Abstract
Hyperhomocysteinemia is a well-known cause of cognitive impairment and neurodegeneration. Increased oxidative stress in the brain has a major possible role in hyperhomocysteinemia-induced pathogenesis. Edaravone is a potent free radical scavenger that has a neuroprotective effect against memory impairment in several experimental models. The current study investigated the possible protective effect of edaravone in L-methionine-induced vascular dementia in a rat model. L-methionine was given (1.7 mg/kg/day) through oral gavage, while edaravone was given (6 mg/kg/day) intraperitoneally. The administration of methionine and edaravone started concomitantly and continued for a total of 9 weeks. Spatial learning and memory were assessed using the radial arm water maze (RAWM). Changes in the oxidative stress-related biomarkers in the hippocampus were assessed using enzymatic assays. Chronic L-methionine administration resulted in short-term and long-term memory impairment, whereas edaravone prevented such effect. Furthermore, edaravone ameliorated L-methionine induced decrease in the activity of the antioxidant enzymes catalase and glutathione peroxidase as well as the ratio of reduced glutathione to oxidized glutathione (GSH/GSSG ratio). Edaravone also prevented increase in the oxidized glutathione (GSSG) secondary to chronic L-methionine administration. In conclusion, the current study suggests that memory impairment and oxidative stress secondary to chronic L-methionine administration can be prevented by edaravone, probably via enhancing antioxidant mechanisms in the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
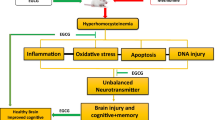
Methionine (Meth) is an essential amino acid; it has to be supplied from food as it cannot be synthesized endogenously (Miller 2003). Meat, fish, poultry, and cheese are among the major sources of methionine (Górska-Warsewicz et al. 2018). A major by-product of methionine metabolism is the amino acid homocysteine (Hcy) which is derived from Meth demethylation (Miller 2003). Increased homocysteine blood level named as hyperhomocysteinemia (HHcy) is a well-established independent risk factor for cardiovascular, cerebrovascular, as well as neurodegenerative diseases including vascular dementia (Koladiya et al. 2009; Pandey and Pradhan 2010). The mechanisms of homocysteine neurotoxicity are diverse, but it is thought to primarily occur via increasing oxidative stress in the brain along with reducing the bioavailability of nitric oxide, leading to endothelial dysfunction, reducing cerebral blood supply and eventually memory decline (Alzoubi et al. 2014; El-Dessouki et al. 2017).
Edaravone, 3-methyl-1-phenyl-2-pyrazolin-5-one (Li et al. 2017), is a potent free radical scavenger (Watanabe et al. 2018). It has been widely used since 2001 for acute ischemic stroke and was approved for amyotrophic lateral sclerosis (ALS) (Watanabe et al. 2018). Edaravone protects the vascular system and the neuronal cells from being damaged through its multi-target pharmacology (Lapchak 2010). Edaravone has multiple mechanisms of actions with the anti-oxidizing mechanism as the most prominent one (Kikuchi et al. 2013; Watanabe et al. 2018). Edaravone was shown to be effective in different neurologic diseases including but not limited to Parkinson’s disease, subarachnoid hemorrhage, and traumatic brain injury (Kikuchi et al. 2011). Recently, a wave of research showed a possible protective effect of edaravone on memory and learning. It has been shown that edaravone has the potential to rescue memory impairment induced by several experimental models such as Alzheimer’s disease (Jiao et al. 2015), post-traumatic stress disorder (Alzoubi et al. 2019b), and cerebral hypoperfusion-induced memory impairment (Li et al. 2017). Giving the aforementioned data above about the protective effect of edaravone against memory impairment induced by different experimental models, this study was conducted in a rat model to test the ability of edaravone to protect against memory impairment induced by chronic L-methionine administration.
Methods
Subjects
Adult male Wistar rats (n = 60), weighing between 200 and 220 g, were provided from the animal house of Jordan University of Science and Technology. The animals were group-housed (five rats/cage) in metallic cages with free access to water and standard chow diet. Wood shaving was provided as bedding and was changed three times weekly. Throughout all the experiment, rats were weighed twice weekly. Optimum hygienic conditions and pathogen-free environment were kept. Temperature was maintained at 23 ± 2 °C with 12-h light/dark cycle. The light cycle started at 7 a.m. All the procedures were performed during the light cycle. Maximum efforts were done to minimize the number of animals used and minimize their suffering. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC; approval number: IACUC-522/2018, 25-11-2018).
The rats were allowed for 1-week acclimatization before manipulations and before starting the experiments. Rats were numbered (on their tails) and randomly assigned into four groups (15 rats/group) as following: the first group (control) received carboxymethyl cellulose (CMC) 0.5% by oral gavage and received dimethyl sulfoxide (DMSO)/distilled water intraperitoneally (IP); the second group (L-methionine only group/L-Meth) received L-methionine (1.7 g/kg/day) suspended in 0.5% CMC by oral gavage and received DMSO with distilled water IP; the third group (the edaravone group) in which rats received edaravone IP in a dose of 6 mg/kg/day along with 0.5% CMC orally; and the last group, L-methionine and edaravone group (L-Meth/edaravone), which received L-methionine 1.7 g/kg/day orally and 6 mg/kg/day of edaravone IP. L-methionine and edaravone administration was started from the same day and continued concomitantly for a total of 9 weeks. Both L-methionine and edaravone were obtained from Sigma-Aldrich (St Louis, Michigan, USA). L-methionine dose was based on previous studies (Alzoubi et al. 2014; El-Dessouki et al. 2017; Hemanth Kumar et al. 2017; Koladiya et al. 2008). Edaravone regimen was also based on previous work that showed beneficial effect of this dose (Alzoubi et al. 2019b; Zhang et al. 2018). Edaravone and L-methionine administration was continued throughout the behavioral test (the radial arm water maze test).
The radial arm water maze
To test spatial learning and memory, all rats from the four groups were involved in the radial arm water maze (RAWM) test as previously described (Alzoubi et al. 2018; Alzoubi et al. 2012). This test was started at the beginning of the ninth week. Briefly, the RAWM is a black circular stainless steel pool, consists of six (V-shape) labeled and equally sized arms creating six swimming paths. The steel pool was filled with water (temperature was maintained at 22 ± 1 °C), which was changed daily with tap water. A hidden platform was submerged (in the goal arm) under water by around 2 cm, which allowed the animals to escape from water. The hidden platform’s location was changed by the experimenter for different rats but kept at the same arm (the target arm) for each rat in both the learning and the memory tests. One day before starting the maze test, the rats were allowed to learn how to swim and to acclimatize the swimming pool. The experiment was performed in a dimly lit room (acclimatization was done with full light) with two pictures on fixed locations on the wall to serve as visual cues to help the rats. First, each rat was tested for learning phase (acquisition phase) by conducting 12 trials of learning (two sessions, each session consisted of 6 trials with 5 min rest between the 2 sessions). For a particular rat, each trial was started in a different arm with the exception of the target arm. The rats first were allowed to swim freely for 1 min in order to find the hidden platform; if failed to reach the target, rats were gently guided by the experimenter and allowed to stay on the platform for 15 s before the next trial took place. A short-term memory test was performed 30 min after ending the learning phase, whereas the long-term memory tests were performed 5 h and 24 h of ending the learning phase. Each time the rat entered incorrect arm, an error was counted. Correct entry was considered if the rat entered all its body, including the tail, in the correct arm and reached to the hidden platform.
Animals decapitation and hippocampus dissection
At the end of the experiment, animals were killed by decapitation (without anesthesia). Trunk blood samples were collected from the decapitation site. Blood samples were, then, centrifuged and stored at −20 °C for later analysis. The brains were removed out of the skulls and placed immediately on filter paper filled with normal saline. The hippocampus was taken from the brain (two hippocampi) and stored in pre-labeled Eppendorf tubes, set immediately in liquid nitrogen, and then transferred to −80 °C freezer until time of tissue processing and analysis.
Molecular assays
Stored hippocampus tissues were homogenized manually using small plastic pestles and homogenization buffer (Sigma-Aldrich CO., Saint Louis, MO). The homogenized buffer was prepared by reconstitution of one tablet of phosphate-buffered saline (Sigma Chemical CO., Saint Louis, MO) with two protease inhibitor tablets (Sigma Chemical) in 200 ml of distilled water (Alzoubi et al. 2013; Alzoubi et al. 2016). After that, the homogenized hippocampal samples were centrifuged for 15 min at 1000 x g at 4 °C. After centrifugation, the supernatant was collected and stored at −80 °C for later analysis of oxidative stress biomarkers. Total protein concentration obtained from the supernatant was estimated using a commercially available kit (Bio-Rad, Hercules, CA, USA).
Glutathione (total glutathione) level was measured based on its available assay kit (Sigma-Aldrich, MI, USA). First of all, the samples were deproteinized using 5% of 5-sulfosalicylic acid (SSA) followed by centrifugation at 1000x for 10 min at 4 °C in order to remove the precipitated protein. Then, total glutathione was assayed spectrophotometrically as instructed in its kit sheet. To measure GSSG, 10 μl of 2-vinylpyridine (Glutathione Assay Kit, Sigma- Aldrich CO., MI, USA) was added to 1 ml of the supernatant of the homogenized samples; then the same steps mentioned above for total glutathione measurement were used for GSSG level. Measuring reduced glutathione was then obtained from substracting the oxidized glutathione from the total glutathione level. The activity of glutathione peroxidase (GPx) was measured using the cellular activity assay kit (CGPI, Sigma-Aldrich, MI, USA). Catalase activity was measured using the commercially available kit as indicated by the manufacturer’s instructions (Cayman Chemical Co., Ann Arbor, MI, USA). For each assay kit, the wavelengths specified in the kit sheet were followed to read the microplates using an Epoch Microplate Spectrophotometer at specified wavelength in each of the after-mentioned kits (Bio-tek instruments, Highland Park, Winooski, USA).
Serum homocysteine level was measured using the commercially available rat homocysteine kit (catalog # MBS 703069) (MyBioSource, California, USA). The measurement was done according to the manufacturer’s instructions.
Statistical analysis
The number of errors was compared via two-way ANOVA followed by posttest for multiple comparisons. The repeated measures factor was time and interaction were independent group dimensions. For the biochemical assays, one-way ANOVA plus Tukey’s posttest was used to achieve comparisons. The statistical software used was GraphPad Prism version 4.0. Significance was set at P < 0.05. Values all over the study were reported as mean ± SEM.
Results
Effect of L-methionine and/or edaravone on serum homocysteine level
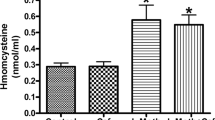
The administration of L-methionine resulted in significant increase in serum Hcy level in both L-methionine and L-methionine/edaravone groups compared to the control and edaravone groups (F(3, 56) = 5.54, P < 0.05). Edaravone administration did not normalize the elevated homocysteine level (Fig. 1).
Effect of L-meth and edaravone on serum homocysteine level. Comparison of control (control), L-meth, edaravone and L-meth/edaravone group. L-meth administration resulted in significant increase in serum homocysteine level in both L-meth and in L-meth/edaravone groups. *Indicates significant difference from the control and edaravone groups (P < 0.05). Data are expressed as mean ± SEM (n = 15/ group). L-meth: L-methionine; Hcy: homocysteine
The effect of L-methionine and/or edaravone on learning and memory
At the beginning of the learning phase, rats from all the experimental groups made high number of errors. With time, the number of errors decreased gradually with training in all groups. During this phase, no statistically significant difference was observed among the four groups (Fig. 2). As learning continued, a gradual decrease in errors made by animals was observed, with no significant interaction among treatment groups, suggesting that neither chronic L-methionine administration nor edaravone influenced learning.
Animals’ performance in the RAWM during the acquisition phase (learning phase). Comparison of control, L-meth, edaravone, and L-meth/edaravone groups. Each animal was trained for six consecutive trials, resting for 5 min, then additional six consecutive trials. No significant difference was observed among the treatment groups. Values are represented as mean ± SEM. (n = 15/group). RAWM: radial arm water maze; L-meth: L-methionine
In memory tests, both short-term and long-term memory tests (at 5 and 24 h), there was a significant interaction between L-methionine and edaravone, as the number of errors committed by the L-methionine group was significantly higher compared to those committed by the control, edaravone, and L-methionine/edaravone groups. Thus, the presence of these two factors together causes significant interaction in all memory tests (short-term memory test: F(3, 54) = 4.51, P < 0.05, Fig. 3A, long-term 5-h memory test: F(3, 56) = 6.14, P < 0.05, Fig. 3B, long-term 24-h memory test: F(3, 53) = 7.57, P < 0.05, Fig. 3C). In conclusion, L-methionine administration resulted in increased the number of errors in both the short-term and the long-term memory tests, whereas edaravone prevented these impairments in memory.
Effect of edaravone and L-meth on memory function in the RAWM. Number of errors in (A) short-term memory (30 min) and long-term memory test after (B) 5 h and (C) after 24 h of ending the learning phase. L-meth administration induced both short-term and long-term memory impairment. On the other hand, edaravone prevented such effect. *Indicates significant difference compared with control, edaravone, and L-meth/ edaravone groups (P < 0.05). Data are expressed as mean ± SEM (n = 15/ group). RAWM: Radial arm water maze; L-meth: L-methionine
The effect of L-methionine and/or edaravone on the levels of oxidative stress biomarkers in the hippocampus
Given the findings that L-methionine administration resulted in both short-term and long-term memory impairment, the next step was to determine whether this impairment was secondary to increase oxidative stress in the hippocampus. First, the level of reduced and oxidized glutathione was measured (GSH and GSSG, respectively). Although GSH level was not changed among the different experimental groups (F(3, 52) = 0.68, P > 0.05), GSSG level was significantly higher in L-methionine group compared to all other groups (F(3, 51) = 6.04, P < 0.05). Moreover, the GSH/GSSG ratio was significantly lower in the L-methionine group compared to the control, edaravone, and L-methionine/edaravone groups (F(3, 53) = 7.57, P < 0.05). No significant difference was observed in GSSG or GSH/GSSG ratio between the control, edaravone, and L-methionine/edaravone groups, suggesting a possible protective effect of edaravone in reducing/preventing the oxidation of GSH to GSSG (Fig. 4).
Effect of L-meth and edaravone on (A) GSH level, (B) GSSG level, and (C) GSH/GSSG ratio. *Significant difference compared to control, edaravone, and L-Meth/Edaravone groups (P < 0.05). Values are represented as mean ± SEM (n = 15/ group). GSH, reduced glutathione; GSSG, oxidized glutathione; L-meth, L-methionine
In further confirming the potential role of increased oxidative stress in L-methionine-induced memory impairment, the activity of the antioxidant enzymes (GPx and catalase) was measured. The activity of these two enzymes was significantly lower in L-methionine group compared with all the other groups (Gpx: F(3, 50) = 6.04, P < 0.05, catalase: (F(3, 50) = 6.72, P < 0.05). On the contrary, no significant difference was observed in their levels in the control, edaravone, and L-methionine/edaravone groups (Fig. 5).
Effect of L-meth and edaravone on (A) GPx activity, (B) catalase activity. Chronic L-methionine administration resulted in significant reduction in the activity of these antioxidants, whereas edaravone prevented these changes in the L-meth/edaravone group.*Indicates significant difference compared with compared with control, edaravone, and L-meth/edaravone groups (P < 0.05). Each point is the mean ± SEM (n = 15/ group). GPx: glutathione peroxidase; L-meth: L-methionine
Discussion
The current study showed that edaravone prevented memory impairment in chronic L-methionine-induced hyperhomocysteinemia, suggesting an interaction between L-methionine and edaravone. Chronic L-methionine administration has been shown to induce memory impairment in several animal studies (Alzoubi et al. 2014; El-Dessouki et al. 2017; Hemanth Kumar et al. 2017; Koladiya et al. 2009). This impairment in memory has been explained via increasing the production of Hcy as a by-product of methionine metabolism (Baydas et al. 2005; El-Dessouki et al. 2017; Miller 2003). The Hcy is a well-known independent risk factor for cognitive impairment including dementia (Ansari et al. 2014; Garcia and Zanibbi 2004; McVeigh and Passmore 2006). Increased oxidative stress in the brain is the most possible mechanism through which Hcy causes neuronal damage, leading to memory impairment (Moretti and Caruso 2019; Obeid and Herrmann 2006; Price et al. 2018). The findings of the present study are in accordance, as significant increase in serum Hcy level was observed in L-methionine-treated groups compared to non-L-methionine groups, with no significant effect of edaravone on Hcy level. Furthermore, L-methionine administration resulted in memory impairment that was manifested by increasing the number of errors committed in both the short-term and the long-term memory tests by the L-methionine group compared to other groups. On the contrary, edaravone was able to prevent these impairments.
Several previous studies have shown that edaravone protects against memory impairment induced by multiple disease models including Alzheimer’s disease (Jiao et al. 2015), traumatic brain injury (TBI) (Wang et al. 2011), intraventricular hemorrhage (Chen et al. 2014), and surgery-induced memory impairment (Tian et al. 2017). The results of these studies are in accordance with the findings of this study where edaravone was able to prevent memory impairment secondary to chronic L-methionine administration.
The mechanisms through which edaravone exerts its neuroprotective effect against memory impairment are diverse but primarily involve attenuating increased oxidative stress in the hippocampus (Ahmad et al. 2012; Banno et al. 2005; Higashi 2009; Nagase et al. 2016; Saini et al. 2007). Edaravone has a potent antioxidant and free radical scavenging effect (Watanabe et al. 2018).
Increased oxidative stress in the hippocampus secondary to Hcy has been linked to the pathophysiology of hyperhomocysteinemia (Alzoubi et al. 2014; Cervellati et al. 2014; Hainsworth et al. 2016). The findings of the current study support the above contention, as significant decrease in the antioxidants levels/activities (GPx, catalase, and GSH/GSSG ratio) along with increased oxidative stress biomarker (GSSG) in the hippocampus has been detected in L-methionine-treated group, hence reflecting oxidative stress-induced memory impairment.
In the present study, edaravone significantly ameliorated chronic L-methionine-induced oxidative damage, which was manifested by normalizing the reduction in the antioxidant activities (catalase, GPx), improving the GSH/GSSG ratio and reducing GSSG. Current findings are consistent with previous studies, which revealed reducing oxidative stress-related memory impairment with edaravone in several conditions including PTSD (Alzoubi et al. 2019b), neonatal hypoxic encephalopathy (Noor et al. 2005), and Alzheimer’s disease (He et al. 2014). As these studies showed protective effect of edaravone on memory impairment induced by oxidative stress, these findings can be extended to the current study where edaravone prevented memory impairment induced by chronic L-methionine administration, possibly, via its antioxidant/ free radical scavenging effect.
It was previously shown that one of the prominent pharmacological properties of Edaravone is its strong antioxidant properties (Alzoubi et al. 2019a; Alzoubi et al. 2019b). On the other hand, L-methionine was shown to induce memory impairment that is related, among multiple other mechanisms, to oxidative stress (Alzoubi et al. 2014). The current study has shown that edaravone protects against L-methionine-induced memory impairment, which was correlated with restored oxidative stress status in the hippocampus. This is a novel fact that was not known before carrying out the current study.
In conclusion, edaravone prevents against memory impairment induced by chronic L-methionine administration, probably, through its ability to normalize oxidative stress in the hippocampus.
References
Ahmad A et al (2012) Edaravone ameliorates oxidative stress associated cholinergic dysfunction and limits apoptotic response following focal cerebral ischemia in rat. Mol Cell Biochem 367:215–225
Alzoubi KH, Khabour OF, Rashid BA, Damaj IM, Salah HA (2012) The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res 226:205–210
Alzoubi KH, Khabour OF, Tashtoush NH, Al-Azzam SI, Mhaidat NM (2013) Evaluation of the effect of pentoxifylline on sleep-deprivation induced memory impairment. Hippocampus 23:812–819. https://doi.org/10.1002/hipo.22135
Alzoubi H, Khabour F, Al-azzam SI, Tashtoush MH, Mhaidat NM (2014) Metformin eased cognitive impairment induced by chronic L-methionine administration: potential role of oxidative stress. Curr Neuropharmacol 12:186–192
Alzoubi KH, Mayyas FA, Khabour OF, Bani Salama FM, Alhashimi FH, Mhaidat NM (2016) Chronic Melatonin Treatment Prevents Memory Impairment Induced by Chronic Sleep Deprivation. Mol Neurobiol 53:3439–3447. https://doi.org/10.1007/s12035-015-9286-z
Alzoubi KH, Khabour OF, Ahmed M (2018) Pentoxifylline prevents post-traumatic stress disorder induced memory impairment. Brain Res Bull 139:263–268. https://doi.org/10.1016/j.brainresbull.2018.03.009
Alzoubi KH, Al Mosabih HS, Mahasneh AF (2019a) The protective effect of edaravone on memory impairment induced by chronic sleep deprivation. Psychiatry Res 281:112577. https://doi.org/10.1016/j.psychres.2019.112577
Alzoubi KH, Shatnawi A, Al-Qudah MA, Alfaqih MA (2019b) Edaravone prevents memory impairment in an animal model of post-traumatic distress. Behav Pharmacol 30:201–207. https://doi.org/10.1097/FBP.0000000000000479
Ansari R, Mahta A, Mallack E, Luo JJ (2014) Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol 10:281–288. https://doi.org/10.3988/jcn.2014.10.4.281
Banno M et al (2005) The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology 48:283–290
Baydas G, Ozer M, Yasar A, Tuzcu M, Koz ST (2005) Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res 1046:187–194. https://doi.org/10.1016/j.brainres.2005.04.011
Cervellati C et al (2014) Oxidative balance, homocysteine, and uric acid levels in older patients with late onset Alzheimer's disease or vascular dementia. J Neurol Sci 337:156–161. https://doi.org/10.1016/j.jns.2013.11.041
Chen Z, Zhang J, Chen Q, Guo J, Zhu G, Feng H (2014) Neuroprotective effects of edaravone after intraventricular hemorrhage in rats. Neuroreport 25:635–640. https://doi.org/10.1097/wnr.0000000000000050
El-Dessouki AM, Galal MA, Awad AS, Zaki HF (2017) Neuroprotective Effects of Simvastatin and Cilostazol in L-Methionine-Induced Vascular Dementia in Rats. Mol Neurobiol 54:5074–5084. https://doi.org/10.1007/s12035-016-0051-8
Garcia A, Zanibbi K (2004) Homocysteine and cognitive function in elderly people. Cmaj 171:897–904
Górska-Warsewicz H, Laskowski W, Kulykovets O, Kudlińska-Chylak A, Czeczotko M, Rejman K (2018) Food Products as Sources of Protein and Amino Acids-The Case of Poland. Nutrients 10:1977. https://doi.org/10.3390/nu10121977
Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM (2016) Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta (BBA) - Mol Basis Dis 1862:1008–1017. https://doi.org/10.1016/j.bbadis.2015.11.015
He F, Cao YP, Che FY, Yang LH, Xiao SH, Liu J (2014) Inhibitory effects of edaravone in beta-amyloid-induced neurotoxicity in rats. Biomed Res Int 2014:370368. https://doi.org/10.1155/2014/370368
Hemanth Kumar B, Dinesh Kumar B, Diwan PV (2017) Hesperidin, a citrus flavonoid, protects against l-methionine-induced hyperhomocysteinemia by abrogation of oxidative stress, endothelial dysfunction and neurotoxicity in Wistar rats. Pharm Biol 55:146–155
Higashi Y (2009) Edaravone for the treatment of acute cerebral infarction: role of endothelium-derived nitric oxide and oxidative stress. Expert Opin Pharmacother 10:323–331. https://doi.org/10.1517/14656560802636888
Jiao SS et al (2015) Edaravone alleviates Alzheimer's disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A 112:5225–5230. https://doi.org/10.1073/pnas.1422998112
Kikuchi K et al (2011) Potential of edaravone for neuroprotection in neurologic diseases that do not involve cerebral infarction. Exp Ther Med 2:771–775. https://doi.org/10.3892/etm.2011.281
Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, Tanaka E (2013) The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci 14:13909–13930. https://doi.org/10.3390/ijms140713909
Koladiya RU, Jaggi AS, Singh N, Sharma BK (2008) Ameliorative role of Atorvastatin and Pitavastatin in L-Methionine induced vascular dementia in rats. BMC Pharmacol 8:14
Koladiya RU, Jaggi AS, Singh N, Sharma BK (2009) Beneficial effects of donepezil on vascular endothelial dysfunction-associated dementia induced by L-methionine in rats. J Health Sci 55:215–225
Lapchak PA (2010) A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother 11:1753–1763. https://doi.org/10.1517/14656566.2010.493558
Li X et al (2017) Edaravone injection reverses learning and memory deficits in a rat model of vascular dementia. Acta Biochim Biophys Sin 49:83–89. https://doi.org/10.1093/abbs/gmw116
McVeigh C, Passmore P (2006) Vascular dementia: prevention and treatment. Clin Interv Aging 1:229–235
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8:7–19
Moretti R, Caruso P (2019) The controversial role of Homocysteine in neurology: from labs to clinical practice Int J Mol Sci 20 doi:https://doi.org/10.3390/ijms20010231
Nagase M, Yamamoto Y, Miyazaki Y, Yoshino H (2016) Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep 21:104–112
Noor JI et al (2005) Short-term administration of a new free radical scavenger, edaravone, is more effective than its long-term administration for the treatment of neonatal hypoxic-ischemic encephalopathy. Stroke 36:2468–2474. https://doi.org/10.1161/01.STR.0000185653.49740.c6
Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580:2994–3005. https://doi.org/10.1016/j.febslet.2006.04.088
Pandey P, Pradhan S (2010) Homocysteine: A possible modifiable risk factor in vascular dementia. Ann Neurosci 13:12–17
Price BR, Wilcock DM, Weekman EM (2018) Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Front Aging Neurosci 10:350. https://doi.org/10.3389/fnagi.2018.00350
Saini AK, Hs AK, Sharma SS (2007) Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur J Pharmacol 568:164–172
Tian A, Ma H, Zhang R, Cui Y, Wan C (2017) Edaravone improves spatial memory and modulates endoplasmic reticulum stress-mediated apoptosis after abdominal surgery in mice. Exp Ther Med 14:355–360. https://doi.org/10.3892/etm.2017.4489
Wang G-H et al (2011) Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma 28:2123–2134. https://doi.org/10.1089/neu.2011.1939
Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y (2018) How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr 62:20–38. https://doi.org/10.3164/jcbn.17-62
Zhang D, Xiao Y, Lv P, Teng Z, Dong Y, Qi Q, Liu Z (2018) Edaravone attenuates oxidative stress induced by chronic cerebral hypoperfusion injury: role of ERK/Nrf2/HO-1 signaling pathway. Neurol Res 40:1–10. https://doi.org/10.1080/01616412.2017.1376457
Funding
Deanship of Research/Jordan University of Science and Technology, grant number 522/2018.
Author information
Authors and Affiliations
Contributions
KA and FM conceived and designed research. ZA conducted experiments. KA and FM contributed new reagents, analytical tools, and in supervising the experimental procedure. KA and ZA analyzed data. KA, ZA, and FM participated in interpreting study results and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experimental protocol was approved by the animal care and use committee (ACUC) of the Jordan University of Science and Technology (approval number: IACUC-522/2018, 25-11-2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alzoubi, K.H., Aburashed, Z.O. & Mayyas, F. Edaravone protects from memory impairment induced by chronic L-methionine administration. Naunyn-Schmiedeberg's Arch Pharmacol 393, 1221–1228 (2020). https://doi.org/10.1007/s00210-020-01827-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01827-z