX-2 antibody (rabbit polyclonal, dilution 1:300; Abcam [ab15191]) or anti-β-actin antibody (mouse monoclonal, dilution 1:5000; Abcam [ab20272]) added to the membrane. After 6 washes with TBST, membranes were incubated with a rabbit polyclonal secondary antibody to mouse IgG HRP; dilution 1:10000) for 2 h at room temperature. Labelled proteins were detected using a chemiluminescence Western blotting system. The expression of studied proteins was semiquantified by ImageJ analysis software and the values were normalized to β-actin.
Evaluation of effects of crocin and pantoprazole pretreatment on gastric mucus production
To evaluate mucus production, Tan et al.’s method was used [26]. Before snap-freezing the gastric mucosa of the indomethacin-induced gastric ulcers in the rats (pretreated with crocin and pantoprazole) for molecular assessment, the mucus was gently scraped using a glass slide and the obtained mucus weighed with an accurate digital device.
Pylorus ligation experiments
To determine the antisecretory and antioxidant effects of crocin, 5 separate groups of fasted rats (n = 5) were subjected to a pylorus ligation procedure as described by Shay et al. [27]. The control and four pretreated groups received a single intraperitoneal injection of saline, crocin at doses of 7.5, 15, 30 mg/kg, or pantoprazole at 50 mg/kg 30 min prior to pylorus ligation, respectively. Five hours after pylorus ligation, animals were killed by an overdose of diethyl ether and their stomachs removed, and the entire gastric contents were transferred into centrifuge tubes for measuring pH, volume and total acidity.
Determination of pH and total acidity of gastric contents
The pH and acidity of the gastric contents were measured with an autotitrator pH meter (Radiometer, Copenhagen, Denmark) by automatic potentiometric titration to pH 7 with 0.01 N NaOH, and was expressed as μEqH+/5 h.
Determination of lipid peroxidation
After collecting the gastric effluents for determining pH, volume and total acidity, the gastric mucus was gently scraped to measure the MDA level in pylorus-ligated rats. The MDA level was measured to assess the level of lipid peroxidation. Briefly, in this method, MDA reacts with thiobarbituric acid (TBA), as a thiobarbituric acid reactive substance (TBARS), to generate a red-colored complex that has peak absorbance at 532 nm. Three millilitres of phosphoric acid (1 %) and 1 ml TBA (0.6 %) was added to 0.5 ml of homogenate and the mixture was heated for 60 min in a boiling water bath. Then 25 µl HCl was added to the ice-cooled mixture and vortexed. Finally, 3.5 ml of n-butanol was added to the mixture and incubated for 5 min and centrifuged at 15,000 rpm for 10 min to separate the n-butanol phase. The supernatant was transferred to a new tube and its absorbance was measured at 532 nm. A standard curve of MDA was constructed over the concentration range of 0–40 μM [15] and used for measurement of MDA levels.
Statistical analysis
Data are shown as mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by post hoc Tukey’s test. Significance was set at P < 0.05.
Results
Macroscopic evaluation of the effect of crocin and pantoprazole pretreatment on indomethacin-induced gastric lesions
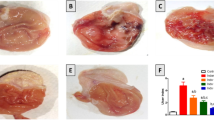
Severe mucosal lesions were evident 5 h after intra-gastric administration of indomethacin, showing multiple erosions, exfoliation and necrosis of superficial cells, hemorrhages in the mucosal layer and severe alterations in the architecture of glandular parts of the gastric mucosa (Fig. 1a). Crocin pretreatment (7.5, 15 and 30 mg/kg) demonstrated moderate to mild disruption of the surface epithelium and decreased the mucosal lesions induced by indomethacin (Fig. 1b–d, respectively). Pantoprazole pretreatment almost completely prevented mucosal lesions by indomethacin (Fig. 1e). Normal appearance of gastric mucosa was observed in both crocin alone and non-treated intact gastric mucosa and no gastric damage was recognized in the gastric mucosa of the normal intact rats and the group treated with crocin alone (Fig. 1f, g).
Gross appearance of the stomachs. a Severe mucosal lesions 5 h after intra-gastric administration of indomethacin (40 mg/kg); b–d crocin pretreatment (7.5, 15 and 30 mg/kg, respectively) decreased the mucosal lesions induced by indomethacin; e pantoprazole pretreatment almost completely prevented mucosal lesions induced by indomethacin; f normal appearance of gastric mucosa following crocin alone; and g normal intact gastric mucosa
The calculated ulcer index in indomethacin-treated control rats was 33 ± 4 mm2, which was significantly higher than in the 7.5, 15 and 30 mg/kg crocin-pretreated groups (22 ± 2, 8 ± 1 and 15 ± 2 mm2 respectively). The area of lesions in pantoprazole-treated group (4 ± 0.4 mm2) was significantly lower than in both crocin- and indomethacin-treated control groups (P < 0.05, P < 0.001 respectively) (Fig. 2). The optimal dose of crocin was 15 mg/kg, which was still significantly higher than pantoprazole (P < 0.05). No gastric lesions were observed in crocin-alone-treated and normal intact groups (Fig. 2f, g, respectively).
The calculated ulcer index in C control indomethacin; Cr7.5, Cr15 and Cr30 crocin pretreatment (7.5, 15 and 30 mg/kg respectively); Pan pretreatment with pantoprazole (50 mg/kg, i.p.); Cr crocin alone (15 mg/kg, i.p.); and NC normal intact control. *P < 0.05, ***P < 0.001 relative to indomethacin-treated control group; α P < 0.05 relative to groups crocin-pretreated at doses of 7.5 and 30 mg/kg. β P < 0.05 significant decrease as compared with the optimal dose of crocin (15 mg/kg). Data are expressed as mean ± SEM
Microscopic observation of the effect of crocin and pantoprazole pretreatment on indomethacin-induced gastric lesions
Microscopic examination showed gastric lesions with multiple erosions, exfoliation and necrosis of superficial cells, hemorrhages in the mucosal layer, marginal inflammation and neutrophil aggregation and severe alterations in the architecture of glandular parts of the gastric mucosa 5 h after indomethacin administration (Fig. 3a). Thirty minutes pretreatment with crocin (7.5, 15 and 30 mg/kg, Fig. 3b–d) and pantoprazole (Fig. 3e) attenuated the gastric lesions induced by indomethacin. No gastric damage was observed in the gastric mucosa of the normal intact rats and crocin-alone-treated group (Fig. 3f, g respectively).There was an overall close correlation between the macroscopic and microscopic findings.
Representative gastric sections were obtained 5 h after indomethacin administration. a Indomethacin-treated control group; b–d rats pretreated with crocin (7.5, 15 and 30 mg/kg, 30 min before intervention); e rats pretreated with pantoprazole (50 mg/kg, 30 min prior to intervention); f–g normal intact rats and crocin-alone-treated groups. All sections stained with hematoxylin and eosin; ×100 magnification
Effects of indomethacin and pretreatment with crocin and pantoprazole on mucosal mRNA and protein expressions of iNOS and caspase-3
Indomethacin-treated control rats showed significantly higher (P < 0.01) levels of mRNA of iNOS and caspase-3 than crocin- and pantoprazole-pretreated animals (P < 0.01) (Figs. 5, 6). The optimal dose for maximum reduction in expression and protein synthesis of caspase-3 conferred by crocin was found at 15 mg/kg. These levels were comparable with those recorded for pantoprazole (50 mg/kg) (Figs. 5, 6). However, pantoprazole produced a more significant reduction in mRNA and protein expression of iNOS level than the optimal dose of crocin (Figs. 5, 6).
Effects of indomethacin and pretreatment with crocin and pantoprazole on mucosal mRNA and protein expressions of COX-2
The levels of mRNA and protein expression of COX-2 in indomethacin-treated control rats were significantly higher than in normal intact animals (P < 0.05). Messenger RNA and protein expression of COX-2 in crocin-pretreated animals were variably higher than in the indomethacin-treated group. The maximum increase was measured at a dose of 15 mg/kg crocin (P < 0.01). Pretreatment with pantoprazole did not produce a change in mRNA or protein expression of COX-2. Crocin alone, compared with intact mucosa, slightly but not significantly increased mRNA and protein expression of COX-2 (Figs. 4a, 5a).
PCR agarose gel bands of the effect of pretreatment with C control indomethacin; Cr7.5, Cr15 and Cr30 crocin (7.5, 15 and 30 mg/kg, i.p.); Pan pantoprazole (50 mg/kg, i.p.); Cr crocin (15 mg/kg, i.p.) alone; and saline (Nor normal intact control) on the gastric mucosal mRNA expression of COX-2 (a), iNOS (b), and caspase-3 (c). *P < 0.05, **P < 0.01 and ***P < 0.001 versus indomethacin-treated control group. α P < 0.01 relative to normal intact rats. β P < 0.05 compared with crocin (15 mg/kg)-pretreated rats. Data are expressed as mean ± SEM. The agarose bands were normalized with GAPDH
Western blot analysis for protein synthesis of COX-2 (a), iNOS (b) and caspase-3 (c) in the rat gastric mucosa following C indomethacin (40 mg/kg, p.o.); Cr7.5, Cr15 and Cr30 crocin (7.5, 15 and 30 mg/kg, i.p.); Pan pantoprazole (50 mg/kg, i.p.); Cr crocin (15 mg/kg, i.p.) alone; and Nor normal intact group. β P < 0.01 indicates significant increase versus normal intact group; α P < 0.01 indicates significant increase versus normal intact group; *P < 0.05, **P < 0.01 and **P < 0.01 versus indomethacin-treated control group. µ P < 0.05 relative to optimal dose of crocin. Data are expressed as mean ± SEM. The protein levels were normalized to β-actin
Effects of indomethacin and pretreatment with crocin and pantoprazole on mucosal protein expression of COX-1
The level of protein expression of COX-1 in indomethacin-treated control rats was significantly lower than in normal untreated rats (Fig. 6). Pantoprazole, but not crocin, significantly inhibited the reduction of COX-1 induced by indomethacin. However, crocin when administered alone did not produce a change in expression of COX-1.
Western blot analysis for protein synthesis of COX-1 in normal untreated (Nor) rat gastric mucosa, and following crocin alone (Cr: 15 mg/kg, i.p.), indomethacin (C: 40 mg/kg, p.o.), and 30 min pretreatment with pantoprazole (Pan: 50 mg/kg, i.p.) or crocin (Cr7.5, Cr15 and Cr30: 7.5, 15 and 30 mg/kg, i.p.). *P < 0.01 indicates significant decrease as compared with normal, crocine-alone- and pantoprazole-pretreated groups; β P < 0.05 indicates significant increase versus control and crocin-pretreated groups. Data are expressed as mean ± SEM. The protein levels were normalized to β-actin
Effect of crocin pretreatment and pantoprazole on mucus production in indomethacin-induced gastric ulcer rats
The gastric mucus content in control indomethacin-treated rats was lower than in crocin- and pantoprazole-treated animals (Table 3). The effect on mucus production of crocin at 15 and 30 mg/kg was equivalent to pantoprazole at 50 mg/kg (Table 3).
Effect of crocin and pantoprazole on the profile of gastric secretion induced by pylorus-ligated rats
As shown in Table 2, volume, pH and total acidity of the gastric contents were not affected by the studied doses of crocin (7.5, 15 and 30 mg/kg, i.p.). Crocin pretreatment partially but not significantly increased the pH and reduced total acidity (Table 2). Pantoprazole significantly decreased the volume and total acidity of the gastric effluents compared with control and crocin-pretreated rats (P < 0.001) (Table 2).
Effect of crocin and pantoprazole on MDA level induced by pylorus ligation
The mucosal level of MDA in the control pylorus-ligated group was significantly increased compared with crocin- and pantoprazole-pretreated rats. Free-radical-induced lipid peroxidation was significantly decreased, as indicated by a reduction in the MDA levels of gastric mucosal tissue by crocin and pantoprazole pretreatment (Table 2).
Discussion
This in vivo study was designed to identify the role of MDA, total acidity and pH of gastric effluent, mucus content, COX-1, COX-2, iNOS and caspase-3 as possible mediators for the gastro-protective effects offered by crocin. Pantoprazole was tested similarly and employed as a standard anti-ulcer agent. For this purpose two series of experiments were carried out. In the first, the indomethacin-induced gastric ulceration model was employed. The results showed that indomethacin induced profound gastric lesions accompanied by significant increases in mRNA and protein expression of iNOS, caspase-3 and COX-2, and reduction in mucus contents. On the other hand, indomethacin caused a significant decrease in COX-1 protein expression. Macroscopic and microscopic observations showed that mucosal erosions induced by indomethacin were significantly inhibited by pantoprazole and crocin. Crocin and pantoprazole significantly inhibited mRNA and protein expression of iNOS and caspase-3 and increased the mucus content. However, unlike pantoprazole, crocin, when administered alone and 30 min prior to indomethacin, had variable increasing effects on mRNA and protein expression of COX-2 and failed to increase the protein expression of COX-1. In the second series of experiments, the pylorus ligation technique was employed. The results showed that pretreatment with crocin and pantorpazole produced an equipotent significant reduction in MDA level. However, a more significant increase in the pH of gastric effluent was measured for pantoprazole than for crocin.
The pathophysiology of NSAID-induced mucosal lesions is associated with inflammatory responses that elicit tissue damage and gastric ulceration. Both prostaglandin-dependent and prostaglandin-independent mechanisms have been proposed to mediate the development of gastric ulceration [2]. Other endogenous mediators involved in ulcer formation are the pro-inflammatory iNOS and caspase-3, through an increase in cellular apoptosis [1, 23]. Besides their role in the inhibition of prostaglandins, NSAIDs have also been shown to have direct cytotoxic effects [28].
The macroscopic and microscopic observations showed that both crocin and pantoprazole conferred significant gastro-protective effects and successfully inhibited the development of gastric lesions induced by indomethacin. Additionally, when administered alone, crocin produced no adverse effects.
The results from the measurement of mRNA expression and protein synthesis of iNOS showed that both crocin and pantoprazole were effective in preventing the rise in mRNA expression and protein synthesis of iNOS. Nitric oxide synthase (NOS) has been shown to have a dual role in both ulcer production and protection, while it was shown to promote ulcer healing by enhancing angiogenesis and mucosal blood flow and stimulating bicarbonate secretion [5]. This protective role is believed to be mediated by the constitutive form of NOS (eNOS), which generates low nanomolar concentrations of NO. Conversely, when NO is generated at micromolar concentrations by the inducible form (iNOS), it promotes mucosal injury by enhancing apoptosis and inflammation [29]. Our results showed that the level of iNOS mRNA expression and protein synthesis in indomethacin-treated control rats was significantly increased as compared with the crocin- and pantoprazole-treated groups. The results showed that crocin, when administered alone, did not produce any change in the level of either mRNA expression or iNOS protein synthesis. This finding is in agreement with the findings of Nam et al., where no change in basal NO level was observed in the microglial cells (macrophages resident in the central nervous system) following exposure to crocin when administered alone but markedly and dose-dependently protected these cells following lipopolysaccharide-induced stimulation [30]. Crocin dose-dependently produced a significant inhibitory effect on mRNA expression and protein synthesis of iNOS. However, at the optimal dose of 15 mg/kg, its reduction efficacy was less than pantoprazole. Pantoprazole almost completely abolished mRNA expression and protein synthesis of iNOS. These findings suggest that the beneficial gastro-protective effects conferred by pantoprazole and crocin are similar and are partly mediated by inhibition of expression of mRNA and protein synthesis of iNOS.
In order to gain insight into other possible mechanisms that mediate the beneficial effects of crocin on indomethacin-induced gastric damage, measurement of the expression of caspase-3, the molecular marker for apoptosis, was undertaken. The results showed that indomethacin significantly increased the expression of mRNA and protein synthesis of caspase-3. The mechanism of NSAID-induced apoptosis is believed to be mediated by reducing the level of survival [1], inhibiting the activity of NF-κB and ubiquitin proteosomes which lead to a common pathway of activation of the apoptotic protease, caspase-3 [31–34]. However, which of these pathways mediates the protective effects of crocin and pantoprazole, which leads to the decrease in expression of mRNA and protein synthesis of caspase-3, could not be ascertained from the results of the present study. Crocetin, another active constituent of saffron, has been shown to inhibit apoptosis at the early stages of brain injury following cerebral contusion through up-regulation of Bcl-2 expression [35]. Moreover, crocin has been reported to prevent retinal ischemia/reperfusion-induced apoptosis in retinal ganglion cells in rats [36]. The expression of mRNA and protein synthesis of caspase-3 with crocin alone was not different from control baseline values. However, our results showed that crocin, similar to pantoprazole, was equally effective in preventing the marked increase in expression of mRNA and protein synthesis of caspase-3 following administration of indomethacin. Taken together, these findings imply that, at the doses employed, crocin, similarly to pantoprazole, also provides its gastro-protective effects partly though its capacity to inhibit caspase-3.
The results from the experiment on indomethacin’s effects on protein expression of COX-1 showed that it significantly decreased the protein synthesis of COX-1, which is in agreement with previous reports [37, 38]. COX-1 has been shown to decrease acid output [38, 39]. Additionally, it has been reported that NSAIDs, through inhibition of COX-1, result in an increase in gastric acid secretion in the rat [38]. The results of the present study showed that crocin pretreatment could not significantly inhibit the of protein expression of COX-1 but could partially modify the pH of gastric effluent. In contrast, pantoprazole significantly prevented the indomethacin-induced reduction of COX-1 and increased the pH of gastric effluent in pylorus-ligated rats. These findings suggest that crocin’s gastro-protective activity is independent of COX-1 and the acid secretory effects induced by indomethacin. The involvement of COX enzymes and prostaglandins in the ulcer healing mechanisms activated by PPIs has been previously investigated, with conflicting results [22]. When administered alone, crocin did not change the level of COX-1 protein expression, suggesting that crocin is a safe agent.
Importantly, for induction of gastric damage, concurrent inhibition of COX-1 and up-regulation of COX-2 seem to be necessary [40]. The results of the present experiments showed that crocin increased COX-2 without affecting the expression of COX-1. This finding indirectly supports and is in agreement with the proposed notion of Tanaka et al. [40]. A review article by Wallace suggested that up-regulation of COX-2 appears to be a defensive and anti-inflammatory response to various gastric irritants [41].
Unexpectedly, these levels in crocin-alone-treated rats were significantly higher than in normal intact rats. The stimulatory effect of the effective dose of crocin on COX-2 expression was equivalent to those measured for indomethacin-treated rats. Previous studies have demonstrated that mRNA and protein of COX-2 are constitutively expressed in gastric mucosa in human and rabbit [42]. Moreover, our histological and macroscopic results showed that crocin alone did not produce erosions in the gastric mucosa. Coupled with these observations, the expression and protein synthesis of iNOS and caspase-3 in the presence of crocin alone did not differ from that of intact control mucosa. These findings suggest that in order to produce ulceration concurrent stimulation of COX-2 and iNOS as well as caspase-3 pathways are needed.
In addition, crocin seems to confer additional protective effects by stimulating mRNA and protein synthesis of COX-2, and therefore production of PGE2, and lays the ground for more rapid healing. In agreement with this suggestion, the levels of mRNA and protein synthesis of COX-2 in the crocin-pretreatment group were found to be more significantly increased than either crocin-alone- or indomethacin-treated groups. These findings are in agreement with the notion that COX-2 has a wider range of biological roles than was previously proposed as a mediator of inflammation [43]. In addition, these findings suggest that the mechanisms of crocin’s gastro-protective effect, at least in respect to COX-2, are not similar to pantoprazole’s.
Previous reports have shown that the administration of black tea and ellagic acid, through up-regulating COX-2 expression, protected the gastric mucosa against indomethacin-induced gastric mucosal lesions in mice [37, 44]. Consistent with these findings, the present results showed that crocin pretreatment significantly increased mRNA and protein expression of COX-2 in gastric mucosa. Messenger RNA and protein expression of COX-2 in crocin-pretreated rats were found to be significantly higher than in indomethacin-treated control animals. Similar findings have been observed with omeprazole, where, in addition to its acid secretory effects, it upregulated mRNA expression of COX-2 and PGE2 synthesis [45]. However, this observation was not consistently reported with pantoprazole [10]. Furthermore, our macroscopic and microscopic results were found to support the anti-ulcer potential of crocin. Taken together, these findings suggest that crocin, without affecting COX-1 protein expression, protected the gastric mucosa against indomethacin-induced gastric lesions through up-regulation of protein synthesis and mRNA expression of COX-2.
In order to gain more insight into the mechanisms that govern the gastro-protective effects of crocin, pylorus ligation experiments were carried out. The results from these experiments showed that the volume and total acidity of gastric contents were not affected by crocin pretreatment at all the tested doses. Pretreatment with crocin produced a partial increase in the pH, while pantoprazole significantly increased pH and also decreased the volume and total acidity of gastric contents as compared with control pylorus-ligated and crocin-pretreated rats. This finding comes in contrast to previous reports. While a 5-day pretreatment with aqueous extract of saffron at a dose of 100 mg/kg stimulated the gastric acid output [46], a single administration of aqueous extract of saffron at 250 or 500 mg/kg reduced it [21]. The reasons for this discrepancy may lie in the dose, duration of administration and employment of crude saffron extract or pharmacologically active constituent.
Furthermore, results from pylorus ligation experiments provided further insight into the role of mucosal MDA, which was employed as a marker for lipid peroxidation. The mucosal level of MDA in crocin- and pantoprazole-pretreated rats was significantly lower than in control pylorus-ligated rats. The mucoprotective activity of crocin at 15 and 30 mg/kg was equivalent to pantoprazole at 50 mg/kg. Similar findings have been reported in diabetic rats [20]. Consistent with these findings, Al-Mofleh et al. showed that saffron protected the gastric mucosa against ethanol-induced gastric ulcers through increasing mucus production in the rat [21]. Taken together, these findings suggest that the gastro-protective effect of crocin, similar to pantoprazole, is partly mediated through an increase in mucus production.
What are the clinical implications of the present findings? Although the results of the present study were not wholly conclusive and extrapolation of the results from animal studies to human beings needs to be carefully guarded, the results showed crocin is safe and effective and comparable to pantoprazole in promoting an increase in mucus content, an indirect parameter for PG release, inhibiting the induction of apoptosis and ameliorating the rise of iNOS induced by indomethacin administration. In addition and interestingly, crocin, similar to pantoprazole, was found to produce a significant reduction in lipid peroxidation. These findings suggest that the primary gastro-protective action of crocin is mediated by its antioxidant effect. However, future clinical studies are prudently needed to support its use in clinical practice. The findings of this study are only small steps towards assessing the potential of crocin as a novel agent for protection of acute gastric erosion associated with the use of NSAIDs.
References
Musumba C, Pritchard D, Pirmohamed M (2009) Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther 30(6):517–531
Gudis K, Sakamoto C (2005) The role of cyclooxygenase in gastric mucosal protection. Dig Dis Sci 50(1):S16–S23
Tomisato W, K-i Tanaka, Katsu T, Kakuta H, Sasaki K, Tsutsumi S et al (2004) Membrane permeabilization by non-steroidal anti-inflammatory drugs. Biochem Biophys Res Comm 323(3):1032–1039
Tomisato W, Tsutsumi S, Rokutan K, Tsuchiya T, Mizushima T (2001) NSAIDs induce both necrosis and apoptosis in guinea pig gastric mucosal cells in primary culture. Am J Physiol 281(4):G1092–G1100
Guo JS, Cho CH, Wang JY, Koo MWL (2006) Differential effects of selective and non-selective inhibition of nitric oxide synthase on the expression and activity of cyclooxygenase-2 during gastric ulcer healing. Eur J Pharmacol 536(3):301–308
Wood AJ, FitzGerald GA, Patrono C (2001) The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 345(6):433–442
Lazzaroni M, Bianchi Porro G (2001) Prophylaxis and treatment of non-steroidal anti-inflammatory drug-induced upper gastrointestinal side-effects. Dig Liver Dis 33:S44–S58
Becker JC, Domschke W, Pohle T (2004) Current approaches to prevent NSAID-induced gastropathy––COX selectivity and beyond. Br J Clin Pharmacol 58(6):587–600
Piotrowski J, Slomiany A, Slomiany B (1999) Activation of apoptotic caspase-3 and nitric oxide synthase-2 in gastric mucosal injury induced by indomethacin. Scand J Gastroenterol 34(2):129–134
Fornai M, Natale G, Colucci R, Tuccori M, Carazzina G, Antonioli L et al (2005) Mechanisms of protection by pantoprazole against NSAID-induced gastric mucosal damage. Naunyn-Schmiedeberg’s Arch Pharmacol 372(1):79–87
Melnyk JP, Wang S, Marcone MF (2010) Chemical and biological properties of the world’s most expensive spice: saffron. Food Res Int 43(8):1981–1989
Abdullaev F (1993) Biological effects of saffron. BioFactors 4(2):83–86
Zheng Y-Q, Liu J-X, Wang J-N, Xu L (2007) Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res 1138:86–94
Goyal S, Arora S, Sharma A, Joshi S, Ray R, Bhatia J et al (2010) Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine 17(3):227–232
Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A (2005) Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia–reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci 8(3):387–393
Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y et al (2008) Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: a relationship investigation between antioxidant activity and crocin contents. Food Chem 109(3):484–492
Hosseinzadeh H, Younesi HM (2002) Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2(1):7
Inoue E, Shimizu Y, Shoji M, Tsuchida H, Sano Y, Ito C (2005) Pharmacological properties of N-095, a drug containing red ginseng, polygala root, saffron, antelope horn and aloe wood. Am J Chin Med 33(01):49–60
Xu G-L, Li G, Ma H-P, Zhong H, Liu F, Ao G-Z (2009) Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem 57(18):8325–8330
Plants A, Karaj I (2009) Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J Med Plant 8:30–38
Al-Mofleh L, Alhaider A, Mossa J, Al-Sohaibani M, Qureshi S, Rafatullah S (2006) Antigastric ulcer studies on ‘saffron’ Crocus sativus L. in rats. Pak J Biol Sci 9(6):1009–1013
Fornai M, Colucci R, Antonioli L, Awwad O, Ugolini C, Tuccori M et al (2011) Effects of esomeprazole on healing of nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers in the presence of a continued NSAID treatment: characterization of molecular mechanisms. Pharmacol Res 63(1):59–67
Maity B, Banerjee D, Bandyopadhyay SK, Chattopadhyay S (2009) Regulation of arginase/nitric oxide synthesis axis via cytokine balance contributes to the healing action of malabaricone B against indomethacin-induced gastric ulceration in mice. Int Immunopharmacol 9(4):491–498
Pérez Y, Oyárzabal A, Mas R, Molina V, Jiménez S (2013) Protective effect of D-002, a mixture of beeswax alcohols, against indomethacin-induced gastric ulcers and mechanism of action. J Nat Med 67(1):182–189
Xing L, Karinch AM, Kauffman GL Jr (1998) Mesolimbic expression of neurotensin and neurotensin receptor during stress-induced gastric mucosal injury. Am J Physiol 274(1):R38–R45
Tan PV, Nyasse B, Dimo T, Mezui C (2002) Gastric cytoprotective anti-ulcer effects of the leaf methanol extract of Ocimum suave (Lamiaceae) in rats. J Ethnopharmacol 82(2):69–74
Shay H (1945) A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5:43–61
Tomisato W, Tsutsumi S, Hoshino T, Hwang H-J, Mio M, Tsuchiya T et al (2004) Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem Pharmacol 67(3):575–585
Calatayud S, Barrachina D, Esplugues JV (2001) Nitric oxide: relation to integrity, injury, and healing of the gastric mucosa. Microsc Res Tech 53(5):325–335
Nam KN, Park Y-M, Jung H-J, Lee JY, Min BD, Park S-U et al (2010) Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 648(1):110–116
Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR (2006) Aspirin induces apoptosis through the inhibition of proteasome function. J Biol Chem 281(39):29228–29235
Power JJ, Dennis MS, Redlak MJ, Miller TA (2004) Aspirin-induced mucosal cell death in human gastric cells: evidence supporting an apoptotic mechanism. Dig Dis Sci 49(9):1518–1525
Jana N (2008) NSAIDs and apoptosis. Cell Mol Life Sci 65(9):1295–1301
Chiou S-K, Mandayam S (2007) NSAIDs enhance proteasomic degradation of survivin, a mechanism of gastric epithelial cell injury and apoptosis. Biochem Pharmacol 74(10):1485–1495
Bie X, Chen Y, Zheng X, Dai H (2011) The role of crocetin in protection following cerebral contusion and in the enhancement of angiogenesis in rats. Fitoterapia 82(7):997–1002
Qi Y, Chen L, Zhang L, Liu W-B, Chen X-Y, Yang X-G (2013) Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3 K/AKT signalling pathway. Exp Eye Res 107:44–51
Chatterjee A, Chatterjee S, Das S, Saha A, Chattopadhyay S, Bandyopadhyay SK (2012) Ellagic acid facilitates indomethacin-induced gastric ulcer healing via COX-2 up-regulation. Acta Biochem Biophys Sin 44(7):565–576
Barnett K, Bell CJ, McKnight W, Dicay M, Sharkey KA, Wallace JL (2000) Role of cyclooxygenase-2 in modulating gastric acid secretion in the normal and inflamed rat stomach. Am J Physiol Gastrointest Liver Physiol 279(6):G1292–G1297
Kato S, Aihara E, Yoshii K, Takeuchi K (2005) Dual action of prostaglandin E2 on gastric acid secretion through different EP-receptor subtypes in the rat. Am J Physiol Gastrointest Liver Physiol 289(1):G64–G69
Tanaka A, Hase S, Miyazawa T, Takeuchi K (2002) Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J Pharmacol Exp Ther 300(3):754–761
Wallace JL (2008) Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev 88(4):1547–1565
Zimmermann KC, Sarbia M, Schrör K, Weber A-A (1998) Constitutive cyclooxygenase-2 expression in healthy human and rabbit gastric mucosa. Mol Pharmacol 54(3):536–540
Halter F, Tarnawski A, Schmassmann A, Peskar B (2001) Cyclooxygenase 2—implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut 49(3):443–453
Adhikary B, Yadav SK, Roy K, Bandyopadhyay SK, Chattopadhyay S. Black tea and the aflavins assist healing of indomethacin-induced gastric ulceration in mice by antioxidative action. Evid Based Complement Alternat Med. 2011; 2011. doi: 10.1155/2011/546560
Poonam D, Vinay CS, Gautam P (2005) Cyclo-oxygenase-2 expression and prostaglandin E2 production in experimental chronic gastric ulcer healing. Eur J Pharmacol 519(3):277–284
Fatemeh N, Ehsan S, Zahra S, Seyed MK, Jalal V (2009) Saffron (Crocus sativus) increases gastric acid and pepsin secretions in rats: role of nitric oxide (NO). Afr J Pharm Pharmacol 3(5):181–184
Acknowledgments
The authors would like to gratefully acknowledge the financial support of the Vice Chancellor of Research Affairs of Ahvaz Jundishapur University of Medical Sciences and Dr Esrafil Mansori for confirmation of the histopathological results. This project has been registered under grant number #PRC106.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mard, S.A., Pipelzadeh, M.H., Teimoori, A. et al. Protective activity of crocin against indomethacin-induced gastric lesions in rats. J Nat Med 70, 62–74 (2016). https://doi.org/10.1007/s11418-015-0938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-015-0938-0