Abstract
Gastric ulcer disease is associated with significant morbidity and mortality rates. The most two common causes of the ulcer are Helicobacter pylori infection and non-steroidal anti-inflammatory drugs. In the past few decades, a significant decrease in the morbidity and mortality rate has been observed probably due to the discovery of proton pump inhibitors. However, the medications used to treat gastric ulcers impose several nauseous side effects. Therefore, recent studies focus on the use of natural products to treat gastric ulcers. In the current study, gastric ulcer was effectively induced using indomethacin, and the protective effect of apigenin, a potent antioxidant flavonoid, was assessed in comparison to omeprazole. The administration of a single oral indomethacin (50 mg/kg) induced gastric ulcer as manifested by hemorrhagic lesions in the gastric mucosa, increased ulcer index, and histopathological alterations. Indomethacin also increased lipid peroxidation, decreased the activities of the antioxidant enzymes superoxide dismutase (SOD) and catalase, increased the immunoreactivity of the inflammatory markers cyclo-oxygenase-2 (COX-2), tumor necrosis factor-alpha (TNF-α), and nuclear factor-kappa B (NF-κB), increased the transcription of the apoptotic marker, Bax, and decreased that of the antiapoptotic Bcl-2. Indomethacin also decreased the immunoreactivity of transforming growth factor-beta 1 (TGF-β1). On the other hand, pretreatment with apigenin (10 and 20 mg/kg) resulted in a dose-dependent improvement in the macroscopic and microscopic features of the gastric mucosa in a manner comparable to that of omeprazole. The gastroprotective effects of apigenin may be attributed to its anti-inflammatory, anti-antioxidant, and anti-apoptotic activities as well as enhancing the expression of TGF-β1. Further experimental and clinical research is required to confirm activity of apigenin as anti-ulcer agent.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptic ulcer disease is associated with significant morbidity and mortality rates. The outcomes of the disease may range from epigastric pain to life-threatening conditions such as gastric perforation or obstruction (Kavitt et al. 2019). The disease most commonly affects the stomach and the proximal part of the duodenum due to the imbalance occurring between the aggressive factors, the excessive release of hydrochloric acid (HCl) or pepsin, and the factors responsible for maintaining mucosal integrity, decreased prostaglandins that are responsible for inducing the production of bicarbonates and mucus (Freston 1988). The hallmark of the disease is the disruption in the inner lining of the affected part that may extend to the muscularis propria layer of the gastric epithelium (Sverdén et al. 2019). Helicobacter pylori infection and the administration of non-steroidal anti-inflammatory drugs (NSAIDs) are the major risk factors involved in the development of gastric ulcers (Zhang et al. 2014a). NSAIDs contribute to mucosal damage by inhibiting the activity of the constitutive enzyme cyclooxygenase-1 (COX-1) and subsequently decreasing the production of prostaglandins and subsequently decreased mucosal blood supply, bicarbonate and mucus secretion, and inhibiting cell proliferation (Bhala et al. 2013).
Proton pump inhibitors (PPIs) are the major class of drugs that are used to treat gastric ulcers by decreasing the production of HCl. However, as a result of decreased HCL production, serious side effects are reported with PPI administration due to decreased HCL-induced protection against pathogens that increases the risk of patients developing enteric infections such as salmonella, and Clostridium difficile (Lambert et al. 2015). This provoked an emerging need to find a safe and effective alternative, preferably from natural origin.
Apigenin is a natural molecule that belongs to the well-known therapeutically effective flavonoids. Flavonoids are well known for their antioxidant, anti-inflammatory, and anti-mutagenic activities that qualify them to be beneficial therapeutic candidates against multiple disorders (Falcone Ferreyra et al. 2012). Apigenin or 4′,5,7-trihydroxyflavone is widely found in its glycosylated form in many plants including onions, celery, parsley, oranges, thyme, basil, chamomile, beer, and tea (Hostetler et al. 2017). Moreover, apigenin was proven to promote wound healing in vivo (Lopez-Jornet et al. 2014). The current study aims to investigate the potential preventive effects of apigenin against indomethacin-induced gastric ulcers in rats, as well as the possible underlying mechanism.
Materials and methods
Drugs and chemicals
Apigenin and indomethacin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals used were of the finest commercially available grade.
Animals
Male apparently healthy Wistar rats (8 week-old, 180 ± 20 g) were obtained from the animal facility, Faculty of Pharmacy, King Abdulaziz University. One week before the commencement of the experiment, animals were left to acclimatize to the laboratory conditions. During animal housing, they were maintained under a standard laboratory environment with room temperature (22 ˚C ± 2), relative humidity ˜ 50%, and 12/12 hours dark/light cycles and fed with standard chow and water ad libitum. All procedures and animal interventions were approved by our local Research Ethics Committee (permit # PH-1444-34).
Experimental design
For the induction of gastric ulcer, animals were deprived of food for 24 h and of water for one hour before the administration of a single dose of indomethacin (50 mg/kg) suspended in 0.5% sodium carboxymethyl cellulose (CMC) by gastric lavage The control animals received plain CMC (0.5% in water, p.o., 10 ml/kg/day for 7 consecutive days) (Bampidis et al. 2020; AlKreathy et al. 2020).
Treatments were given to five animal groups (n = 6) as follows: Group one, which served as the control group, received the respective vehicles; Group two, received 0.5% CMC for seven days and indomethacin (50 mg/kg, p.o.) after the last dose; Groups three and four, received apigenin (10 and 20 mg/kg, respectively, p.o.) suspended in 0.5% CMC for seven days then indomethacin (50 mg/kg, p.o.) after the last dose; Group five, received omeprazole (30 mg/kg, p.o.) suspended in 0.5% CMC for seven days then indomethacin (50 mg/kg, p.o.). The chosen doses are based on a pilot experiment and consistent with those in the literature (Park et al. 2008; Johnson and Greenwood-Van Meerveld 2017).
Six hours after the induction of gastric ulcer using indomethacin, animals were anesthetized (Ketamine, 80 mg/kg, IP) and sacrificed by decapitation. Stomachs were dissected out, incised at the longer curvature, and washed using normal saline. Half of the stomachs were flash frozen in liquid nitrogen and kept at – 80 ˚C for further biochemical analysis and the other halves were preserved in 10% formaldehyde for the histopathological and immunohistochemical assessments.
Gross morphological investigation of the gastric mucosa and calculation of ulcer index
After washing and drying the stomach specimens, the inner lining of the stomach was photographed using a digital camera. The stomachs were investigated for inflammatory and hemorrhagic lesions. The lengths of the bleeding strikes were measured for the calculation of the ulcer index using the following equation:
Ulcer index = 10 X total ulcerated area/ total mucosal area (AlKreathy et al. 2020).
Histopathological examination of the rats’ stomachs
Stomach tissues fixed in 10% formalin were molded into paraffin blocks, and then 5 μm sections were cut using tissue microtome (LEICA RM2125RT, LEICA, Wetzlar, Germany). Tissue sections were then stained with Hematoxylin and Eosin (H and E) and examined using an electric light microscope (Bancroft and Gamble 2008). A tissue scoring system as previously described (AlKreathy et al. 2020) was utilized to assess the histopathological alterations according to the lesions’ severity in the stomachs as follows: [1] epithelial cell loss (score: 0–3), [2] hemorrhage (score: 0–4), [3] inflammatory cell infiltration (score: 0–2) and [4] mucosal erosions (score: 0–4).
Assessment of mucin content
Mucous secretion was assessed by assessing gastric mucin content using alcian blue and periodic acid-Schiff mixture. The stomach sections were hydrated and then immersed in 3% acetic acid for three minutes and then in 1% alcian blue stain (pH 2.5) for 15 min. Slides were washed and put in 0.5% periodic acid for 5 min then in Schiff’s reagent for another 10 min, washed with tap water, and then slightly stained with hematoxylin. The quantitative image analysis of mucin content as optical density (OD) was performed using ImageJ, 1.48a, NIH, USA.
Assessment of the immunohistochemical reactivity of the pro-inflammatory markers and transforming growth factor-β1
The protein expression of the markers of inflammation, cyclo-oxygenase-2 (COX-2), tumor necrosis factor-alpha (TNF-α), and nuclear factor-kappa B (NF-κB), and the fibrosis marker transforming growth factor-β1 (TGF-β1) were assessed. Briefly, tissue Sect. (5 μm) were deparaffinized using xylene (Fisher Scientific, Waltham, MA, US). This was followed by antigen retrieval l by immersing the tissue sections in citrate buffer (0.1 M, pH 6) and boiling in a water bath for 10 min. After five minutes of washing using 0.1 M PBS at pH 7.4, tissue sections were incubated at 4 °C for 18 h with diluted (1:100) rabbit primary antibodies: anti-COX-2, anti- TNF-α, anti- NF-κB, or TGF-β1 (Abcam, Cambridge, UK, Catalogue numbers, ab179800, ab307164, ab16502, and ab215715, respectively). The slides were then washed and incubated with the secondary antibody, HRP Anti-Rabbit IgG antibody (Abcam, Cambridge, UK, Catalogue number, ab288151) for 1 h. A tissue staining anti-rabbit kit (Abcam, Cambridge, UK, Cat # ab64261) was used to stain the target antigens. Two non-overlapping fields from each slide (n = 3) were analyzed using ImageJ, 1.48a (NIH, USA), and the protein expressions were represented as OD.
Assessment of oxidative stress biomarkers
Tissues were thawed and homogenized in phosphate buffer saline (0.1 M, pH 7.4) as 10% homogenate. Lipid peroxidation induced by indomethacin were assessed by detecting the levels of the major thiobarbituric acid reactive species, malondialdehyde (MDA) using the MDA colorimetric assay kit, MD2529, purchased from Biodiagnostics, (Cairo, Egypt). The activities of the antioxidant enzymes catalase and superoxide dismutase (SOD) were also assessed using the commercially available kits CA2517 and SD252, respectively, purchased from Biodiagnostics, (Cairo, Egypt). The manufacturer’s instructions were strictly adhered to during assessments.
Assessment of mRNA expression of the apoptotic markers Bax and Bcl-2 using real time-polymerase chain reaction (RT-PCR)
The TRIzol method was used to extract RNA from stomach tissues. Purity (A260/A280 ratio) and concentration of RNA were determined using spectrophotometric analyses (dual-wavelength Beckman spectrophotometer, USA). The Omniscript RT kit (Catalogue number: 205,113, Qiagen, MD, USA) used the cDNA. This was followed by quantification of mRNA using qRT-PCR with a SYBR Green Master Mix (Catalogue number: 180,830, Qiagen, MD, USA). The used primer nucleotide sequences are shown in Table 1. Gene expression changes were calculated by the comparative cycle threshold (Ct) method and the values were normalized to endogenous reference β-actin.
Statistical analysis
The non-parametric data, lesion score, were analyzed using the Kruskal Wallis test followed by Dunn’s post hoc test, and the results are presented as medians and interquartile ranges. Otherwise, all other data are parametric as confirmed by Kolmogorov–Smirnov test and were analyzed using one-way ANOVA followed by the Tukey post hoc test, and the results are presented as means and standard deviations (SD). At p-value < 0.05, differences between treatments were considered statistically significant. GraphPad Prism software version 8.0.2 for Windows was used for all analyses (GraphPad Software Inc., San Diego, CA, USA).
Results
The effect of apigenin on the macroscopic features of the gastric mucosa and the ulcer index of indomethacin-treated rats
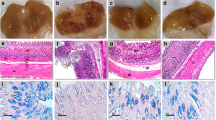
Specimens from the control group showed a normal macroscopic picture of the gastric mucosa (Fig. 1A). However, indomethacin treatment induced gastric inflammation and the development of hemorrhagic strikes (Fig. 1B). On the other hand, pretreatment with apigenin induced dose-dependent significance in the gastric mucosa. Animals pretreated with apigenin 10 mg/kg showed mild improvement with smaller and fewer lesions (Fig. 1C), while pretreatment with apigenin 20 mg/kg induced more obvious improvement (Fig. 1D). However, pretreatment with omeprazole 30 mg/kg completely restored the normal macroscopic features of the gastric mucosa (Fig. 1E). These results are further emphasized by the assessment of the ulcer index (Fig. 1F), where indomethacin significantly increased the ulcer index compared to the control animals. Pretreatment with apigenin (10 and 20 mg/kg) induced a significant dose-dependent decrease in the ulcer index compared to indomethacin-only-treated animals. Pretreatment with omeprazole decreased the ulcer index to the control levels.
Photographs of the rats’ stomachs: A control group; B indomethacin (50 mg/kg) only treated group; C indomethacin and APG 10 mg/kg treated group; D indomethacin and APG 20 mg/kg treated group; E indomethacin and omeprazole (30 mg/kg) treated group; F Ulcer index analyzed by one-way ANOVA followed by Tukey’s as a post-hoc test (n = 6), where a, b, c, and d are considered statistically significant from the control, indomethacin-only, indomethacin plus apigenin 10 mg/kg, and indomethacin plus apigenin 20 mg/kg, respectively, at P < 0.05
The effect of apigenin on the histopathological alterations in the gastric mucosa of indomethacin-treated rats
The control group showed the normal architecture of the gastric tissues (Fig. 2A). However, gastric tissue from animals treated with indomethacin showed less thick mucosa with non-intact epithelium lining of the mucosal layer and congested blood vessels (Fig. 2B). Pretreatment with apigenin 10 mg/kg revealed milder disruption of the gastric tissue and epithelium lining when compared to the indomethacin-only treatment (Fig. 2C). Pretreatment with apigenin 20 mg/kg greatly protected against indomethacin-induced deleterious effects with mildly disrupted epithelium lining (Fig. 2D). Pretreatment with omeprazole (30 mg/kg) conserved the normal architecture of the gastric tissue (Fig. 2E) with. These findings were further quantified by assessing the histological scoring (Fig. 2F) where the treatment with indomethacin alone significantly increased the histological score when compared to the control group. The pretreatment with apigenin (10 and 20 mg/kg) induced a significant dose-dependent decrease in the histological score compared to the indomethacin-only-treated group. Pretreatment with omeprazole decreased the score to the control levels.
Photomicrographs of the rats’ stomachs stained with hematoxylin and eosin showing the histological features of A control group; B indomethacin (50 mg/kg) only treated group; C indomethacin and APG 10 mg/kg treated group; D indomethacin and APG 20 mg/kg treated group; E indomethacin and omeprazole (30 mg/kg) treated group; F histological score analyzed by Kruskal Wallis followed by Dunn’s post hoc test (n = 6), where a, b, and c are considered statistically significant from the control, indomethacin-only, and indomethacin plus apigenin 10 mg/kg, respectively, at P < 0.05
Effect of apigenin on the mucin content of the stomachs of indomethacin-treated rats
As illustrated in Fig. 3, indomethacin treatment alone significantly reduced the mucin secretion by the gastric mucosa. However, pretreatment with apigenin 10 and 20 mg/kg, before indomethacin administration, significantly restored the mucin secretion in a manner comparable to that induced by omeprazole (30 mg/kg).
Photomicrographs showing the effects of APG and omeprazole on the mucin secretion, detected after reacting with Alcian blue, in the gastric mucosa of indomethacin-treated rats. A control group; B indomethacin (50 mg/kg) only treated group; C indomethacin and APG 10 mg/kg treated group; D indomethacin and APG 20 mg/kg treated group; E indomethacin and omeprazole (30 mg/kg) treated group; F statistical analysis of the stained mucin optical density (OD). Mucin reactivity was analyzed by one-way ANOVA followed by Tukey’s as a post-hoc test (n = 6), where a and b are considered statistically significant from the control, and indomethacin-only, respectively, at P < 0.05
Effect of apigenin on the immunohistochemical reactivity of the proinflammatory mediators in the stomachs of indomethacin-treated rats
Immunohistochemical staining of the gastric tissue revealed that the administration of a single dose of indomethacin increased the immunoreactivity of the pro-inflammatory mediators, TNF-α, COX-2, and NF-κB compared to the control animals. However, gastric tissue of the rats pretreated with apigenin 10, and 20 mg/kg significantly reduced the immunoreactivity of the three biomarkers as compared to the indomethacin-only-treated group. The pretreatment with apigenin 20 mg/kg and omeprazole showed a more significant reduction in the expression of TNF-α, and NF-κB compared to the animals pretreated with apigenin 10 mg/kg (Fig. 4).
The effect of APG on the gastric oxidative stress biomarkers, MDA (A), catalase (CAT) (B), and superoxide dismutase SOD (C). Results were analyzed by one-way ANOVA followed by Tukey’s as a post-hoc test (n = 6). where a, b, and c are considered statistically significant from the control, indomethacin-only, and indomethacin plus APG 10 mg/kg, respectively, at P < 0.05
Effect of apigenin on the biomarkers of oxidative stress in the stomachs of indomethacin-treated rats
As illustrated by Fig. 5, the administration of a single indomethacin dose significantly increased lipid peroxidation in the gastric tissue as evidenced by the increased levels of MDA and decreased the antioxidant capacity of the gastric mucosa through decreasing the activities of the antioxidant enzymes, catalase, and SOD. In contrast, the pretreatment with apigenin 10 and 20 mg/kg significantly attenuated lipid peroxidation and maintained catalase, SOD activities. Apigenin 20 mg/kg showed a more significant reduction in MDA levels and an increase in the catalase activity compared to the lower dose, 10 mg/kg, in a manner comparable to that induced by omeprazole 30 mg/kg.
The effect of APG on the immunohistochemical reactivity of proinflammatory mediators, tumor necrosis factor-alpha (TNF-α), cyclooxygenase-2 (COX-2), and nuclear factor-kappa B (NF-κB), in gastric tissue of the indomethacin-treated rats. Optical density (OD) results were analyzed by one-way ANOVA followed by Tukey’s as a post-hoc test (n = 6), where a, b, c, and d are considered statistically significant from the control, indomethacin-only, indomethacin plus APG 10 mg/kg, and indomethacin plus APG 20 mg/kg, respectively, at P < 0.05
The effect of apigenin on the apoptosis markers in the stomachs of indomethacin-treated rats
As shown in Fig. 6, the administration of a single indomethacin dose, significantly increased the gene transcription of apoptotic marker, Bax and decreased that of the antiapoptotic, Bcl-2. However, the pretreatment with apigenin 10 and 20 mg/kg significantly reduced Bax and increased Bcl-2 transcription. Pretreatment with apigenin 20 mg/kg showed a more significant decrease in the Bax transcription compared to apigenin 10 mg/kg.
The effect of APG on the Bax and Bcl-2 gene transcription in the gastric tissue of the indomethacin-treated rats. Results were analyzed by one-way ANOVA followed by Tukey’s as a post hoc test (n = 6), where a, b, c, and d are considered statistically significant from the control, indomethacin-only, indomethacin plus APG 10 mg/kg, and indomethacin plus APG 20 mg/kg, respectively, at P < 0.05
The effect of apigenin on the immunohistochemical reactivity of the fibrosis marker, TGF-β1, in the stomachs of indomethacin-treated rats
The immunohistochemical staining of the fibrosis marker, TGF-β1, revealed that the treatment with indomethacin significantly decreased its expression in the gastric tissue. On the other hand, the pretreatment with apigenin significantly attenuated this effect in a dose-dependent manner. Apigenin 20 mg/kg administration was found to induce a more significant increase in the immunohistochemical reactivity of TGF-β1 compared to omeprazole as shown in Fig. 7.
Photomicrographs showing the effects of APG and omeprazole on the immunohistochemical reactivity of TGF-β1, in the gastric mucosa of indomethacin-treated rats. A control group; B indomethacin (50 mg/kg) only treated group; C indomethacin and APG 10 mg/kg treated group; D indomethacin and APG 20 mg/kg treated group; E indomethacin and omeprazole (30 mg/kg) treated group; F statistical analysis of the optical density (OD) of the stained TGF-β1. Data were analyzed by one-way ANOVA followed by Tukey’s as a post hoc test (n = 6), where a, b, c, and d are considered statistically significant from the control, indomethacin-only, indomethacin plus APG 10 mg/kg, and indomethacin plus APG 20 mg/kg, respectively, at P < 0.05
Discussion
For many years, the prevalence of gastric ulcer had been greatly expanding resulting in high morbidity and mortality rates until a significant decrease in the disease epidemiology occurred in the few past decades. This decline can be attributed to the discovery of the drug classes that reduce the release of HCL, besides, encountering that one of the major causes of peptic ulcer is the H pylori infection, and subsequently, more directed and effective treatments were used (Malfertheiner et al. 2009). However, the observation that the commercially available treatments are associated with noxious side effects together with the evidence that the disease pathogenesis involves inflammation and oxidative damage emerged the need for finding a new safe and effective alternative, preferably of natural origin possessing antioxidant and anti-inflammatory activities (Prayoga et al. 2024).
Indomethacin, one of the effective NSAIDs, is widely used for pain relief and decreasing fever and inflammation. However, one of its major side effects is inducing peptic ulcer due to decreasing the gastroprotective prostaglandins and subsequently decreasing mucous and bicarbonate secretion and decreasing the blood supply. Indomethacin is the most commonly used in the induction of peptic ulcers in experimental animals due to its highest ulcerogenic potential among all NSAIDs (Suleyman et al. 2010). These effects can be attributed to its inhibitory effect on the constitutional cyclooxygenase-1 (COX-1) and the subsequent decrease in the production of the gastroprotective, PGE2, which leads to disruption of the gastric mucosa through a sequence of events starting with reducing bicarbonate release, going through disrupted mucosal barriers and finally cytotoxicity (Ibrahim et al. 2018). These cytotoxic effects induce the recruitment of reactive oxygen species (ROS)-releasing inflammatory cells, decreased gastric mucosal blood supply, and finally apoptosis (Matsui et al. 2011; Uc et al. 2012).
In the current study, a single, oral indomethacin dose induced the hallmark macroscopic and microscopic features indicating the induction of peptic ulcers in the rats’ gastric mucosa. These investigations were further emphasized by assessing the quantitative ulcer index and index score, respectively, where indomethacin was found to significantly increase both values compared to the untreated animals. The pretreatment with apigenin, especially the higher dose (20 mg/kg), greatly attenuated the indomethacin-induced ulcerogenic effect and maintained the gastric mucosal integrity to a great extent, in a way similar to that of the omeprazole.
Under physiological conditions, the small amounts of ROS produced have protective effects through modulating the immune-mediated attack against pathogens and activating protective anti-inflammatory signals. However, the overproduction of ROS mediates the inflammatory processes involved in gastric mucosal conditions including peptic ulcer and cancer (Jaeschke 2011; Kim et al. 2012). The interplay between oxidative stress and inflammation is reciprocal reinforcing each other, where ROS induces inflammation and recruitment of inflammatory cells including cells that further contribute to the production of ROS, neutrophils (Yoshikawa and Naito 2000). Neutrophil infiltration induced by indomethacin leads to increased TNF-α levels in gastric mucosa contributing to the pathogenesis of peptic ulcer (Ding et al. 1998). When TNF-α binds to its receptors, it leads to the recruitment of adaptor proteins and downstream signaling events that result in the activation of the transcriptional factor, NF-κB (Bajaj and Sharma 2006) that regulates the expressions of several proteins including pro-inflammatory cytokines, inducible nitric oxide synthase, and the inducible COX-2 (O’Neill and Kaltschmidt 1997). This explains the increased immunoreactivity of the proinflammatory markers TNF-α, NF-κB, and COX-2 in the gastric mucosa of the indomethacin-treated rats in the current study. On the other hand, apigenin administration has proven to have a significant anti-inflammatory activity as evidenced by its effect on decreasing the expression of the inflammatory markers. These results come in line with a previous study that showed that apigenin decreased LPS-induced inflammation by decreasing the expression of COX-2 and NF-κB (Wang et al. 2014).
One of the mechanisms that has been proven to be a major contributor to the pathogenesis of mucosal injury is oxidative stress. Cellular damage occurs due to the overproduction of ROS, including superoxide, hydrogen peroxide, and peroxynitrite radicals. When the levels of the accumulated ROS overwhelm the antioxidant capacity of the cell, lipid peroxidation and protein oxidation occur leading to genetic material, membrane, and organelles damage and subsequently mucosal ulceration, erosion, and bleeding (Wiseman and Halliwell 1996; Tandon et al. 2004). Indomethacin-induced gastric ulcer can be attributed to its disturbing effects on the oxidative balance in the gastric mucosa (Adriana et al. 2008; Suleyman et al. 2010). In the current study, indomethacin was found to increase lipid peroxidation as evidenced by the increased MDA levels and decreased the antioxidant capacity of the gastric mucosal cells through decreasing the activities of the antioxidant enzymes, catalase, and SOD contributing to the overproduction of the peroxide, and superoxide radicals. Therefore, antioxidants of natural origin represent potential therapeutic candidates for decreasing the severity of peptic ulcer disease. Apigenin is well known for its in vivo antioxidant activities against a number of diseases, such as cancer (Singh et al. 2004), sepsis (Karamese et al. 2016), hepatotoxicity (Rašković et al. 2017) and neurotoxicity (Yadav et al. 2022). Similarly, in the current study, the pretreatment of the rats with apigenin restored the antioxidant enzyme activities and decreased lipid peroxidation.
Studies have proven that oxidative stress and inflammation play pivotal roles in apoptosis (Zamzami et al. 1995; Hsu et al. 1996). Antioxidants are proven to block apoptosis through increasing the expression of the antiapoptotic, Bcl-2 (Korsmeyer et al. 1995; Hockenbery 1995). Moreover, inflammation is an important modulator of apoptosis (Takeda et al. 1993). Indomethacin-induced gastric ulcer, was previously demonstrated through its effect on decreasing the expression of the antiapoptotic gene, Bcl-2, and increasing that of the apoptotic gene, Bax (Maity et al. 2009; Neamatallah 2024). Similarly, indomethacin promoted apoptosis in the current study. However, the pretreatment with apigenin significantly reduced gastric mucosal apoptosis by decreasing the transcription of Bax and increasing that of Bcl-2. Apigenin previously showed similar activities against doxorubicin-induced toxicity (Zare et al. 2019), and spinal cord injury (Zhang et al. 2014b).
The TGF-β protein family plays a pivotal role in growth and development, inflammation, and repair including angiogenesis. In vivo, TGF-β was found to enhance wound strength and promote epithelial cell growth (Myoken et al. 1990; Clark and Coker 1998). TGF-β1 is a fibrogenic cytokine, that enhances the extracellular matrix gene expression (Verrecchia et al. 2001). In gastric ulcers, TGF-β1 was found to induce remarkable acceleration of the lesion healing regulating connective tissue formation and angiogenesis, and its levels were found to reach the peak after 24 h of injury then declined during healing (Ernst et al. 1996). In the current study, indomethacin decreased the expression of TGF-β1 in gastric mucosa. This comes in accordance with previous studies that proved the inhibitory effect of indomethacin on TGF-β1 in peptic ulcer (Lempinen et al. 2002), and other conditions like cardiovascular diseases (Guo et al. 2013). Treatment with apigenin in the current study is believed to prevent gastric ulcers by increasing the expression of TGF-β1 in the gastric mucosa of the treated animals. Likewise, apigenin was found to induce collagen synthesis in the skin through activating TGF- β1signaling (Zhang et al. 2015).
It is noteworthy to report that this study suffers several limitations including the use of limited number of animals and a relatively high dose of indomethacin as compared to human diose. Finally, apigenin attenuates the ulcerogenic effects induced by indomethacin in rats. These effects can be attributed to its anti-inflammatory, antioxidant, antiapoptotic and TGF-β1-enhancing activities. These data warrant further experimental and clinical research is required to confirm activity of apigenin as anti-ulcer agent.
Data availability
No datasets were generated or analysed during the current study.
References
Adriana M, Şoimiţa S, Daniela-Rodica M, Camelia A, Doiniţa C, Doina D (2008) Oxidative stress implications in experimental gastric ulcer induced by indomethacin. Bulletin of the University of Agricultural Sciences & Veterinary Medicine Cluj-Napoca. Veterinary Medicine 65(1)
AlKreathy HM, Alghamdi MK, Esmat A (2020) Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm J 28:916–926. https://doi.org/10.1016/j.jsps.2020.06.012
Bajaj G, Sharma RK (2006) TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun 345:1558–1564. https://doi.org/10.1016/j.bbrc.2006.05.059
Bampidis V, Azimonti G, Bastos MD, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F (2020) Safety and efficacy of sodium carboxymethyl cellulose for all animal species. EFSA Journal 18(7):e06211
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences
Bhala N, Emberson J, Merhi A et al (2013) Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet (London England) 382:769–779
Clark DA, Coker R (1998) Molecules in focus transforming growth factor-beta (TGF-β). Int J Biochem Cell Biol 30:293–298. https://doi.org/10.1016/S1357-2725(97)00128-3
Ding SZ, Lam SK, Yuen ST et al (1998) Prostaglandin, tumor necrosis factor alpha and neutrophils: causative relationship in indomethacin-induced stomach injuries. Eur J Pharmacol 348:257–263. https://doi.org/10.1016/s0014-2999(98)00162-9
Ernst H, Konturek PC, Brzozowski T et al (1996) Subserosal application of transforming growth factor-beta 1 in rats with chronic gastric ulcers: effect on gastric ulcer healing and blood flow. J Physiol Pharmacol 47:443–454
Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222. https://doi.org/10.3389/fpls.2012.00222
Freston JW (1988) The pathophysiological and pharmacological basis of peptic ulcer therapy. Toxicol Pathol 16:260–266. https://doi.org/10.1177/019262338801600219
Guo G, Ott C-E, Grünhagen J et al (2013) Indomethacin prevents the progression of thoracic aortic aneurysm in Marfan Syndrome mice. Aorta (Stamford) 1:5–12. https://doi.org/10.12945/j.aorta.2013.13.007
Hockenbery DM (1995) bcl-2, a novel reguator of cell death. BioEssays 17:631–638. https://doi.org/10.1002/bies.950170709
Hostetler GL, Ralston RA, Schwartz SJ (2017) Flavones: food sources, bioavailability, metabolism, and Bioactivity. Adv Nutr 8:423–435. https://doi.org/10.3945/an.116.012948
Hsu H, Shu HB, Pan MG, Goeddel DV (1996) TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299–308. https://doi.org/10.1016/s0092-8674(00)80984-8
Ibrahim R, Allam M, El-Gohary O et al (2018) Protective effect of obestatin on indomethacin-induced acute gastric ulcer in rats: role of VEGF and TNF-α. Benha Med J 35:369
Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26 Suppl 1173–179. https://doi.org/10.1111/j.1440-1746.2010.06592.x
Johnson AC, Greenwood-Van Meerveld B (2017) Critical evaluation of Animal models of Gastrointestinal disorders. Handb Exp Pharmacol 239:289–317. https://doi.org/10.1007/164_2016_120
Karamese M, Erol HS, Albayrak M et al (2016) Anti-oxidant and anti-inflammatory effects of apigenin in a rat model of sepsis: an immunological, biochemical, and histopathological study. Immunopharmacol Immunotoxicol 38:228–237. https://doi.org/10.3109/08923973.2016.1173058
Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM (2019) Diagnosis and treatment of peptic Ulcer Disease. Am J Med 132:447–456. https://doi.org/10.1016/j.amjmed.2018.12.009
Kim YJ, Kim E-H, Hahm KB (2012) Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol 27:1004–1010. https://doi.org/10.1111/j.1440-1746.2012.07108.x
Korsmeyer SJ, Yin XM, Oltvai ZN et al (1995) Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta 1271:63–66. https://doi.org/10.1016/0925-4439(95)00011-r
Lambert AA, Lam JO, Paik JJ et al (2015) Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE 10:e0128004. https://doi.org/10.1371/journal.pone.0128004
Lempinen M, Inkinen K, Wolff H, Ahonen J (2002) Connective tissue growth factor in indomethacin-induced rat gastric ulcer. Eur Surg Res 34:232–238. https://doi.org/10.1159/000063394
Lopez-Jornet P, Camacho-Alonso F, Gómez-Garcia F et al (2014) Effects of potassium apigenin and verbena extract on the wound healing process of SKH-1 mouse skin. Int Wound J 11:489–495. https://doi.org/10.1111/j.1742-481X.2012.01114.x
Maity P, Bindu S, Dey S et al (2009) Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J Pineal Res 46:314–323. https://doi.org/10.1111/j.1600-079X.2009.00663.x
Malfertheiner P, Chan FKL, McColl KEL (2009) Peptic ulcer disease. Lancet 374:1449–1461. https://doi.org/10.1016/S0140-6736(09)60938-7
Matsui H, Shimokawa O, Kaneko T et al (2011) The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 48:107–111. https://doi.org/10.3164/jcbn.10-79
Myoken Y, Kan M, Sato GH et al (1990) Bifunctional effects of transforming growth factor-beta (TGF-beta) on endothelial cell growth correlate with phenotypes of TGF-beta binding sites. Exp Cell Res 191:299–304. https://doi.org/10.1016/0014-4827(90)90018-6
Neamatallah T (2024) Caffeic acid phenethyl ester attenuates indomethacin-induced gastric ulcer in rats. Naunyn Schmiedebergs Arch Pharmacol 397:1791–1801. https://doi.org/10.1007/s00210-023-02730-z
O’Neill LA, Kaltschmidt C (1997) NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20:252–258. https://doi.org/10.1016/s0166-2236(96)01035-1
Park JA, Ha SK, Kang TH et al (2008) Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci 82:1217–1223. https://doi.org/10.1016/j.lfs.2008.03.021
Prayoga DK, Aulifa DL, Budiman A, Levita J (2024) Plants with anti-ulcer activity and mechanism: a review of preclinical and clinical studies. Drug Des Devel Ther 18:193–213. https://doi.org/10.2147/DDDT.S446949
Rašković A, Gigov S, Čapo I et al (2017) Antioxidative and protective actions of apigenin in a Paracetamol-Induced Hepatotoxicity Rat Model. Eur J Drug Metab Pharmacokinet 42:849–856. https://doi.org/10.1007/s13318-017-0407-0
Singh JPV, Selvendiran K, Banu SM et al (2004) Protective role of apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine 11:309–314. https://doi.org/10.1078/0944711041495254
Suleyman H, Albayrak A, Bilici M et al (2010) Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 33:224–234. https://doi.org/10.1007/s10753-009-9176-5
Sverdén E, Agréus L, Dunn JM, Lagergren J (2019) Peptic ulcer disease. BMJ 367:l5495. https://doi.org/10.1136/bmj.l5495
Takeda Y, Watanabe H, Yonehara S et al (1993) Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-alpha. Int Immunol 5:691–694. https://doi.org/10.1093/intimm/5.6.691
Tandon R, Khanna HD, Dorababu M, Goel RK (2004) Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J Physiol Pharmacol 48:115–118
Uc A, Zhu X, Wagner BA et al (2012) Heme oxygenase-1 is protective against nonsteroidal anti-inflammatory drug-induced gastric ulcers. J Pediatr Gastroenterol Nutr 54:471–476. https://doi.org/10.1097/MPG.0b013e3182334fdf
Verrecchia F, Chu ML, Mauviel A (2001) Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 276:17058–17062. https://doi.org/10.1074/jbc.M100754200
Wang J, Liu Y-T, Xiao L et al (2014) Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37:2085–2090. https://doi.org/10.1007/s10753-014-9942-x
Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313(Pt 1):17–29. https://doi.org/10.1042/bj3130017
Yadav RK, Mehan S, Sahu R et al (2022) Protective effects of apigenin on methylmercury-induced behavioral/neurochemical abnormalities and neurotoxicity in rats. Hum Exp Toxicol 41:9603271221084276. https://doi.org/10.1177/09603271221084276
Yoshikawa T, Naito Y (2000) The role of neutrophils and inflammation in gastric mucosal injury. Free Radic Res 33:785–794. https://doi.org/10.1080/10715760000301301
Zamzami N, Marchetti P, Castedo M et al (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377. https://doi.org/10.1084/jem.182.2.367
Zare MFR, Rakhshan K, Aboutaleb N et al (2019) Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci 232:116623. https://doi.org/10.1016/j.lfs.2019.116623
Zhang B-B, Li Y, Liu X-Q et al (2014a) Association between vacA genotypes and the risk of duodenal ulcer: a meta-analysis. Mol Biol Rep 41:7241–7254. https://doi.org/10.1007/s11033-014-3610-y
Zhang F, Li F, Chen G (2014b) Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol Sci 35:583–588. https://doi.org/10.1007/s10072-013-1566-7
Zhang Y, Wang J, Cheng X et al (2015) Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. Eur J Histochem 59:2467. https://doi.org/10.4081/ejh.2015.2467
Acknowledgements
Special thanks and great appreciation to Prof. Ashraf Abdel-Naim and Dr. Gamal Abd El-Aziz, King Abdulaziz University, for their assistance in the animal handling procedures and histopathological studies.
Funding
No specific fund.
Author information
Authors and Affiliations
Contributions
Zaenah Zuhair Alamri is a single author. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• In this study, indomethacin was used to induce gastric ulcers in rats.
• Apigenin protected against macroscopic and gastric microscopic pathological changes.
• Apigenin showed anti-inflammatory, anti-antioxidant, and anti-apoptotic activities.
• Apigenin enhanced the expression of TGF-β1.
• Apigenin possesses a promising potential as an anti-ulcer agent.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alamri, Z.Z. Apigenin attenuates indomethacin-induced gastric ulcer in rats: emphasis on antioxidant, anti-inflammatory, anti-apoptotic, and TGF-β1 enhancing activities. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03200-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03200-w