Abstract
Vascular dementia (VaD) is a degenerative cerebrovascular disorder that leads to progressive decline in cognitive abilities and memory. Several reports demonstrated that oxidative stress and endothelial dysfunction are principal pathogenic factors in VaD. The present study was constructed to determine the possible neuroprotective effects of simvastatin in comparison with cilostazol in VaD induced by l-methionine in rats. Male Wistar rats were divided into four groups. Group I (control group), group II received l-methionine (1.7 g/kg, p.o.) for 32 days. The remaining two groups received simvastatin (50 mg/kg, p.o.) and cilostazol (100 mg/kg, p.o.), respectively, for 32 days after induction of VaD by l-methionine. Subsequently, rats were tested for cognitive performance using Morris water maze test then sacrificed for biochemical and histopathological assays. l-methionine induced VaD reflected by alterations in rats’ behavior as well as the estimated neurotransmitters, acetylcholinesterase activity as well as increased brain oxidative stress and inflammation parallel to histopathological changes in brain tissue. Treatment of rats with simvastatin ameliorated l-methionine-induced behavioral, neurochemical, and histological changes in a manner comparable to cilostazol. Simvastatin may be regarded as a potential therapeutic strategy for the treatment of VaD. To the best of our knowledge, this is the first study to reveal the neuroprotective effects of simvastatin or cilostazol in l-methionine-induced VaD.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular dementia (VaD) is a degenerative illness caused by different vascular lesions that restrict blood supply to different brain regions [1]. It is usually associated with cognitive dysfunctions as well as impairments of memory and executive function [2]. Persistent reduction in cerebral blood flow induces hypoxia/ischemia of the brain tissue and deprivation of oxygen and nutrients, and can contribute to cell death [3].
It has been suggested that oxidative stress might play a major role in the pathogenesis of VaD [4]. Certainly, free radicals, including reactive oxygen species (ROS), can react with substrates essential for the survival of neurons such as proteins, lipids, and nucleic acid, leading to neuropathological lesions and brain damage [5]. Moreover, the brain is highly sensitive to oxidative damage, mostly because of its high level of polyunsaturated fatty acid, its high oxygen requirement for metabolic processes, in addition to its low concentration of antioxidant defenses [6].
Homocysteine (Hcy) is a non-essential sulfur-containing amino acid that is derived from methionine metabolism [7]. Induction of neurological dysfunction via oxidative stress has been shown by Hcy. This effect can be elucidated by enhancing the production of ROS and oxidative deactivation of nitric oxide (NO) [8]. Hyperhomocysteinemia is coupled with increased asymmetric dimethylarginine (ADMA) plasma concentrations, a potent endogenous inhibitor of NO synthase [9]. Furthermore, Hcy can be toxic to neurons and can increase their damage by amyloid beta (Aβ), as the accumulation of intracellular and extracellular Aβ 42 in neuronal cells results from homocysteic acid [10].
Simvastatin inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme in the cascade of cellular cholesterol biosynthesis [11]. Statins, in addition to their cholesterol-lowering action, are known to possess many cholesterol-independent actions including favorable effect on vascular endothelium [12]. Simvastatin has antioxidant effect by direct scavenging of free radical molecules, independently of their effects on lipid metabolism [13]. Simvastatin was shown to reduce the expression of inflammatory cytokines and interfere with leukocyte recruitment and migration to the CNS [14]. Additionally, it was associated with a pronounced reduction in the incidence of dementia in Parkinsonism disease, with the improvement of behavioral outcomes and decrease of the cognitive deficits [15].
Cilostazol, a potent inhibitor of type III phosphodiesterase (PDE3), has been shown to increase cellular levels of cAMP, and to thereby inhibit platelet aggregation [16]. Moreover, it has been approved as a therapeutic agent for intermittent claudication [17]. Cilostazol increases cerebral blood flow and decreases the size of cerebral infarcted area through edema reduction [18]. It also protects against brain white matter damage and improves neurologic deterioration, spatial memory, and learning [19]. Furthermore, cilostazol has been shown to inhibit lipid peroxidation and apoptosis in a model of cerebral ischemia [20].
The current study aimed to explore the possible neuroprotective effects of simvastatin in comparison with cilostazol in VaD induced in rats by l-methionine. To the best of our knowledge, this is the first study to explore the protective roles of simvastatin or cilostazol in VaD. The study design included understanding the mechanisms underlying the pathophysiology of the disease and the actions of simvastatin and cilostazol.
Material and Methods
Animals
Adult male Wistar rats, weighing 150–200 g each, were used in the present study. Animals were allocated in groups and were allowed to accommodate for 1 week in the animal house at the Faculty of Pharmacy, Ahram Canadian University, before subjecting them to experimentation. They were provided with a standard diet and water. The animals were kept at a temperature of 22 ± 3 °C and a 12-h light/dark cycle, as well as a constant relative humidity. The study was conducted in accordance to the Ethics Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University, Egypt, PT (1048).
Drugs and Chemicals
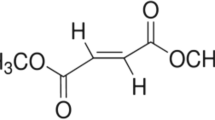
l-methionine was purchased from Cornell Lab (Cairo, Egypt). It was dissolved in saline and administered orally in a dose of 1.7 g/kg [21]. Simvastatin and cilostazol were purchased from Hikma Company (Cairo, Egypt) and Al Debeiky Pharma (Cairo, Egypt), respectively. They were suspended in 1 % tween 80 and administered orally in doses of 50 [22] and 100 mg/kg [23], respectively. Kits for acetylcholinesterase (AChE) enzyme, endothelial nitric oxide synthase (eNOS) enzyme, β-amyloid protein (Aβ 42), acetylcholine (ACh), and catecholamines were purchased from Kamiya Biomedical Company (USA), LifeSpan BioSciences Inc. (USA), Immuno-Biological Laboratories Inc.(USA), USCN Life Science Inc. (China), and Mybiosource Company (USA), respectively. Kits for total cholesterol and reduced glutathione (GSH) were purchased from Biodiagnostic (Egypt). Finally, kits for malondialdehyde (MDA), interleukin-6 (IL-6), and interleukin-10 (IL-10) were purchased from Mybiosource Company (USA), RayBiotech Inc. (USA), and Immuno-Biological Laboratories Inc. (USA), respectively.
Experimental Design
The animals were randomly divided into four experimental groups (ten rats each). Group I served as control group, group II received l-methionine (1.7 g/kg, p.o.) for 32 days. The remaining two groups received simvastatin (50 mg/kg, p.o.) and cilostazol (100 mg/kg, p.o.), respectively, for 32 days after induction of VaD by l-methionine. Memory and cognitive performance of the animals were assessed by Morris water maze (MWM) test on the last 5 days of the experiment. Immediately after performing the behavioral test, the rats were sacrificed by decapitation; the brains were carefully isolated and chilled, and one of the hemispheres was homogenized in ice-cold 50 mM phosphate buffer (pH 7.4) for the estimation of dementia-related parameters such as AChE activity, eNOS, ACh, and Aβ 42, in addition to oxidative stress parameters such as MDA and GSH. Furthermore, total cholesterol, IL-6, and IL-10 were also assessed. The other hemisphere was homogenized in ice cold-acidified butanol (0.85 ml HCl per 1 l n-butanol) for the determination of brain neurotransmitters (NA, DA, and 5-HT). Finally, the brains of 2–3 rats from each group were preserved in 10 % formalin and kept for histological examination of cortical and hippocampal tissues.
Morris Water Maze
Morris water maze test was employed to assess learning and memory of rats [24]. Morris water maze apparatus consists of a large circular pool (150 cm in diameter, 45 cm in height), filled to a depth of 30 cm with water at 28 ± 1 °C. The water was made opaque with white-colored dye. The tank was divided into four equal quadrants with the help of two threads. A submerged platform (10 cm2) was placed inside the target quadrant of this pool 1 cm below the surface of the water. Each animal was subjected to four consecutive trials on each day with a gap of 5 min. Starting position on each day to conduct four acquisition trials was changed as described below, and Q4 was maintained as target quadrant in all acquisition trials.
-
1.
Day l Q1Q2Q3Q4
-
2.
Day 2 Q2Q3Q4Q1
-
3.
Day 3 Q3Q4Q1Q2
-
4.
Day 4 Q4Q1Q2Q3
The rat was gently placed in the water between quadrants (Q) and allowed 120 s to locate the submerged platform. Then, it was allowed to stay on the platform for another 20 s. Escape latency time to find the hidden platform in the target quadrant was noted as an index of acquisition or learning. On the 5th day, the platform was removed, and each animal was allowed to explore in the pool for 120 s. Mean time spent in each quadrant was noted. The mean time spent in the target quadrant for finding the hidden platform was noted as an index of retrieval (memory).
Estimation of Brain Contents of ACh and Catecholamines
Brain ACh, noradrenaline (NA), dopamine (DA), and serotonin (5-HT) contents were measured according to the methods described by Mathew et al. [25], Aviles et al. [26], Kobori et al. [27], and Song et al. [28], respectively, using rat-specific ELISA kit. ACh and NA were expressed as nmol/g tissue and pg/g tissue, respectively; meanwhile, DA and serotonin were expressed as ng/g tissue. All the procedures of the used kits were performed according to the manufacturer’s instructions.
Estimation of Brain AChE and eNOS
Brain AChE (ng/g tissue) and eNOS (pg/g tissue) were determined according to the method described by Den Blaauwen et al. [29] and Frick et al. [30], respectively, using rat-specific ELISA kit. All the procedures of the used kits were performed according to the manufacturer’s instructions.
Estimation of Aβ 1–42 in the Brain
Aβ 1–42 activity was determined according to the method described by Wang et al. [31] using ELISA kit and was expressed as pg/g tissue.
Estimation of Brain GSH
Brain GSH (mg/g wet tissue) was determined using Ellman’s reagent according to the method described by [32] after deproteinizing homogenates with 5-sulfuosalicylic acid.
Estimation of Lipid Peroxidation
As a marker of lipid peroxidation, MDA was determined in brain homogenates according to the method described by Armstrong, Browne [33] using ELISA kit and expressed as pmol/g tissue.
Estimation of Total Cholesterol Levels
Total cholesterol levels were determined in the brain based on the method described by Allain et al. [34] using commercial reagent kits and were expressed as mg/g tissue.
Estimation of Brain Contents of IL-6 and IL-10
IL-6 and IL-10 were measured according to the methods described by Venihaki et al. [35] and Eskdale et al. [36], respectively, using ELISA kits and were expressed as pg/g tissue.
Histological Examination of Cortical and Hippocampal Tissues
Histological assessment was performed on the brains of 2–3 rats randomly selected from each group. The brains were immediately fixed in 10 % phosphate buffered formaldehyde, subsequently embedded in paraffin, and 5-μm longitudinal sections were performed. The sections were stained with hematoxylin and eosin (H & E) and examined microscopically.
Statistical Analysis
Data were expressed as means ± S.E.M., and comparisons between means were carried out using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test. A probability level of less than 0.05 was accepted as being significant in all types of statistical tests.
Results
The rats in each group were trained daily for 4 days in Morris water maze apparatus starting from 28th day of drugs administration. The rats that received l-methionine showed a significant increase in mean escape latency time by 38.01, 56.67, 84.37, and 138.29 %, respectively, during the 4 days as compared to that of the normal control group (Fig. 1). Treatment with cilostazol showed a significant decrease in mean escape latency time by 18.89, 30.79, 40.04, and 56.51 %, respectively, as compared to the l-methionine control group. Meanwhile, treatment with simvastatin resulted in a significant decrease in mean escape latency time by 13.76, 27.00, 36.42, and 53.02 %, respectively, as compared to the l-methionine control group (Fig. 1).
Effect of simvastatin and cilostazol on mean escape latency time in l-methionine-induced vascular dementia in rats. Water maze test was performed for four successive days starting from 28th day of drugs administration. Each point with vertical line represents the mean of escape latency time ± S.E. within each testing session for each group (eight rats); *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
The mean time spent in the target quadrant in water maze was measured on the last day of the experiment after 4 days of training. The rats that received l-methionine showed significant decrease in time spent in target quadrant by 64.40 %, as compared to that of the normal control group. Treatment with cilostazol and simvastatin showed significant increase in time spent in target quadrant by 95.23 and 72.19 %, respectively, as compared to the l-methionine control group (Fig. 2).
Effect of simvastatin and cilostazol on mean time spent in target quadrant in l-methionine-induced vascular dementia in rats. Time spent in target quadrant in water maze was measured at day 5 after 4 days of training. Each bar with vertical line represents the mean of eight rats ±S.E. *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
l-methionine resulted in a significant increase in NA and DA brain contents by 181.15 and 253.18 %, respectively, and a significant decrease in 5-HT brain content by 63.60 %, as compared to that of the normal control group. Meanwhile, treatment with cilostazol showed a significant decrease in NA and DA brain contents by 26.12 and 30.71 %, respectively, and a significant increase in 5-HT brain content by 65.61 % as compared to the l-methionine control group. In the same context, treatment with simvastatin showed a significant decrease in NA and DA brain contents by 45.78 and 50.92 %, respectively, and a significant increase in 5-HT brain content by 93.87 % as compared to the l-methionine control group. Moreover, treatment with simvastatin resulted in a further decrease in brain NA and DA contents by 26.61 and 29.16 %, respectively, as compared to the cilostazol control group (Table 1).
Administration of l-methionine significantly increased brain AChE activity and decrease in brain ACh content by 388.11 and 72.87 %, respectively, as compared to that of the normal control group. Whereas, administration of cilostazol significantly decreased brain AChE activity and increased brain ACh content by 42.88 and 58.81 %, respectively, as compared to the l-methionine control group. Similarly, simvastatin decreased brain AChE activity and increased brain ACh content by 61.19 and 86.14 %, respectively, as compared to the l-methionine control group. Moreover, administration of simvastatin resulted in a further decrease in brain AChE activity by 32.06 % as compared to the cilostazol control group (Fig. 3).
Effect of simvastatin and cilostazol on brain acetylcholinesterase (AChE) activity (a) and ACh content (b) in l-methionine-induced vascular dementia in rats. Each bar with vertical line represents the mean of eight rats ±S.E. *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group, using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
l-methionine resulted in a significant decrease in brain eNOS content by 84.83 % as compared to that of the normal control group. Whereas, treatment with cilostazol and simvastatin showed significant increase in brain eNOS content by 137.22 and 302.18 %, respectively, as compared to the l-methionine control group. Moreover, administration of simvastatin resulted in a further increase in brain eNOS activity by 69.53 % as compared to the cilostazol control group (Fig. 4).
Effect of simvastatin and cilostazol on brain endothelial nitric oxide synthase activity in l-methionine-induced vascular dementia in rats. Each bar with vertical line represents the mean of eight rats ±S.E. *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group, using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
In the present experiment, l-methionine increased brain Aβ-42 content by 141.44 % as compared to that of the normal control group. On the other hand, treatment with cilostazol and simvastatin caused a significant decrease in brain Aβ-42 content by 26.28 and 43.91 %, respectively, compared to the l-methionine control group. Simvastatin even decreased brain Aβ-42 content by 23.92 % as compared to the cilostazol control group (Fig. 5).
Effect of simvastatin and cilostazol on brain amyloid beta-42 content in l-methionine-induced vascular dementia in rats. Each bar with vertical line represents the mean of eight rats ±S.E. *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group, b p < 0.05 vs cilostazol treated group at p < 0.05 using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
The rats that received l-methionine showed a significant increase in brain MDA and a decrease in GSH contents by 419.77 and 79.54 %, respectively, as compared to that of the normal control group. Meanwhile, treatment with cilostazol showed a significant decrease in brain MDA and increase in GSH contents by 39.09 and 120.63 %, respectively, as compared to the l-methionine control group. In the same context, treatment with simvastatin decreased brain MDA and increased GSH contents by 52.92 and 81 %, respectively, as compared to the l-methionine control group. Even more, simvastatin decreased brain MDA content by 22.72 % as compared to the cilostazol control group (Table 2).
l-methionine caused a significant elevation in brain total cholesterol content by 233.27 % as compared to that of the normal control group. Meanwhile, treatment with cilostazol and simvastatin reduced l-methionine-induced elevation in brain total cholesterol content by 43.17 and 58.24 %, respectively. Furthermore, treatment with simvastatin decreased brain total cholesterol content by 26.51 % as compared to the cilostazol control group (Fig. 6).
Effect of simvastatin and cilostazol on brain total cholesterol content in l-methionine-induced vascular dementia in rats. Each bar with vertical line represents the mean of eight rats ±S.E. *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group, b p < 0.05 vs cilostazol-treated group at p < 0.05 using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
Administration of l-methionine significantly increased brain IL-6 and decreased IL-10 content by 356.73 and 78.65 %, respectively, as compared to that of the normal control group. Treatment with cilostazol, on the other hand, significantly decreased brain IL-6 content and increased brain IL-10 content by 40.56 and 120.81 %, respectively, as compared to the l-methionine control group. Moreover, simvastatin decreased brain IL-6 and increased IL-10 contents by 46.42 and 80.65 %, respectively, as compared to the l-methionine control group (Fig. 7).
Effect of simvastatin and cilostazol on brain interleukin-6 (IL-6) (a) and interleukin-10 (IL-10) (b) contents in l-methionine-induced vascular dementia in rats. Each bar with vertical line represents the mean of eight rats ±S.E. *p < 0.05 vs normal control group, @ p < 0.05 vs l-methionine control group using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
Histological examination of stained brain sections revealed serious damaging effects of l-methionine on brain tissues compared to a normal brain sections (Fig. 8a, b) manifested as hemorrhages in the meninges covering the cerebral cortex, damage in the lining endothelium of the cerebral blood capillaries with encephalomalacia in the cerebral matrix, focal gliosis in the cerebrum in addition to focal hemorrhage in the cerebellum (Fig. 8c–f). Administration of cilostazol showed moderate protection against l-methionine-induced brain injury, where brain sections showed only focal gliosis in the cerebrum associated with congestion in the meningeal blood vessels and cerebral capillaries with restoration of the normal histological structure of the cerebellum (Fig. 9a, b). Sections from simvastatin-treated group displayed mild pathological changes compared to those from the l-methionine group that appeared as focal gliosis in the cerebrum with congestion in the blood vessels with restoration of the normal structure of the cerebellum (Fig. 9c, d).
Photomicrograph of sections of brain tissue of control rat showing (a) the normal histological structure of the meninges (m), cerebral cortex (c), and cerebrum (cr), and (b) the normal histological structure of the cerebellum (cl). l-methionine-induced demented rat showing (c) hemorrhages in the meninges covering the cerebral cortex, (d) damage in the lining endothelium of the cerebral blood capillaries (v) with encephalomalacia in the cerebral matrix, (e) focal gliosis (g) in the cerebrum, and (f) the cerebellum with focal hemorrhage (h) (H & E ×40)
Photomicrograph of sections of brain tissue of l-methionine-induced demented rat treated with cilostazol showing (a) focal gliosis (g) in the cerebrum associated with congestion in the meningeal blood vessels and cerebral capillaries and (b) normal histological structure of the cerebellum (cl). l-methionine-induced demented rat treated with simvastatin showing (c) focal gliosis in the cerebrum with congestion in the blood vessels (v) and (d) normal histological structure of the cerebellum (cl) (H & E ×40)
Discussion
Homocysteine (Hcy) is a non-essential sulfur-containing amino acid derived from methionine metabolism [7]. Autoxidation of Hcy results in the disruption of redox homeostasis that affects the redox signaling pathways in vascular and neuronal cells resulting in the induction of neurological dysfunction via oxidative stress [37]. Hcy can thus be toxic to neurons and can increase their susceptibility to Aβ damage [38] causing profound memory impairment, neural dysfunction, and impaired brain energy metabolism [39].
Indeed, in the current study, l-methionine administration for 32 days resulted in significant increase in escape latency time of control animals during ongoing acquisition trials, denoting abnormal acquisition of memory, and a decrease in time spent in target quadrant at day 5 in searching of missing platform during retrieval trials. This was coupled with increased activity of AChE and oxidative stress markers together with marked histopathologic changes in the brain.
l-methionine-induced changes, in the current study, were attenuated by treatment of the rats with simvastatin. Simvastatin have been shown to exhibit important immunomodulatory and anti-inflammatory activity independent of lipid-lowering effects [40]. Statins have pleiotropic effects on endothelium, platelets, smooth muscle cells, and inflammation. These effects included the improvement of endothelial and microvascular function, the reduction of inflammation through decreased expression of pro-inflammatory transcriptional factors that in turn decrease cytokines, chemokines, and inducible nitric oxide synthase (iNOS) expression [41]. Statins also possess antioxidant effects by direct scavenging of free radical molecules, independently of their effects on lipid metabolism [42].
Simvastatin was not previously investigated in l-methionine-induced VaD; however, in a study performed by Tramontina et al. [43], simvastatin showed neuroprotective effect in streptozotocin-induced model of Alzheimer’s disease (AD) in rats manifested by improved learning and spatial memory deficits in Morris water maze test. Moreover, simvastatin was found to strongly reduce levels of β-amyloid peptides (Aβ42 and Aβ40) in vitro and in vivo [44]. The reported protective effects of simvastatin in models of cerebral ischemia [45] were attributed to the ability of simvastatin to reduce the size of the infarct, in addition to activation of eNOS as well as its anti-atherosclerotic, anti-thrombotic, and anti-inflammatory effects [46]. Recently, simvastatin was shown to restore vascular reactivity and endothelial function coupled with reducing vessel pathology in a mouse model of cerebrovascular disease [47].
Cilostazol was also not previously investigated in l-methionine-induced VaD; however, the observed neuroprotective effects and the improvement of neurological function with cilostazol can be related to its ability to increase cerebral blood flow and decrease the size of cerebral infarcted area [48]. Moreover, in a study performed by Lee et al. [49], cilostazol was found to possess neuroprotective effect against apoptotic white matter changes in rats after chronic cerebral hypoperfusion. Besides, cilostazol improved cognition and neurologic deterioration in patients with Alzheimer’s and cerebrovascular diseases [50].
The reported protective effects of cilostazol were attributed to its ability to scavenge the hydroxyl and peroxyl radicals and inhibit their cell damage [51]. In addition, cilostazol significantly decreases ischemic brain infarction, inhibits apoptotic and oxidative cell death [52], and attenuates gray and white matter damage after focal cerebral ischemia in rats [53]. Reduction of the brain ischemic infarction and edema by cilostazol was confirmed by magnetic resonance imaging (MRI) in rats [54].
In the current experiments, l-methionine-induced pathological and behavioral changes were coupled by increase in NA and DA contents as well as decrease in 5-HT content that was prevented by treatment with simvastatin or cilostazol. Increased brain noradrenergic activity may contribute to the agitated behaviors or cognitive deficits of patients with advanced AD as NA concentration was significantly higher in the patients with advanced AD than in those with mild to moderate severity [55]. DA concentration was found to be increased in the cerebrospinal fluid of patients with subcortical ischemic VaD [56]. In VaD, it has been shown that DA uptake sites were dramatically reduced in the caudate nucleus [57]. Serotonergic function has been shown to decrease in patients with AD [58]. Concentration of 5-HT and 5-HT metabolites has been shown to be reduced in a number of brain regions including the striatum, hippocampus, hypothalamus, and caudate nucleus in VaD [59].
Neurotransmitters play a critical role in the brain circuits involved in various aspects of memory. The importance of acetylcholine is illustrated by its role in both the pathology and cognitive deficits. Stimulation of cholinergic neurons in the nucleus basalis of Meynert causes vasodilatation thus, increasing cortical and hippocampal blood flow in rats [60, 61]. Indeed, in the present study, administration of l-methionine decreased ACh content that was attenuated by treatment with simvastatin or cilostazol.
In the present study, l-methionine-induced deficits were accompanied by increased MDA, a marker of lipid peroxidation together with a decrease in GSH content. GSH provides major protection in oxidative injury by participating in the cellular defense systems against oxidative damage [62]. Treatment of rats with simvastatin or cilostazol reduced l-methionine-induced increase in brain MDA, coupled with increased brain GSH.
NO synthesized in endothelial cells by eNOS is the major source of NO in brain vessels and an important signaling molecule in vasculogenesis [63] and cerebral blood flow regulation [64]. Thus, intact endothelial function via NO confers key homeostatic balance in normal brain physiology, which raises the possibility that endothelial dysfunction associated with deficiency of eNOS and endothelial NO causes cerebrovascular pathology and neurological disease [65]. Correlations between eNOS polymorphisms and decreased NO bioavailability or endothelial dysfunction have also been reported [66], and reduced eNOS expression has been shown in AD [67]. In the current experiments, l-methionine-induced endothelial dysfunction was associated with decrease in brain eNOS content, an effect that was not shown in groups receiving simvastatin or cilostazol.
The expression of IL-6 and other inflammatory factors in atherosclerotic lesions can be upregulated by the nuclear factor-κappaB (NF-κB). The proliferation of vascular smooth muscle cells can lead to excessive production of extracellular matrix, and generation of growth factors and cytokines, including IL-6 [68]. One of the most ubiquitous eukaryotic transcription factors that regulate expression of genes involved in controlling cellular proliferation/growth, inflammatory responses, cell adhesion, and so forth is NF-κB [69]. The transcriptional activity of NF-κB is regulated via an elaborate series of intracellular signal transduction events in response to external stimuli. In addition to its central roles in mediating inflammation, NF-κB is important in control via cell proliferation, and cell transformation [70].
Reactive oxygen metabolites (ROM) have been reported to be a crucial event in the activation of NF-κB [71]. Therefore, it has been proposed that antioxidants, as simvastatin or cilostazol, by reducing ROM, may in turn block NF-κB activation and finally inhibit NF-κB-mediated increase in the production of pro-inflammatory cytokines [72]. Indeed, in the current experiments, treatment of the rats with simvastatin or cilostazol prevented l-methionine-induced increases in IL-6. Whereas, IL-10, an anti-inflammatory cytokine, was suppressed by l-methionine administration, and this effect was prevented by treatment with simvastatin or cilostazol.
Amyloid precursor protein (APP)-Aβ metabolism is possibly regulated by lipids. An essential role has been played by cholesterol in regulating the enzyme activity that is involved in Aβ protein production and APP metabolism [73]. Proposed mechanisms include secretase enzyme regulation, Aβ transport disruption, Aβ aggregation promotion, and modulating the neurotoxicity of Aβ plaque [74, 75]. Hyperlipidemia has been shown to cause memory impairment, cholinergic dysfunction, inflammation, and microbleedings [76] and thus may have an important role in the progression of age-related cognitive decline, mild cognitive impairment, VaD, and AD [77]. Certainly, in the present study, treatment of the rats with simvastatin or cilostazol prevented l-methionine-induced increase in brain cholesterol and Aβ42.
In conclusion, the prevalence of dementia has increased significantly nowadays. As none of the available medications appears to be able to stop the disease progression, there is an enormous medical need for the development of novel therapeutic strategies that target the underlying pathogenic mechanisms. Simvastatin and cilostazol improved learning and memory in l-methionine-induced model of dementia. Keeping in mind that both agents showed antioxidants, anti-inflammatory and neuroprotective effects in current study, their use might slow progression of VaD.
References
Gupta S, Sharma B (2014) Pharmacological modulation of I 1-imidazoline and α 2-adrenoceptors in sub acute brain ischemia induced vascular dementia. Eur J Pharmacol 723:80–90
Simard M, van Reekum R, Cohen T (2015) A review of the cognitive and behavioral symptoms in dementia with Lewy bodies. The Journal of neuropsychiatry and clinical neurosciences
Reischl S, Li L, Walkinshaw G, Flippin LA, Marti HH, Kunze R (2014) Inhibition of HIF prolyl-4-hydroxylases by FG-4497 reduces brain tissue injury and edema formation during ischemic stroke.
Cervellati C, Romani A, Seripa D, Cremonini E, Bosi C, Magon S, Passaro A, Bergamini CM, et al. (2014) Oxidative balance, homocysteine, and uric acid levels in older patients with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci 337(1):156–161
Akyol S, Erdogan S, Idiz N, Celik S, Kaya M, Ucar F, Dane S, Akyol O (2014) The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: an in-depth analysis. Redox Rep 19(5):180–189
Chen CT, Trépanier M-O, Hopperton KE, Domenichiello AF, Masoodi M, Bazinet RP (2014) Inhibiting mitochondrial β-oxidation selectively reduces levels of nonenzymatic oxidative polyunsaturated fatty acid metabolites in the brain. J Cereb Blood Flow Metab 34(3):376–379
Rauch BJ, Gustafson A, Perona JJ (2014) Novel proteins for homocysteine biosynthesis in anaerobic microorganisms. Mol Microbiol 94(6):1330–1342
Wang X, Cui L, Joseph J, Jiang B, Pimental D, Handy DE, Liao R, Loscalzo J (2012) Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress. J Mol Cell Cardiol 52(3):753–760
Keil U, Bonert A, Marques CA, Strosznajder JB, Muller-Spahn F, Muller WE, Eckert A (2004) Elevated nitric oxide production mediates beta-amyloid-induced mitochondria failure. Pol J Pharmacol 56(5):631–634
Mendoza-Oliva A, Ferrera P, Arias C (2013) Interplay between cholesterol and homocysteine in the exacerbation of amyloid-β toxicity in human neuroblastoma cells. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 12(6):842–848
Qi X, Zheng L, Lee K, Kim D, Kim C, Cai D, Wu Z, Qin J, et al. (2013) HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis 4(2):e518
Margaritis M, Channon KM, Antoniades C (2014) Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering. Antioxid Redox Signal 20(8):1198–1215
Abbas AM, Sakr HF (2013) Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J Physiol Biochem 69(4):737–750
Wang K-W, Chen H-J, Lu K, Liliang P-C, Liang C-L, Tsai Y-D, Cho C-L (2014) Simvastatin attenuates the cerebral vascular endothelial inflammatory response in a rat traumatic brain injury. Annals of Clinical & Laboratory Science 44(2):145–150
Burn DJ, Yarnall AJ (2014) Dementia in Parkinson’s disease. Non-motor Symptoms of Parkinson’s Disease:158
Saito S, Hata K, Iwaisako K, Yanagida A, Takeiri M, Tanaka H, Kageyama S, Hirao H, et al. (2014) Cilostazol attenuates hepatic stellate cell activation and protects mice against carbon tetrachloride-induced liver fibrosis. Hepatol Res 44(4):460–473
Association AD (2014) Peripheral arterial disease in people with diabetes.
Hase Y, Okamoto Y, Fujita Y, Kitamura A, Nakabayashi H, Ito H, Maki T, Washida K, et al. (2012) Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol 233(1):523–533
Sakurai H, Hanyu H, Sato T, Kume K, Hirao K, Kanetaka H, Iwamoto T (2013) Effects of cilostazol on cognition and regional cerebral blood flow in patients with Alzheimer’s disease and cerebrovascular disease: a pilot study. Geriatrics & gerontology international 13(1):90–97
Toda Y, K-i K, Saito M, Inaba T, Sakurazawa M, Katayama Y (2014) The effect of cilostazol and aspirin pre-treatment against subsequent transient focal cerebral ischemia in rat. Neurol Res 36(11):1011–1019
Sharma B, Singh N (2012) Salutary effect of NFκB inhibitor and folacin in hyperhomocysteinemia–hyperlipidemia induced vascular dementia. Prog Neuro-Psychopharmacol Biol Psychiatry 38(2):207–215
Kou J, Kim H-D, Jin J, Cao D, Li L, Lalonde R, K-i F (2010) Simvastatin enhances immune responses to Aβ vaccination and attenuates vaccination-induced behavioral alterations. Brain Res 1356(0):102–111
Hiramatsu M, Takiguchi O, Nishiyama A, Mori H (2010) Cilostazol prevents amyloid β peptide25-35-induced memory impairment and oxidative stress in mice. Br J Pharmacol 161(8):1899–1912
Parle M, Singh N (2004) Animal models for testing memory. Asia Pacific J Pharmacol 16:101–120
Mathew JS, Saliki JT, Ewing SA, Lehenbauer TW, Panciera RJ, Malayer JR, Cummings CA, Kocan AA (2001) An indirect enzyme-linked immunosorbent assay for diagnosis of American canine hepatozoonosis. J Vet Diagn Investig 13(1):17–21
Aviles H, Belay T, Vance M, Sonnenfeld G (2005) Effects of space flight conditions on the function of the immune system and catecholamine production simulated in a rodent model of hindlimb unloading. Neuroimmunomodulation 12(3):173–181. doi:10.1159/000084850
Kobori N, Clifton GL, Dash PK (2006) Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma 23(7):1094–1102. doi:10.1089/neu.2006.23.1094
Song G, Gwee K, Moochhala S, Ho K (2005) Melatonin attenuates stress-induced defecation: lesson from a rat model of stress-induced gut dysfunction. Neurogastroenterology & Motility 17(5):744–750
Den Blaauwen D, Poppe W, Tritschler W (1983) Cholinesterase (EC 3.1. 1.8) with butyrylthiocholine-iodide as substrate: references depending on age and sex with special reference to hormonal effects and pregnancy. Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie 21(6):381–386
Frick M, Dulak J, Cisowski J, Józkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher SP, et al. (2003) Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis 170(2):229–236
Wang R, Sweeney D, Gandy S, Sisodia S (1996) Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem 271:31894–31902
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Armstrong D, Browne R (1994) The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. In: Free radicals in diagnostic medicine. Springer, pp 43–58
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20(4):470–475
Venihaki M, Dikkes P, Carrigan A, Karalis KP (2001) Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J Clin Invest 108(8):1159–1166. doi:10.1172/JCI12869
Eskdale J, Kube D, Tesch H, Gallagher G (1997) Mapping of the human IL10 gene and further characterization of the 5’flanking sequence. Immunogenetics 46(2):120–128
Perna A, Ingrosso D, De Santo N (2003) Homocysteine and oxidative stress. Amino Acids 25(3–4):409–417
Ho PI, Collins SC, Dhitavat S, Ortiz D, Ashline D, Rogers E, Shea TB (2001) Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem 78(2):249–253
Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580(13):2994–3005
McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY (2004) A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol 172(5):2903–2908
Jasiñska M, Owczarek J, Orszulak-Michalak D (2007) Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep 59(5):483
Ungureanu D, Filip C, Artenie A, Artenie R (2003) Evaluation of simvastatin antioxidant effects. Rev Med Chir Soc Med Nat Iasi 107(1):66–71
Tramontina AC, Wartchow KM, Rodrigues L, Biasibetti R, Quincozes-Santos A, Bobermin L, Tramontina F, Goncalves CA (2011) The neuroprotective effect of two statins: simvastatin and pravastatin on a streptozotocin-induced model of Alzheimer’s disease in rats. J Neural Transm 118(11):1641–1649. doi:10.1007/s00702-011-0680-z
Fassbender K, Simons M, Bergmann C, Stroick M, Lütjohann D, Keller P, Runz H, Kühl S, et al. (2001) Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci 98(10):5856–5861
Shabanzadeh AP, Shuaib A, Wang CX (2005) Simvastatin reduced ischemic brain injury and perfusion deficits in an embolic model of stroke. Brain Res 1042(1):1–5
Cimino M, Balduini W, Carloni S, Gelosa P, Guerrini U, Tremoli E, Sironi L (2005) Neuroprotective effect of simvastatin in stroke: a comparison between adult and neonatal rat models of cerebral ischemia. Neurotoxicology 26(5):929–933
Tong XK, Hamel E (2015) Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease. J Cereb Blood Flow Metab 35(3):512–520. doi:10.1038/jcbfm.2014.226
Yuzawa I, Yamada M, Fujii K (2008) An oral administration of cilostazol before focal ischemia reduces the infarct volume with delayed cerebral blood flow increase in rats. J Stroke Cerebrovasc Dis 17(5):281–286
Lee JH, Park SY, Shin YW, Hong KW, Kim CD, Sung S-M, Kim KY, Lee WS (2006) Neuroprotection by cilostazol, a phosphodiesterase type 3 inhibitor, against apoptotic white matter changes in rat after chronic cerebral hypoperfusion. Brain Res 1082(1):182–191
Arai H, Takahashi T (2009) A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: pilot follow-up study. Am J Geriatr Psychiatry 17(4):353–354
Kim KY, Shin HK, Choi JM, Hong KW (2002) Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther 300(2):709–715
Choi JM, Shin HK, Kim KY, Lee JH, Hong KW (2002) Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther 300(3):787–793
Honda F, Imai H, Ishikawa M, Kubota C, Shimizu T, Fukunaga M, Saito N (2006) Cilostazol attenuates gray and white matter damage in a rodent model of focal cerebral ischemia. Stroke 37(1):223–228
Lee JH, Lee Y-K, Ishikawa M, Koga K, Fukunaga M, Miyakoda G, Mori T, Hosokawa T, et al. (2003) Cilostazol reduces brain lesion induced by focal cerebral ischemia in rats—an MRI study. Brain Res 994(1):91–98
Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA (1997) Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatr 154(1):25–30
Tohgi H, Ueno M, Abe T, Takahashi S, Nozaki Y (1992) Concentrations of monoamines and their metabolites in the cerebrospinal fluid from patients with senile dementia of the Alzheimer type and vascular dementia of the Binswanger type. J Neural Transm Park Dis Dement Sect 4(1):69–77
Allard P, Englund E, Marcusson J (1999) Reduced number of caudate nucleus dopamine uptake sites in vascular dementia. Dement Geriatr Cogn Disord 10(2):77–80 doi:17105
Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi-Jadid K, Cole GM, Satyamurthy N, et al. (2006) Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci U S A 103(3):702–707. doi:10.1073/pnas.0510237103
Elliott MS, Ballard CG, Kalaria RN, Perry R, Hortobágyi T, Francis PT (2009) Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain:awp069
Sato A, Sato Y, Uchida S (2004) Activation of the intracerebral cholinergic nerve fibers originating in the basal forebrain increases regional cerebral blood flow in the rat’s cortex and hippocampus. Neurosci Lett 361(1–3):90–93. doi:10.1016/j.neulet.2004.01.004
Sato A, Sato Y, Uchida S (2001) Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Int J Dev Neurosci 19(3):327–337
Jefferies H, Coster J, Khalil A, Bot J, McCauley RD, Hall JC (2003) Glutathione. ANZ J Surg 73(7):517–522
Dai X, Faber JE (2010) Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106(12):1870–1881
Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J (2009) Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience 159(2):744–750
Tan X-L, Xue Y-Q, Ma T, Wang X, Li JJ, Lan L, Malik KU, McDonald MP, et al. (2015) Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol Neurodegener 10(1):1–14
Leeson C, Hingorani A, Mullen M, Jeerooburkhan N, Kattenhorn M, Cole T, Muller D, Lucas A, et al. (2002) Glu298Asp endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ Res 90(11):1153–1158
Jeynes B, Provias J (2009) Significant negative correlations between capillary expressed eNOS and Alzheimer lesion burden. Neurosci Lett 463(3):244–248
Zhang L, Jin M, Hu X-s, Zhu J-h (2006) Homocysteine stimulates nuclear factor κB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol Int 30(7):592–597
Chen F, Castranova V, Shi X, Demers LM (1999) New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 45(1):7–17
Surh Y-J, Chun K-S, Cha H-H, Han SS, Keum Y-S, Park K-K, Lee SS (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 480:243–268
Gloire G, Legrand-Poels S, Piette J (2006) NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72(11):1493–1505
Tak PP, Firestein GS (2001) NF-κB: a key role in inflammatory diseases. J Clin Investig 107(1):7–11
Duron E, Hanon O (2008) Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag 4(2):363–381
Di Paolo G, Kim TW (2011) Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 12(5):284–296. doi:10.1038/nrn3012
Moreira ELG, de Oliveira J, Prediger RDS, Farina M, de Bem AF (2015) Chapter 68—cholesterol levels and cognitive impairments. In: Preedy CRMR (ed) Diet and nutrition in dementia and cognitive decline. Academic Press, San Diego, pp. 743–751
Ullrich C, Pirchl M, Humpel C (2010) Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci 45(4):408–417. doi:10.1016/j.mcn.2010.08.001
Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, et al. (2010) Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev 9(4):399–417
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was conducted in accordance to the Ethics Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University, Egypt, PT (1048).
Rights and permissions
About this article
Cite this article
El-Dessouki, A.M., Galal, M.A., Awad, A.S. et al. Neuroprotective Effects of Simvastatin and Cilostazol in l-Methionine-Induced Vascular Dementia in Rats. Mol Neurobiol 54, 5074–5084 (2017). https://doi.org/10.1007/s12035-016-0051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0051-8