Abstract
Melatonin, as a neuroendocrine hormone, is produced primarily by the pineal gland. Melatonin, a pleotropic molecule, acts as a free radical scavenger, antioxidant, and regulator of circadian rhythm in mammals via several receptor-mediated and non-receptor-mediated mechanisms. This inexpensive, well-tolerated, and multi-target molecule has a great therapeutic potential against many diseases. Many evidences have proposed that melatonin plays a key role in the pathophysiology of various cardiovascular diseases. The aim of this paper is to discuss the data and experiments regarding the effects of melatonin in management of cardiovascular risk factors. PubMed, EMBASE, and Scopus have been searched for data collection using related keywords. Two hundred ten articles were included in this review from 2253 founded documents. Using these documents, the main mechanisms of action of melatonin are discussed and summarized in this article. Also, recent progresses regarding melatonin’s effects on cardiovascular risk factors and diseases including diabetes, hypertension, hyperlipidemia, obesity, myocardial ischemia-reperfusion injury, pulmonary hypertension, and atherosclerosis have been reviewed. Many studies have demonstrated the beneficial effects of melatonin in prevention and improving cardiovascular risk factors, and this inexpensive and well-tolerated drug can be strongly proposed in different cardiovascular diseases as well as metabolic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are the most important cause of death and account for about one third of all deaths. Considering that cardiovascular diseases are the major causes of mortality worldwide, preventive measures to reduce the creation and development of these disorders are very important (Mozos 2017).
Melatonin (N-acetyl-5-methoxytryptamine) is a very old molecule which is produced in many organisms such as bacteria, fungi, plants, and animals. In vertebrates, melatonin is the main hormone of pineal gland that is secreted on a base of a circadian pattern and synchronized to the dark phase of light/dark cycle (Cecon et al. 2018). In the other words, the secretion of melatonin is inversely regulated by light. Melatonin can also be produced by extra-pineal tissues such as the retina, the innate immune system, and the gastrointestinal tract (Jockers et al. 2016a). Melatonin regulates the sleep cycle and also has antioxidant and anti-inflammatory properties and controls glucose and lipid metabolism (Espino et al. 2011).
Moreover, melatonin increasingly is being recognized in the pathophysiology of cardiovascular diseases. The reduced levels of melatonin and its major metabolite, 6-sulphatoxymelatonin, have been reported in various cardiovascular diseases including myocardial infarcts, coronary heart disease, congestive heart failure, and nocturnal hypertension (Baker and Kimpinski 2018; Mukherjee et al. 2012). Also, the deficiency in melatonin secretion, as in shift-work, aging and illuminated environments during the night, causes glucose intolerance, insulin resistance, metabolic circadian disorganization and sleep disturbance that threatens the health conditions (Cipolla-Neto et al. 2014).
Melatonin receptors have been identified within the cardiovascular system, including various vascular tissues. In animals, pinealectomy causes hypertension and peripheral vasoconstriction (Zanoboni et al. 1978) (Baker and Kimpinski 2018).
Administration of melatonin has been shown to be efficient in the modulation of oxidative stress, inflammatory marker, hypertension and metabolic syndrome (Gomes Domingos et al. 2019). Exogenous melatonin also has been shown to reduce platelet aggregation, nocturnal hypertension, and serum catecholamine levels (Pandi-Perumal et al. 2017b).
In this review, we discuss the effect of melatonin on cardiovascular risk factors such as hypertension, hyperlipidemia, diabetes, etc. and the recent progresses in the understanding of melatonin’s effects on cardiovascular diseases (Table 1).
Scopus, PubMed, and EMBASE databases were searched with the following keywords:
- Hypertension or “blood pressure” or hypotensive or antihypertensive
- Diabetes or hyperglycemia or insulin or hypoglycemic or antihyperglicemic or antidiabetic or “blood glucose”
- Dyslipidemia or hyperlipidemia or “high cholesterol” or “high triglyceride” or hypercholesterolemia or hypertriglyceridemia
- atherogenic or atherosclerosis
- Obesity or overweight or appetite or anti-obesity or “weight loss”
- “metabolic syndrome”
In total, 2253 studies were found, of which 210 were included in this review.
Melatonin secretion and metabolism
The pineal gland secretes melatonin in response to low light. In low light conditions, a cascade of signal transductions (starting at the suprachiasmatic nucleus and ending at the pineal gland) is activated and induces an increase in melatonin synthesis and secretion (Moore 1996). Initially, it was thought that melatonin production is restricted to the pineal gland. However, the removal of pineal gland did not result in the complete elimination of melatonin, and about 20% of the normal levels remained in serum and urine. Melatonin-synthesizing enzymes have been shown in retina, ovary, gastrointestinal tract and etc. However, the melatonin produced outside the pineal gland generally does not reach the bloodstream and therefore does not show systemic effects (Bubenik and Pang 1994)
Melatonin is metabolized at the site of production or in the liver. In liver, cytosol, mitochondria, and endoplasmic reticulum are involved in melatonin metabolism (Slominski et al. 2017). 6-Sulfatoxymelatonin is the main metabolite of melatonin that produced in the liver and exerted in urine. 6-Sulfatoxymelatonin is a reliable biomarker representing the blood melatonin concentration (Xu and Huang 2017).
Mechanisms of action of melatonin
Melatonin exerts its physiological actions through four mechanisms including:
- 1-
Binding to melatonin receptors in plasma membrane, cytoplasm and nucleolus
- 2-
Binding to orphan nuclear receptors
- 3-
Binding to intracellular proteins such as calcium binding proteins
- 4-
Antioxidant effect.
Melatonin receptors
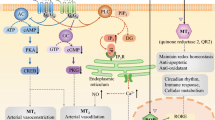
In humans, two types of membrane receptors (MT1 and MT2): one type of cytoplasmic receptor (MT3) and one type of nuclear receptor have been identified for melatonin (Emet et al. 2016) (Fig. 1). Both MT1 and MT2 belong to G protein-coupled receptors, whereas MT3 is a quinone reductases which inhibits the electron transfer reactions of quinones and consequently prevents oxidative stress. MT3 receptor is located in the cytoplasm of liver, lung, kidney, eye, heart, brown fat tissue, and intestine and muscle cells. This receptor is a detoxification enzyme. Some studies indicate the role of MT3 receptor in regulation of intra-ocular pressure (Ekmekcioglu 2006) (Emet et al. 2016). Retinoid-related orphan nuclear hormone receptor (RZR/RORα) is forth type of melatonin receptors. Melatonin, via this receptor, regulates some transcription factors belongs to retinoic acid receptor super-family. (Pandi-Perumal et al. 2008). The GPR50 receptor (a Melatonin-related Orphan receptor) does not bind to melatonin; however, it potentiates the binding of melatonin to MT1 receptors. Its natural ligand has not been defined yet (Ekmekcioglu 2006; Emet et al. 2016).
For G protein-coupled receptors of melatonin, receptor binding affects intracellular signaling through regulatory activities of adenylate cyclase, guanylate cyclase, phospholipase C, and calcium channels. MT1 and MT2 receptors, via activating Gi subunit, inhibit adenylate cyclase, which subsequently reduce cAMP production (Dubocovich 1995). Additionally, through Gq subunit, melatonin increases phospholipase C activity and intracellular calcium concentrations (Dubocovich 1995). Melatonin has the ability to attach to calcium binding proteins (such as calmodulin) with high affinity, thereby inhibits the activation of myosin light-chain kinase, which in turn will decrease the contractile response in different smooth muscles (Fathollahi et al. 2015). MT2 receptors, but not MT1, also modulate guanylyl cyclase and subsequently cGMP production (Baker and Kimpinski 2018).
In the central nervous system including hypothalamus, pituitary, suprachiasmatic nucleus, hippocampus and paraventricular nucleus, the melatonin receptors are highly expressed. (Baker and Kimpinski 2018). Melatonin receptors also have widespread distribution in central and peripheral vasculature. In CNS vessels, melatonin receptors have been identified within the vertebral arteries and Circle of Willis of rats and primates (Capsoni et al. 1994) (Baker and Kimpinski 2018). In peripheral vessels, melatonin receptors have been identified in the coronary arteries of chicks, the caudal artery of rats and internal carotids in primates (Stankov et al. 1993) (Baker and Kimpinski 2018). Melatonin effects on the vascular tonicity depend on the type of activated melatonin receptor. Animal studies reveal that vasorelaxation is mediated through MT2 activation and vasoconstriction through MT1 activation via endothelium and smooth muscle, respectively (Fig. 1) (Ekmekcioglu 2006). Despite numerous studies indicating the presence of different melatonin receptors in vascular tissue, there are few reports specifying the presence of MT1 and MT3 receptors in cardiomyocytes (Slominski et al. 2012). Considering the widespread distribution of receptors within the cardiovascular system, melatonin is expected to play an important role in various cardiovascular diseases (Baker and Kimpinski 2018).
Antioxidant and free radical scavenger activity of melatonin
The antioxidant and mitochondrial-protecting effects of melatonin have been demonstrated in numerous animal studies (Kurhaluk et al. 2018) (Gerush et al. 2018) (Djordjevic et al. 2018) and clinical trials (Raygan et al. 2019b). For example, Raygan et al. showed that administration of 5 mg (twice daily) melatonin to diabetic patients with CHD for 12 weeks has beneficial effects on plasma MDA, GSH, PCO, NO, and serum hs-CRP levels (Raygan et al. 2019b). In elderly diabetic patients, 5 mg/daily of melatonin reduced the glutathione and MDA concentrations and activities of SOD-1, CAT, GPx-1, and GR (Rybka et al. 2016). Two main mechanisms have been suggested to explain the antioxidant and free radical scavenging activity of melatonin: first mechanism is binding of melatonin to the MT3 receptor which prevents from oxidative stress via inhibiting the electron transfer reactions of quinones (Nosjean et al. 2000), and the second mechanism is the scavenging of free radicals, because this hormone is an electron donor (Tan et al. 2015). Depending on the dose of endogenous or exogenous melatonin, receptor-dependent or receptor-independent mechanisms (first or second mechanisms) may be involved (Jockers et al. 2016a).
In addition to the above two mechanisms, melatonin also indirectly stimulates antioxidative enzymes such as glutathione peroxidase, glutathione reductase superoxide dismutase, and glucose-6-phosphate dehydrogenase, and consequently lowers molecular damage under conditions of excessive oxidative stress (Reiter and Tan 2003). This stimulation of antioxidative enzymes is mediated by acting on MT1 and MT2 receptors. Due to its highly lipophilic properties, melatonin easily crosses cell membranes and reaches intracellular compartments, including nuclei and mitochondria. Melatonin specially reduces mitochondrial oxidative stress that preserves normal mitochondrial function and decreases subsequent apoptotic events and cell death (Acuna-Castroviejo et al. 2014).
In addition to the melatonin itself, which is a direct free radical scavenger, the metabolites of melatonin such as cyclic 3-hydroxymelatonin and N1-acetyl-N2-formyl-5-methoxykynuramine, (is deformylated to N1-acetyl-5-methoxykynuramine) are also free radical scavengers (Jockers et al. 2016a). Therefore, a cascade melatonin’s metabolites may contribute to the antioxidant effects of the parent molecule and the total antioxidant capacity of melatonin seems to be higher than that of other known antioxidants such as vitamin E and vitamin C, under in vivo and in vitro conditions (Pandi-Perumal et al. 2017a).
Recent studies have reported that melatonin also modulates the autophagy process via different pathways including the reduction of mammalian Mst1 phosphorylation and enhancement of Sirt3 expression. Autophagy is a lysosomal degradation process that removes damaged organelles and misfolded proteins in order to maintain cellular homeostasis (Roohbakhsh et al. 2018).
Impaired autophagy can cause cardiac hypertrophy (Zaglia et al. 2014), heart failure (Thomas et al. 2013) and ischemia/reperfusion (I/R) injury (Rodella et al. 2013a). Administration of melatonin has alleviated adverse left ventricle remodeling. Exogenous melatonin also reduces cardiac dysfunction in diabetic animals (Zhang et al. 2017) and induces a significant protective effect in ischemia/reperfusion (I/R) injury and hypertension (Rodella et al. 2013a) (Yu et al. 2015b).
Melatonin and aging
Aging causes the morphological and functional deterioration in all living organisms, especially in mitochondria. Age-related impairment of mitochondria leads to the elevated free radicals that obviously cause molecular disfigurement and functional decay in different systems. Melatonin by its scavenging activities reduces the oxidative stress of the mitochondria. It has been reported that surgical removal of the pineal gland of young rats causes a more rapid accumulation of free radicals in different tissues when the animals reached 25 months of age (Reiter et al. 1999). It seems that melatonin deficiency is responsible for the accelerated oxidative damage and therefore one of the factors that contributed to elevated oxidative injury in the elderly, including an increased incidence of diseases that have a significant free radicals component including cardiovascular diseases (Reiter et al. 2018).
Melatonin and diabetes
Diabetes is the greatest risk factor for the development of cardiovascular diseases. The relative risk of fatal cardiovascular events in diabetic patients is 2- to 3-fold higher compared to non-diabetic individuals. The mechanisms of this heightened risk are poorly understood. Diabetes is characterized by hyperglycemia, impaired insulin secretion, insulin resistance and an enhanced inflammatory state. Each of these co-morbidities can contribute to the increased the risk of cardiovascular diseases in diabetic patients (Barrett et al. 2017). Hyperglycemia and insulin resistance increase the susceptibility of the arterial wall to atherosclerosis and cause arterial stiffening at any given age.
In diabetic patients, melatonin levels are lower than healthy people and a functional interaction between insulin and melatonin has been observed (Reutrakul et al. 2018). Moreover, glucose intolerance and insulin resistance can be seen in some physiological or pathophysiological states such as shift work, aging and environmental high level of illumination during the night which are associated with reductions in blood melatonin levels (Cipolla-Neto et al. 2014). A similar situation is also seen in MT1-knockout animals (Contreras-Alcantara et al. 2010). On the basis of these evidences, melatonin may be involved in the development of diabetes. On the other hand, melatonin administration reduces glucose tolerance mainly by decreasing the morning release of insulin (Rubio-Sastre et al. 2014). In addition, various studies have shown a correlation between sleep disorders and a greater risk for type 2 diabetes and a decreased glucose tolerance. Moreover, the role of allelic variations of the MT2 receptors on glycemic control and insulin secretion has been proposed in genome-wide association studies (Andersson et al. 2010). Both insulin and melatonin exhibit a circadian rhythm, but there is negative correlation between melatonin and insulin. In the other words, insulin secretion is inversely affected by melatonin. Collectively, the reduction of melatonin levels due to any reason including aging and exposure to light at night may cause type 2 diabetes. Therefore, circadian system can be considered as a pharmacological target for decreasing the prevalence of insulin resistance and hyperglycemia. According to the results of animal studies, melatonin supplementation has beneficial effects on insulin resistance, insulin secretion, and glucose homeostasis (Zhao et al. 2017; Mayo et al. 2018; Zhao et al. 2017; Zhou et al. 2018a; Hajam et al. 2018; Lardone et al. 2014; Zanuto et al. 2013). It has been shown that melatonin regulates blood glucose levels via direct binding to melatonin receptors and modulating the expression of the glucose uptake transporters that regulate the uptake of glucose in adipocytes (Nduhirabandi et al. 2017).
MT1 and MT2 receptors are expressed in the beta-cells of the pancreas islets and are involved in the modulation of insulin secretion, via the inhibition of the adenylate cyclase (Fig. 2). Cyclic guanosine monophosphate formation is also reduced in the pancreatic beta-cells by inhibiting the soluble guanylate cyclase, via MT2 receptors (Peschke et al. 2015). Melatonin regulates both basal and stimulated insulin secretion through the maintenance of ROS homeostasis in pancreatic islets (Simões et al. 2016). Melatonin, through MT1 receptors, phosphorylates and activates the tyrosine kinase β-subunit of the insulin receptor, and consequently mobilizes several intracellular transduction steps of the insulin-signaling pathways (tyrosine phosphorylation of IRS-1; IRS-1/PI(3)-kinase and downstream AKT and GSK-3 β (Heo et al. 2018). Melatonin also activates MAPK signaling pathway including Raf1 and ERK (Li et al. 2018). The PI3K/AKT pathway is involved in cell metabolism and glycogen synthesis and MEK/ERKs pathway is involved in cell proliferation, growth and differentiation (Sharma et al. 2015). In α-cells of pancreatic islets, melatonin stimulates the secretion of glucagon via PI3K activation (Bähr et al. 2011).
In human studies, few randomized controlled trials have evaluated the antihyperglycemic effect of melatonin. Rezvanfar et al. (Rezvanfar et al. 2017) showed that melatonin administration at a dosage of 6 mg for 12 weeks decreased fasting glucose and HbA1c in diabetic patients. This research group also reported that 10 mg/day of melatonin for 12 weeks could improve glycemic control in diabetic patients with coronary heart disease (Raygan et al. 2019a). Another 12-week clinical trial showed the significant improvement of the inflammatory status and insulin sensitivity in obese patients with acanthosis nigricans receiving melatonin (Sun and Wang 2018). Recently, a systematic review and meta-analysis of 12 randomized controlled indicated that melatonin significantly reduces fasting glucose. However, the insulin and HbA1c levels was not significantly influenced by melatonin (Doosti-Irani et al. 2018).
Totally, the evidence indicates that melatonin supplementation may improve glucose homeostasis and advance the current therapeutic strategy to treat the diabetes. However, more prospective studies using higher doses and longer administration period are recommended to confirm the impact of melatonin on diabetic patients.
Dyslipidemia
Low melatonin levels have been reported in individuals with elevated LDL cholesterol levels (Pandi-Perumal et al. 2017b). On the other hand, exposure to light at night has been associated with impaired lipid parameters in elderly individuals. In a cross-sectional study in 528 elderly individuals, exposure to light at night caused a significant increase in body weight, waist circumference and LDL cholesterol levels (Obayashi et al. 2013).
There are large numbers of animal studies showing that melatonin administration can improve dyslipidemia (Santos et al. 2018; Salari Lak et al. 2011; Ríos-Lugo et al. 2010). In diet-induced hypercholesterolemic rats, the low (1 mg/kg/day) and high (10 mg/kg/day) dose of melatonin decreased the total cholesterol, LDL cholesterol, oxidized LDL, total antioxidant capacity, and TBARS levels specially in higher dose (Butun et al. 2013). Melatonin administration (25 μg/ml) in high-fat diet rats for 9 weeks decreased body weight gain and triglycerides and cholesterol levels from the 3rd week without affecting food intake (Ríos-Lugo et al. 2010).
A study on aluminum-induced toxicity in rats showed that melatonin alleviates the aluminum induced increase in total cholesterol, LDL cholesterol, triglycerides, and oxidized LDL (Allagui et al. 2015).
There are also several clinical studies showing the relation between melatonin and lipid profile in doses ranging between 0.3 and 10 mg/day (Mozaffari et al. 2012). In a study in patients with nonalcoholic fatty liver disease, administration of melatonin (5 mg; 2 times per day) for 14 months significantly reduced LDL cholesterol and triglycerides (Celinski et al. 2014). In cigarette smokers also, 2 weeks of melatonin administration (3 mg/kg) significantly reduced free fatty acids and smoke-induced vascular injury (Wang et al. 2016).
Regarding to the related mechanisms, most of studies propose the antioxidant effect of melatonin as the main mechanism in improving lipid profile. Melatonin increases antioxidative enzymes and glutathione levels, removes free radicals, decreases lipid peroxidation, and prevents electron leakage from the mitochondria (Mozaffari et al. 2012). Melatonin also modulates the macrophage activity and regulates the secretion of cytokines, such as TNF-α, IFN-γ, IL-2, and IL-6, which affects cholesterol metabolism (Mozaffari et al. 2012).
In addition to the antioxidant mechanism, it has been shown that melatonin improves lipid metabolism via gut microbiota communities in animals and humans. In high-fat diet-fed mice, oral administration of melatonin improves lipid metabolism and reverses microbiota dysbiosis of gut (Yin et al. 2018).
Obesity
Obesity is a major risk factor for cardiovascular diseases including hypertension, ischemic heart disease, and diabetes. Melatonin has shown antiobesity effects in different animal and human studies (Prado et al. 2018b; Nduhirabandi et al. 2014; Nduhirabandi et al. 2011). Melatonin supplementation lowers body weight and intra-abdominal visceral fat deposition. This antiobesogenic effect of melatonin is partly due to its regulatory role on the balance of energy and the regulation of the energy store. Moreover, its relationship with the physiological processes of wakefulness/sleep rhythm may impact body weight (Cipolla-Neto et al. 2014). Melatonin also changes the composition of the gut microbiota. It has been shown that high fat diet causes gut microbiota dysbiosis that contributes to obesity in mice. The antiobesity effects melatonin may be mediated by the reversing of high fat diet-induced gut microbiota (Xu et al. 2017).
The potential involvement of brown adipose tissue has also been suggested as another mechanism whereby animals gain weight in the absence of melatonin and lose weight in presence of melatonin (Tan et al. 2011). Brown adipose tissue burns calories for the purpose of heat production, thereby consuming glucose and fatty acids and limiting fat deposition (Richard and Picard 2011). Melatonin also regulates the adipocyte-derived bioactive factors such as adipokines. The dysregulated production or secretion of adipokines is seen in obesity (Favero et al. 2015). Moreover, melatonin is able to improve other complications associated with obesity, such as cutaneous symptoms in obese patients with acanthosis nigricans (Sun et al. 2018), periodontitis (Virto et al. 2018), and metabolic abnormalities (Nduhirabandi et al. 2011).
Melatonin and hypertension
Melatonin has an important role in the regulation of several cardiovascular parameters, including blood pressure, and is considered as a possible antihypertensive agent (Pechanova et al. 2014) In animal and human studies, melatonin has shown an effective and safe antihypertensive effect (Klimentova et al. 2016) (Simko et al. 2018). For example, in L-NAME-induced hypertension male Wistar rats, administration of melatonin (10 mg/kg/day) for 4 weeks, has blunted systolic blood pressure enhancement (Simko et al. 2018). In another study, 6-week administration of melatonin reduced systolic blood pressure in rats with continuous light-induced hypertension.
In human studies, both on healthy controls and patient populations of nocturnal and essential hypertension, melatonin intake has shown clinically significant hypotensive effects. Simko et al. found that melatonin attenuates hypertension caused by continuous light exposure (24 h/day) (Simko et al. 2014b). Continuous light leads to hypertension, left ventricle hypertrophy, increased oxidative stress in the left ventricle and aorta and left ventricle fibrosis. Melatonin treatment reduced these pathological changes. Melatonin also alleviates 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hypertension via decreasing vascular reactivity and renal oxidative stress (Ilhan et al. 2015). A double-blind, placebo-controlled study demonstrated that administration of 2.5 mg/day of melatonin for 3 weeks to hypertensive patients significantly reduced both systolic and diastolic blood pressure (Scheer et al. 2004). Treatment with 2–5 mg/day of melatonin for 7–90 days has decreased nocturnal systolic and diastolic blood pressure. In another related study, melatonin was also able to reduce blood pressure, circulating catecholamines and vascular reactivity in healthy volunteers (Pandi-Perumal et al. 2017a). Recently, a systematic review and meta-analysis of 8 randomized controlled trials, demonstrated that melatonin administration significantly decreases systolic and diastolic blood pressure in patients with metabolic disorders (Akbari et al. 2019).
Non-dipper hypertension
Melatonin also can be useful in hypertension with non-dipper pattern (Zeman et al. 2005). Hypertension can be divided into dipping and non-dipping pattern on the base of day-night blood pressure profile. In the non-dipping pattern, the physiological nocturnal fall in blood pressure is blunted which causes organ damage such as microalbuminuria, left ventricular hypertrophy and worsening the prognosis of cardiovascular events. The surge of melatonin production at night-time is missed in non-dipping hypertensive patients compared to hypertensive patients with normal night-time reduction of blood pressure (dipping pattern) (Dubielski et al. 2016). It has been suggested that repeated melatonin intake at night, can reduce nocturnal blood pressure due to its curing effect on the circadian output of the suprachiasmatic nucleus. It has been proposed that melatonin administration normalizes the regulatory function of this hormone on blood pressure (Pandi-Perumal et al. 2017a).
Non-dipping blood pressure is also a frequent finding in preeclampsia. In a study on 31 patients with preeclampsia, nighttime melatonin levels were significantly lower in comparison to normal pregnant women (48.4 ± 24.7 vs. 85.4 ± 26.9 pg/mL) (Bouchlariotou et al. 2014). A recent study has shown that melatonin attenuates the effects of light-at-night on body temperature and systolic blood pressure (Gubin et al. 2019). On the other hand, a recent randomized, placebo-controlled, crossover pilot study showed that night-time administration of high-dose (24 mg/day of a sustained-release formulation for 4 weeks) did not decrease nocturnal blood pressure in 40 patients with essential hypertension (Rahbari-Oskoui et al. 2019). It seems that further research projects are needed to qualify the effects of administration time on the melatonin antihypertensive properties.
Hypertension in adult offspring
There are also some studies that show maternal melatonin therapy can prevent hypertension in adult offspring. For example, in a study on Sprague-Dawley rats, maternal melatonin administration attenuated maternal high-fructose-induced hypertension in adult rat offspring. This beneficial effect of maternal melatonin therapy was related to regulating several pathways, including Prkaa2, Prkab2, Sirt1, Sirt4, Pparg, and Ppargc1a. Additionally, melatonin decreased plasma asymmetric dimethylarginine and increased the protein levels of mTOR (Tain et al. 2018b). Other studies show that methyl-donor diet-induced programmed hypertension in adult rat offspring is attenuated by maternal melatonin therapy (Tain et al. 2018a). Maternal melatonin therapy also prevents prenatal L-NAME-induced fetal programming of hypertension in adult rat offspring via regulation of hydrogen sulfide-generating pathway and renal transcriptome (Tain et al. 2016).
The precise mechanism(s) of hypotensive effect of melatonin is not fully understood. A recent research has proposed a key role of melatonin in neurovascular blood pressure regulation (Baker and Kimpinski 2018). There are also several pleiotropic receptor-independent properties of melatonin which have a possible impact on blood pressure regulations. These pleiotropic effects include antioxidant defense mechanisms, endothelium-dependent vasodilation, sympatho-vagal autonomic regulation (Baker and Kimpinski 2018), the reduction of myeloperoxidase activity (van der Zwan et al. 2010), preservation of renal L-arginine availability, restoration of the NO pathway by reduction of plasma ADMA, restoration of plasma AAR, (Tain et al. 2010), and sympathetic inhibition (blunting of the excessive catecholamine outflow) (Pechanova et al. 2016).
Totally, since melatonin acts favorably on hypertension has minimal side effects (as we discus below), it can be considered as a suitable alternative in the treatment of this widespread disease. However, additional studies using longer intervention periods and higher number of patients are required to qualify and quantify dosage, duration and time-dependent differences of melatonin effects and to clarify its underlying mechanisms (Gubin et al. 2019) and (Pechanova et al. 2014).
Pulmonary hypertension
Pulmonary hypertension is recognized by elevated pulmonary arterial pressure that eventually leads to right ventricular hypertrophy and heart failure (Vonk-Noordegraaf et al. 2013). Oxidative stress is one of the main mechanisms in the development of pulmonary hypertension (Masri et al. 2008). As discussed above, melatonin has a potent antioxidant activity, which can reduce antioxidant damage in cardiovascular tissues.
Five recent animal studies have suggested that melatonin can be useful in pulmonary hypertension. Maarman et al. reported that melatonin alleviates right ventricular hypertrophy and dysfunction in a rat model of pulmonary hypertension. It also reduces plasma oxidative stress and interstitial fibrosis (Maarman et al. 2015). In another study in pulmonary hypertensive newborn sheep, melatonin reduced pulmonary artery resistance and pressure and significantly reduced pulmonary oxidative stress markers, improved the vasodilator function of small pulmonary arteries and increased enzymatic and non-enzymatic antioxidant capacity (Torres et al. 2015). In Long Evans rats with monocrotaline-induced pulmonary hypertension, Maarman et al. reported that administration of melatonin for 5 days prior to or 14 days after the injection of monocrotaline, improved plasma oxidative stress parameters and right ventricular function and reduced cardiac interstitial fibrosis both in curative and preventive treatment. (Maarman et al. 2015). Another study in lambs (Ovis aries) showed that postnatal administration of melatonin decreases nitrotyrosine (as an oxidative stress marker) and reduces the cardiopulmonary response to hypoxia and the pathological vascular remodeling. (Astorga et al. 2018). Hung et al. evaluated the effect of melatonin (10 mg/kg/day) on the pulmonary hypertension and vascular remodeling in chronically hypoxic rats and found that melatonin significantly attenuated oxidative and inflammatory markers, the levels of RVSP, thickness of the arteriolar wall and increased in the eNOS phosphorylation in the lung (Hung et al. 2017).
Totally, melatonin seems to exert beneficial effects in pulmonary hypertension via antioxidant, anti-inflammatory, and vasodilation mechanisms. Clinical studies to evaluate the effect of melatonin on patients with pulmonary hypertension are needed.
Endothelial dysfunction
The endothelium, by synthesizing and releasing a variety of endothelium-derived relaxing factors, including NO, vasodilator prostaglandins and endothelium-dependent hyperpolarization factors, has an important role in modulating vascular tone. The reduced production of these endothelium-derived relaxing factors causes endothelial dysfunction. Increasing evidence has demonstrated that endothelial dysfunction is the cause of different cardiovascular diseases (Godo and Shimokawa 2017). There are some studies indicating melatonin prevents the endothelial dysfunction via different mechanisms. Most important mechanism is ameliorating the levels of NȮ (Rexhaj et al. 2015; Salmanoglu et al. 2016; Paulis et al. 2010). Hung et al. showed that melatonin restores the vascular responses in diabetic rats through ameliorating the levels of NȮ and inducing the expression of eNOS (Hung et al. 2013). Similar results have been reported by Salmanoglu et al. (Salmanoglu et al. 2016). Melatonin also diminishes blood homocysteine and asymmetric dimethyl arginine levels which are reliable markers of endothelial dysfunction (Kantar et al. 2015). Obstructive sleep apnea induces chronic intermittent hypoxia that is associated with endothelial dysfunction. In rats with chronic intermittent hypoxia, melatonin has protective effects against endothelial dysfunction via anti-inflammatory and antioxidant mechanisms. Melatonin reduces pro-inflammatory mediators (COX-2, TNF-α, and inducible NO synthase) and the expression of NADPH oxidase and adhesion molecules (E-selectin, ICAM-1, and VCAM-1) (Hung et al. 2013). The suppression of the Toll-like receptor 4/nuclear factor kappa B system in vessels with atherosclerotic damage is another mechanism for the protective effects of melatonin in endothelial dysfunction that has been shown in high-fat-fed rabbits (Hu et al. 2013). Nevertheless, it still needs to be demonstrated, whether melatonin can improve already established endothelial dysfunction in human studies.
Melatonin and heart diseases
The results of several studies suggest that low melatonin production is associated with a higher risk of cardiac disease such as coronary heart disease (CHD), left ventricular hypertrophy (Su et al. 2017), infarction, and congestive heart failure (Dominguez-Rodriguez et al. 2016). Exogenous melatonin administration, on the other hand, induces profound protective effects against different cardiac diseases including ischemia-reperfusion injury (Yu et al. 2017), (McMullan et al. 2016), diabetic cardiomyopathy (Zhou et al. 2018b; Kandemir et al. 2018), heart failure (Garakyaraghi et al. 2012; Simko et al. 2014a), aluminum phosphide-induced cardiotoxicity (Asghari et al. 2017), 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cardiac injury (Sarihan et al. 2015), elevated heart rate (Simko et al. 2016), and postural tachycardia syndrome (Green et al. 2014).
Coronary heart disease
In 48 male CHD patients, overnight urinary aMT6s levels (the major metabolite of melatonin in urine) were significantly lower in the patient group versus control group (Sakotnik et al. 1999). In another study on 15 CHD patients versus healthy controls, nighttime serum melatonin levels were more than 5 times lower in CHD patients in comparison to controls (Brugger et al. 1995). Similarly, in 16 patients with coronary artery disease, the secretion of melatonin was lower at 2 am, 4 am, and 8 am compared to controls (Yaprak et al. 2003). In comparing urinary aMT6s levels in patients with stable versus unstable angina and coronary disease, nocturnal urinary aMT6s levels in patients with unstable angina were significantly lower than both healthy controls and patients with stable angina.
Congestive heart failure
The urinary aMT6s excretions in 33 patients with severe CHF were significantly lower in comparison to 146 healthy controls. The mechanisms to explain why melatonin production is reduced in CHF remain to be fully elucidated. It has been proposed that a complex interaction between melatonin production, sympathetic receptor activity, and neuropeptide Y is involved. In addition, some of substances that are increased in CHF patients including prostaglandins, calcitonin-gene-related peptide, and vasopressin may exert a modulatory role on melatonin production (Baker and Kimpinski 2018).
Melatonin and arrhythmias
In an isolated perfused heart model in rat, melatonin has shown antiarrhythmic effects. Melatonin significantly increased the threshold to induce ventricular fibrillation. This protective effect of melatonin against malignant arrhythmias may be related to upregulation of cardiac cell-to-cell electrical coupling protein (connexin-43) especially in individuals with melatonin deficiency (Beňová et al. 2015). Similarly, in another study in SHR rats, melatonin has attenuated abnormal myocardial connexin-43 distribution in SHR, and upregulated connexin-43 mRNA, total connexin-43 protein, and its functional phosphorylated forms. Moreover, melatonin increased cardioprotective PKC expression in SHR rats. These findings indicate that melatonin has antiarrhythmias activity via the upregulation of myocardial connexin-43 and modulation of PKC-related cardioprotective signaling (Benova et al. 2013).
Melatonin also has indirect antiarrhythmic effects via its renal and cardiac protective actions. Melatonin prevents arrhythmogenic myocardial remodeling during unilateral ureteral obstruction via AT1 reduction and Hsp70-VDR increment that reduces oxidative stress, fibrosis, and apoptosis in rat heart (Prado et al. 2018a).
In a clinical study, melatonin has shown beneficial effects in age-dependent disturbances of cardiovascular rhythms in normotensive and hypertensive volunteers. Administration of melatonin (1.5 mg/day) at 22:30 h for 2 weeks attenuated the increased morning of heart rate (Gubin et al. 2016). On the other hand, there is a small clinical report indicating melatonin administration to two patients (with normal myocardium) for treating sleep disorders has shown proarrhythmic effect (premature ventricular contractions). The discontinuation of melatonin stopped premature ventricular contractions in both patients (de Vries et al. 2017).
Collectively, it seems that more studies are needed to determine whether melatonin exerts proarrhythmic or antiarrhythmic effects in human.
Myocardial infarctions
In patients with ST segment elevation myocardial infarctions who underwent primary percutaneous coronary intervention, there is a relation between the intra-platelet melatonin levels and the rate of angiographic no-reflow phenomenon (a phenomenon associated with impaired clinical outcomes). Intra-platelet melatonin levels are lower in patients with angiographic no-reflow compared to patients without no-reflow. Regression analysis shows that intra-platelet melatonin levels are the only predictor of angiographic no-reflow following MI (Dominguez-Rodriguez et al. 2010).
The mechanisms of these protective effects remain incompletely understood. Ghaeli et al. reported that in patients with ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention, the addition of melatonin to the standard treatment significantly reduces the level of creatine kinase-MB compared with the group receiving only standard therapy (Ghaeli et al. 2015). It has been suggested that melatonin may protect against ischemia-reperfusion injury by activating silent information regulator 1 (SIRT1) signaling in a receptor-dependent manner (Yu et al. 2015a). Another study also found that melatonin could protect adipose-derived mesenchymal stem cells (ADSCs) against hypoxia/serum deprivation injury by modulating the SIRT1 signaling pathway. Melatonin treatment also reduces the expression of the apoptotic proteins including acetylated p53, acetylated Ac-Fox01, acetylated nuclear factor kappa-light-chain-enhancer of activated B cells and B cell lymphoma 2-associated X protein, while increasing the expression of the antiapoptotic protein BCL-2 (Han et al. 2016). Similarly, melatonin improved the survival and function of ADSCs in a rat model of myocardial infarction. Its protective effects were due to increased expression of Cu/Zn superoxide dismutase and other antioxidant enzymes (Zhu et al. 2015). Melatonin also enhances Notch1/Hes1/Akt signaling and saves intracellular Trx system via upregulating Hes1, Notch1, N1ICD, and p-Akt expressions which ameliorates myocardial ischemia reperfusion injury (Yu et al. 2017). The activation of PKB, ERK1/2, and STAT-3 during reperfusion and inhibition of the MPTP, are other proposed mechanisms (Lochner et al. 2013). Melatonin suppresses the Syk/COX-1/SERCA axis, which sustains mitochondrial homeostasis, ameliorates myocardial apoptosis, and delays the development of diabetic cardiomyopathy (Zhou et al. 2018b; Xiong et al. 2018). In streptozotocin-induced diabetic cardiomyopathy in rats, melatonin has protective effects via phosphorylation of VEGF-A (Kandemir et al. 2018) and modulation of Mst1/Sirt3 signaling (Zhang et al. 2017) .
Collectively, there are strong evidences of reduced melatonin levels in various cardiovascular diseases. However, to identify whether reduced melatonin levels are the cause or consequence of such conditions, further research including larger scale studies with larger number of patients are needed. The therapeutic role of exogenous melatonin in cardiovascular diseases also needs further studies.
Atherosclerosis
There is no human study about the effectiveness of melatonin in the treatment of atherosclerosis. However, many animal studies show that melatonin is effective in the treatment of atherosclerosis (Rodella et al. 2013b; Li et al. 2019). Cheng et al. reported that melatonin reduces the number and area of atheromatous plaques in a rabbit model of atherosclerosis via modulating MAPK pathway. In addition to MAPK signaling, a recent study showed that melatonin decreases aortic endothelial permeability and atherosclerosis in a mouse model of diabetes by phosphorylation of myosin light-chain and decreasing the expression of MLCK (Cheng et al. 2015). Melatonin also decreases the upstream expression of extracellular signal-related kinase (ERK) and p38 (Tang et al. 2016). Similar results have been reported in a rabbit’s model of atherosclerosis. In this study, melatonin has reversed the increase of MLCK activity and expression via decreasing the levels of phosphorylated JNK, ERK, and p38 (Cheng et al. 2015). Zhu et al. found that via downregulating the expression of MT1 receptors, miR-29b promotes endothelial permeability and apoptosis in high-fat diet-fed apoE knockout mice (Zhu et al. 2014). The anti-inflammatory effects of melatonin improve cigarette smoke-induced restenosis in rat carotid arteries after balloon injury (Yang et al. 2014). Anti-inflammatory and antiproliferative properties of melatonin also have been shown in rat aortic smooth muscle cells. In these cells, melatonin reduces TNF-alpha-induced RASMC inflammation via decreasing VCAM-1 expression and NF-kappaB activity through the inhibition of P38 mitogen-activated protein kinase phosphorylation (Li et al. 2019). Another mechanism for anti-inflammatory properties of melatonin in vascular smooth muscles is inducing autophagy and attenuating NLRP3 inflammasome activation, which is mediated by the Sirt3/Parkin signaling pathway (Ma et al. 2018).
Melatonin dose and safety
Most of clinical trials have shown very low toxicity of melatonin over a wide range of doses (Pandi-Perumal et al. 2017b). In a systematic review on 51 controlled studies of melatonin supplementation in humans, 26 articles found no significant adverse effects while 24 studies reported at least one statistically significant adverse effect. Adverse effects were generally minor and short-lived. The most commonly reported adverse events were fatigue, mood change, and neurocognitive performance. A few studies noted endocrine (e.g., glucose metabolism and reproductive parameters) and cardiovascular (e.g., blood pressure and heart rate) adverse effects. Totally, it seems that the safety profile of oral melatonin supplementation in humans is generally favorable. By dosing in accordance with natural circadian rhythms, most of these adverse effects can be easily managed. (Foley and Steel 2019).
The dose of oral melatonin has ranged between 0.050 and 0.16 mg/kg in most studies (Navarro-Alarcon et al. 2014). However, the considerably larger doses of melatonin have been used in different clinical studies and found to be safe. For example, in the treatment of amyotrophic lateral sclerosis, patients received doses of 300 mg/day for up to 2 years (Weishaupt et al. 2006). In a phase I dose escalation study to assess the tolerability and pharmacokinetics in healthy volunteers, no adverse effects with the oral doses of 20, 30, 50, and 100 mg of melatonin were seen (except mild transient drowsiness with no effects on sleeping patterns) (Galley et al. 2014) (Pandi-Perumal et al. 2017b).
Melatonin analogs
The competence of melatonin as a drug is limited because of its poor oral bioavailability and short half-life. Regarding to the therapeutic efficacy of melatonin in a wide variety of diseases, the development of new selective ligands for melatonin receptors has become an active area of investigations during recent years. Several melatonin receptor agonists with increased receptor affinity or improved pharmacokinetics are under being development including indolic and nonindolic compounds (Carocci et al. 2014).
Although there are some synthetic MT receptor antagonists under investigations, the majority of non-selective MT1–MT2 receptor ligands, including drugs used in humans are agonists, including ramelteon, agomelatine, and tasimelteon (Fig. 3). Ramelteon and tasimelteon are pure MT receptor ligands, while agomelatine is also an antagonist at the 5-HT2C receptors, which contributes to its antidepressant action (Jockers et al. 2016b). Other non-selective MT receptor agonists include 6-hydroxymelatonin, 6-chloromelatonin, 2-iodomelatonin, UCM 793, GR 196429 and 2-methoxy-α,β-didehydro-agomelatine. This latter ligand shows ~ 3500-fold greater potency than melatonin in the melanophore aggregation assay (Jockers et al. 2016a).
Ramelteon (for insomnia), agomelatine (to treat depression), and tasimelteon (for treatment of sleep and circadian disturbances) are the approved analogs of melatonin and currently available as marketed drugs (Cecon et al. 2018). Piromelatine, Neu-P11, and TIK-301 are clinically under investigation compounds (Paulis et al. 2012). The results of preclinical studies show that melatonin receptor agonists can be considered promising agents for the treatment of cardiovascular-related pathologies (Carocci et al. 2014).
Regarding to that attention has been focused on the development of potent long-acting melatonin analogs (ramelteon, agomelatine, piromelatine, and tasimelteon) and considerably high doses of these analogs have been administered in clinical trials in comparison to melatonin itself, clinical trials on melatonin in the range of 50–100 mg/day are needed to further evaluate and compare the therapeutic value of melatonergic agents in cardiovascular diseases (Cardinali and Hardeland 2017).
Conclusion
In summary, the physiological and pharmacological importance of melatonin in prevention and improving cardiovascular risk factors as well as metabolic syndrome is relatively well defined. Many studies have demonstrated that melatonin has beneficial effects on hypertension, myocardial injury, ischemia reperfusion injury, pulmonary hypertension, vascular diseases, lipid metabolism, and other related disorders including the metabolic syndrome. It is therefore not surprising that melatonin, as an inexpensive and well-tolerated drug, has been strongly proposed in many cardiovascular diseases, especially with potential benefits in the reduction of ischemia–reperfusion injury and decreasing nocturnal blood pressure. Decreased production or secretion of melatonin is attributed to the development of hypertension, both essential and nocturnal. Melatonin, as a pleiotropic and multi-target molecule, has the advantage of exerting simultaneously a variety of effects and therefore it can replace the many drugs needed by the patient. The development of potent long-acting melatonin analogs (e.g., ramelteon, agomelatine, piromelatine, and tasimelteon) is another active area of investigations during recent years to increase the potency and selectivity of melatonin therapy. Ramelteon (for insomnia), agomelatine (to treat depression), and tasimelteon (for treatment of sleep and circadian disturbances) are the approved analogs of melatonin and currently available as marketed drugs.
Future prospective
Oral melatonin supplementation has shown promising results in clinical trials of essential and nocturnal hypertension; however, large-size clinical trials are needed to evaluate melatonin’s efficacy as a novel therapeutic intervention in treatment of this condition and also other cardiovascular diseases. In future clinical trials, the administration of larger doses of are needed, because in most previous studies, the dose has ranged between 0.050 and 0.16 mg/kg.
Although melatonin supplementation seems to be relatively safe in humans, but some patients may experience adverse events. The adjustment of dose and administration in accordance with the circadian rhythm of endogenous melatonin may reduce the likelihood of complications. In further clinical research projects, a more comprehensive focus on potential adverse events is required.
References
Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC et al (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71:2997–3025
Akbari M, Ostadmohammadi V, Mirhosseini N, Lankarani KB, Tabrizi R, Keshtkaran Z et al (2019) The effects of melatonin supplementation on blood pressure in patients with metabolic disorders: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens 33:202–209
Allagui MS, Hachani R, Saidi S, Feriani A, Murat JC, Kacem K, el feki A (2015) Pleiotropic protective roles of melatonin against aluminium-induced toxicity in rats. Gen Physiol Biophys 34:415–424
Andersson EA, Holst B, Sparso T, Grarup N, Banasik K, Holmkvist J et al (2010) MTNR1B G24E variant associates with BMI and fasting plasma glucose in the general population in studies of 22,142 Europeans. Diabetes 59:1539–1548
Asghari MH, Moloudizargari M, Baeeri M, Baghaei A, Rahimifard M, Solgi R, Jafari A, Aminjan HH, Hassani S, Moghadamnia AA, Ostad SN, Abdollahi M (2017) On the mechanisms of melatonin in protection of aluminum phosphide cardiotoxicity. Arch Toxicol 91:3109–3120
Astorga CR, González-Candia A, Candia AA, Figueroa EG, Cañas D, Ebensperger G et al (2018) Melatonin decreases pulmonary vascular remodeling and oxygen sensitivity in pulmonary hypertensive newborn lambs. Front Physiol 9:185
Bähr I, Mühlbauer E, Schucht H, Peschke E (2011) Melatonin stimulates glucagon secretion in vitro and in vivo. J Pineal Res 50:336–344
Baker J, Kimpinski K (2018) Role of melatonin in blood pressure regulation: an adjunct anti-hypertensive agent. Clin Exp Pharmacol Physiol 45:755–766
Barrett TJ, Murphy AJ, Goldberg IJ, Fisher EA (2017) Diabetes-mediated myelopoiesis and the relationship to cardiovascular risk. Ann N Y Acad Sci 1402:31–42
Benova T, Viczenczova C, Radosinska J, Bacova B, Knezl V, Dosenko V, Weismann P, Zeman M, Navarova J, Tribulova N (2013) Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can J Physiol Pharmacol 91:633–639
Beňová T, Viczenczová C, Radošinská J, Szeiffová Bačová B, Knezl V, Dosenko V et al (2015) The role of melatonin in protecting the heart against malignant arrhythmias. Cardiol Lett 24:60–69
Bouchlariotou S, Liakopoulos V, Giannopoulou M, Arampatzis S, Eleftheriadis T, Mertens PR et al (2014) Melatonin secretion is impaired in women with preeclampsia and an abnormal circadian blood pressure rhythm. Ren Fail 36:1001–1007
Brugger P, Marktl W, Herold M (1995) Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet 345:1408
Bubenik GA, Pang SF (1994) The role of serotonin and melatonin in gastrointestinal physiology: ontogeny, regulation of food intake, and mutual serotonin-melatonin feedback. J Pineal Res 16:91–99
Butun I, Ekmekci H, Ciftci O, Sonmez H, Caner M, Altug T, Kokoglu E (2013) The effects of different doses of melatonin on lipid peroxidation in diet-induced hypercholesterolemic rats. Bratisl Med J 114:129–132
Capsoni S, Viswanathan M, De Oliveira AM, Saavedra JM (1994) Characterization of melatonin receptors and signal transduction system in rat arteries forming the circle of Willis. Endocrinology 135:373–378
Cardinali DP, Hardeland R (2017) Inflammaging, metabolic syndrome and melatonin: a call for treatment studies. Neuroendocrinology 104:382–397
Carocci A, Catalano A, Sinicropi MS (2014) Melatonergic drugs in development. Clin Pharmac 6:127–137
Cecon E, Oishi A, Jockers R (2018) Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol 175:3263–3280
Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Brzozowski T, Konturek SJ, Korolczuk A (2014) Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol 65:75–82
Cheng X, Wan Y, Xu Y, Zhou Q, Wang Y, Zhu H (2015) Melatonin alleviates myosin light chain kinase expression and activity via the mitogen-activated protein kinase pathway during atherosclerosis in rabbits. Mol Med Rep 11:99–104
Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J Pineal Res 56:371–381
Contreras-Alcantara S, Baba K, Tosini G (2010) Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring) 18:1861–1863
de Vries LJ, Geczy T, Szili-Torok T (2017) Sleep medications containing melatonin can potentially induce ventricular arrhythmias in structurally normal hearts: a 2-patient report. J Cardiovasc Pharmacol 70:267–270
Djordjevic B, Cvetkovic T, Stoimenov TJ, Despotovic M, Zivanovic S, Basic J, Veljkovic A, Velickov A, Kocic G, Pavlovic D, Sokolovic D (2018) Oral supplementation with melatonin reduces oxidative damage and concentrations of inducible nitric oxide synthase, VEGF and matrix metalloproteinase 9 in the retina of rats with streptozotocin/nicotinamide induced pre-diabetes. Eur J Pharmacol 833:290–297
Dominguez-Rodriguez A, Abreu-Gonzalez P, Jimenez-Sosa A, Avanzas P, Bosa-Ojeda F, Kaski JC (2010) Usefulness of intraplatelet melatonin levels to predict angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol 106:1540–1544
Dominguez-Rodriguez A, Abreu-Gonzalez P, Piccolo R, Galasso G, Reiter RJ (2016) Melatonin is associated with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony. Int J Cardiol 221:359–363
Doosti-Irani A, Ostadmohammadi V, Mirhosseini N, Mansournia M, Reiter RJ, Kashanian M, Rahimi M, Razavi M, Asemi Z (2018) The effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 50:783–790
Dubielski Z, Zamojski M, Wiechecki B, Mozenska O, Petelczyc M, Kosior DA (2016) The current state of knowledge about the dipping and non-dipping hypertension. Nadcisnienie Tetnicze 20:33–43
Dubocovich ML (1995) Melatonin receptors: are there multiple subtypes? Trends Pharmacol Sci 16:50–56
Ekmekcioglu C (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 60:97–108
Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A (2016) A review of melatonin, its receptors and drugs. Eurasian J Med 48:135–141
Espino J, Pariente JA, Rodriguez AB (2011) Role of melatonin on diabetes-related metabolic disorders. World J Diabetes 2:82–91
Fathollahi A, Daneshgari F, Hanna-Mitchell AT (2015) Melatonin and its role in lower urinary tract function: an article review. Curr Urol 8:113–118
Favero G, Stacchiotti A, Castrezzati S, Bonomini F, Albanese M, Rezzani R, Rodella LF (2015) Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr Res 35:891–900
Foley HM, Steel AE (2019) Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther Med 42:65–81
Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR (2014) Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res 56:427–438
Garakyaraghi M, Siavash M, Alizadeh MK (2012) Effects of melatonin on left ventricular ejection fraction and functional class of patients with heart failure: a randomized, double-blind, placebocontrolled trial. J Res Med Sci 17:S13–S16
Gerush IV, Bevzo VV, Ferenchuk YO (2018) The effect of melatonin on lipid peroxide oxidation, oxidative modification of proteins and mitochondria swelling in the skeletal muscle tissue of rats under alloxan diabetes. Ukr Biochem J 90:62–69
Ghaeli P, Vejdani S, Ariamanesh A, Hajhossein Talasaz A (2015) Effect of melatonin on cardiac injury after primary percutaneous coronary intervention: a randomized controlled trial. Iran J Pharm Res 14:851–855
Godo S, Shimokawa H (2017) Endothelial functions. Arterioscler Thromb Vasc Biol 37:e108–e114
Gomes Domingos AL, Hermsdorff HHM, Bressan J (2019) Melatonin intake and potential chronobiological effects on human health. Crit Rev Food Sci Nutr 59:133–140
Green EA, Black BK, Biaggioni I, Paranjape SY, Bagai K, Shibao C, Okoye MC, Dupont WD, Robertson D, Raj SR (2014) Melatonin reduces tachycardia in postural tachycardia syndrome: a randomized, crossover trial. Cardiovasc Ther 32:105–112
Gubin DG, Gubin GD, Gapon LI, Weinert D (2016) Daily melatonin administration attenuates age-dependent disturbances of cardiovascular rhythms. Cur Aging Sci 9:5–13
Gubin D, Weinert D, Solovieva SV, Durov AM, Litvinova NS, Danilova LA et al (2019) Melatonin attenuates light-at-night effects on systolic blood pressure and body temperature but does not affect diastolic blood pressure and heart rate circadian rhythms. Biol Rhythm Res. https://doi.org/10.1080/09291016.2018.1564586
Hajam YA, Rai S, Basheer M, Ghosh H, Singh S (2018) Protective role of melatonin in streptozotocin induced pancreatic damages in diabetic wistar rat. Pak J Biol Sci 21:423–431
Han D, Huang W, Li X, Gao L, Su T, Li X, Ma S, Liu T, Li C, Chen J, Gao E, Cao F (2016) Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J Pineal Res 60:178–192
Heo JI, Yoon DW, Yu JH, Kim NH, Yoo HJ, Seo JA et al (2018) Melatonin improves insulin resistance and hepatic steatosis through attenuation of alpha-2-HS-glycoprotein. J Pineal Res 65:e12493
Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y (2013) Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-kappaB system in high-fat-fed rabbits. J Pineal Res 55:388–398
Hung MW, Kravtsov GM, Lau CF, Poon AMS, Tipoe GL, Fung ML (2013) Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J Pineal Res 55:247–256
Hung MW, Yeung HM, Lau CF, Poon AMS, Tipoe GL, Fung ML (2017) Melatonin attenuates pulmonary hypertension in chronically hypoxic rats. Int J Mol Sci 18:1125
Ilhan S, Atessahin D, Atessahin A, Mutlu E, Onat E, Sahna E (2015) 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced hypertension: the beneficial effects of melatonin. Toxicol Ind Health 31:298–303
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G et al (2016a) Update on melatonin receptors: IUPHAR Review 20. Br J Pharmacol 173:2702–2725
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G et al (2016b) Update on melatonin receptors: IUPHAR Review 20. Br J Pharmacol 173:2702–2725
Kandemir YB, Guntekin Ü, Tosun V, Korucuk N, Bozdemir MN (2018) Melatonin protects against streptozotocin-induced diabetic cardiomyopathy by the phosphorylation of vascular endothelial growth factor-A (VEGF-A). Cell Mol Biol 64:47–52
Kantar Ş, Türközkan N, Bircan FS, Pasąoʇlu OT (2015) Beneficial effects of melatonin on serum nitric oxide, homocysteine, and ADMA levels in fructose-fed rats. Pharm Biol 53:1035–1041
Klimentova J, Cebova M, Barta A, Matuskova Z, Vrankova S, Rehakova R, Kovacsova M, Pechanova O (2016) Effect of melatonin on blood pressure and nitric oxide generation in rats with metabolic syndrome. Physiol Res 65:S373–s380
Kurhaluk N, Bojkova B, Radkowski M, Zaitseva OV, Kyriienko S, Demkow U et al (2018) Melatonin and metformin diminish oxidative stress in heart tissue in a rat model of high fat diet and mammary carcinogenesis. Adv Exp Med Biol 1047:7–19
Lardone PJ, Álvarez-Sánchez N, Guerrero JM, Carrillo-Vico A (2014) Melatonin and glucose metabolism: clinical relevance. Curr Pharm Des 20:4841–4853
Li Y, Wu H, Liu N, Cao X, Yang Z, Lu B, Hu R, Wang X, Wen J (2018) Melatonin exerts an inhibitory effect on insulin gene transcription via MTNR1B and the downstream Raf1/ERK signaling pathway. Int J Mol Med 41:955–961
Li HY, Leu YL, Wu YC, Wang SH (2019) Melatonin inhibits in vitro smooth muscle cell inflammation and proliferation and atherosclerosis in apolipoprotein E-deficient mice. Oxidative Med Cell Longev 67:1889–1901
Lochner A, Huisamen B, Nduhirabandi F (2013) Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed) 5(E):305–315
Ma S, Chen J, Feng J (2018) Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid Med Cell Longev 2018:9286458
Maarman G, Blackhurst D, Thienemann F, Blauwet L, Butrous G, Davies N, Sliwa K, Lecour S (2015) Melatonin as a preventive and curative therapy against pulmonary hypertension. J Pineal Res 59:343–353
Masri FA, Comhair SA, Dostanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, Erzurum SC (2008) Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci 1:99–106
Mayo JC, Aguado A, Cernuda-Cernuda R, Álvarez-Artime A, Cepas V, Quirós-González I et al (2018) Melatonin uptake by cells: an answer to its relationship with glucose? Molecules 23:E1999
McMullan CJ, Rimm EB, Schernhammer ES, Forman JP (2016) A nested case-control study of the association between melatonin secretion and incident myocardial infarction. Heart 103:694–701
Moore RY (1996) Neural control of the pineal gland. Behav Brain Res 73:125–130
Mozaffari S, Hasani-Ranjbar S, Abdollahi M (2012) The mechanisms of positive effects of melatonin in dyslipidemia: a systematic review of animal and human studies. Int J Pharmacol 8:496–509
Mozos I (2017) Links between shift work, cardiovascular risk and disorders. In: He W, Yu L (eds) Shift Work: Impacts, Disorders and Studies. Nova Science Pub Inc., New York, pp 23–44
Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D (2012) Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J Pineal Res 53:166–179
Navarro-Alarcon M, Ruiz-Ojeda FJ, Blanca-Herrera RM, A-Serrano MM, Acuna-Castroviejo D, Fernandez-Vazquez G et al (2014) Melatonin and metabolic regulation: a review. Food Funct 5:2806–2832
Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A (2011) Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res 50:171–182
Nduhirabandi F, Huisamen B, Strijdom H, Blackhurst D, Lochner A (2014) Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J Pineal Res 57:317–332
Nduhirabandi F, Huisamen B, Strijdom H, Lochner A, Huisamen B (2017) Role of melatonin in glucose uptake by cardiomyocytes from insulin-resistant Wistar rats. Cardiovasc J Afr 28:362–369
Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F et al (2000) Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem 275:31311–31317
Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N (2013) Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab 98:337–344
Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP (2008) Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 85:335–353
Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, Dhandapany PS, Brown GM, Cardinali DP (2017a) Melatonin and human cardiovascular disease. J Cardiovasc Pharmacol Ther 22:122–132
Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K et al (2017b) Melatonin and human cardiovascular disease. J Cardiovasc Pharmacol Ther 22:122–132
Paulis L, Pechanova O, Zicha J, Liskova S, Celec P, Mullerova M et al (2010) Melatonin improves the restoration of endothelium-derived constricting factor signalling and inner diameter in the rat femoral artery after cessation of L-NAME treatment. J Hypertens 28:S19–S24
Paulis L, Simko F, Laudon M (2012) Cardiovascular effects of melatonin receptor agonists. Expert Opin Investig Drugs 21:1661–1678
Pechanova O, Paulis L, Simko F (2014) Peripheral and central effects of melatonin on blood pressure regulation. Int J Mol Sci 15:17920–17937
Pechanova O, Paulis L, Simko F (2016) Impact of melatonin on central blood pressure regulation. Act Nerv Super Rediviva 58:99–104
Peschke E, Bahr I, Muhlbauer E (2015) Experimental and clinical aspects of melatonin and clock genes in diabetes. J Pineal Res 59:1–23
Prado NJ, Casarotto M, Calvo JP, Mazzei L, Ponce Zumino AZ, Garcia IM et al (2018a) Antiarrhythmic effect linked to melatonin cardiorenal protection involves AT1 reduction and Hsp70-VDR increase. J Pineal Res 65:e12513
Prado NJ, Ferder L, Manucha W, Diez ER (2018b) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr Hypertens Rep 20:45
Rahbari-Oskoui FF, Abramson JL, Bruckman AM, Chapman AB, Cotsonis GA, Johnson SA, Bliwise DL (2019) Nighttime administration of high-dose, sustained-release melatonin does not decrease nocturnal blood pressure in African-American patients: results from a preliminary randomized, crossover trial. Complement Ther Med 43:157–164
Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z (2019a) Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr 38:191–196
Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z (2019b) Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr 38:191–196
Reiter RJ, Tan DX (2003) Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res 58:10–19
Reiter RJ, Tan D, Kim SJ, Manchester LC, Qi W, Garcia JJ, Cabrera JC, el-Sokkary G, Rouvier-Garay V (1999) Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech Ageing Dev 110:157–173
Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018) Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules (Basel, Switzerland) 23:E509
Reutrakul S, Sumritsopak R, Saetung S, Chanprasertyothin S, Chailurkit LO, Anothaisintawee T (2018) Lower nocturnal urinary 6-sulfatoxymelatonin is associated with more severe insulin resistance in patients with prediabetes. Neurobiol Sleep Circadian Rhythms 4:10–16
Rexhaj E, Pireva A, Paoloni-Giacobino A, Allemann Y, Cerny D, Dessen P, et al (2015). Prevention of vascular dysfunction and arterial hypertension in mice generated by assisted reproductive technologies by addition of melatonin to culture media. American journal of physiology. Heart and circulatory physiology 309(7): H1151-1156
Rezvanfar MR, Heshmati G, Chehrei A, Haghverdi F, Rafiee F, Rezvanfar F (2017) Effect of bedtime melatonin consumption on diabetes control and lipid profile. Int J Diabetes Dev Countries 37:74–77
Richard D, Picard F (2011) Brown fat biology and thermogenesis. Front Biosci (Landmark Ed) 16:1233–1260
Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI (2010) Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J Pineal Res 49:342–348
Rodella LF, Favero G, Foglio E (2013a) Front Biosci 5:119–129
Rodella LF, Favero G, Foglio E, Rossini C, Castrezzati S, Lonati C, Rezzani R (2013b) Vascular endothelial cells and dysfunctions: role of melatonin. Front Biosc (Elite Ed) 5:119–129
Roohbakhsh A, Shamsizadeh A, Hayes AW, Reiter RJ, Karimi G (2018) Melatonin as an endogenous regulator of diseases: the role of autophagy. Pharmacol Res 133:265–276
Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M (2014) Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37:1715–1719
Rybka J, Kędziora-Kornatowska K, Kupczyk D, Muszalik M, Kornatowski M, Kędziora J (2016) Antioxidant effect of immediate-versus sustained-release melatonin in type 2 diabetes mellitus and healthy controls. Drug Deliv 23:814–817
Sakotnik A, Liebmann PM, Stoschitzky K, Lercher P, Schauenstein K, Klein W, Eber B (1999) Decreased melatonin synthesis in patients with coronary artery disease. Eur Heart J 20:1314–1317
Salari Lak L, Heidari R, Nejati V (2011) Protective effects of melatonin on lipid profile in fructose induced Dyslipidemia. Iran J Endocrinol Metab 13:406–411
Salmanoglu DS, Gurpinar T, Vural K, Ekerbicer N, Dariverenli E, Var A (2016) Melatonin and L-carnitin improves endothelial disfunction and oxidative stress in type 2 diabetic rats. Redox Biol 8:199–204
Santos RMD, Marani F, Chiba FY, Mattera M, Tsosura TVS, Tessarin GWL, Pereira RF, Belardi BE, Pinheiro BCES, Sumida DH (2018) Melatonin promotes reduction in TNF levels and improves the lipid profile and insulin sensitivity in pinealectomized rats with periodontal disease. Life Sci 213:32–39
Sarihan ME, Parlakpinar H, Ciftci O, Yilmaz F, Sagir M, Yilmaz O, Ceker G (2015) Protective effects of melatonin against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cardiac injury in rats. Eur J Pharmacol 762:214–220
Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM (2004) Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43:192–197
Sharma S, Singh H, Ahmad N, Mishra P, Tiwari A (2015) The role of melatonin in diabetes: therapeutic implications. Arch Endocrinol Metab 59:391–399
Simko F, Bednarova KR, Krajcirovicova K, Hrenak J, Celec P, Kamodyova N, Gajdosechova L, Zorad S, Adamcova M (2014a) Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J Pineal Res 57:177–184
Simko F, Pechanova O, Repova Bednarova K (2014b) Hypertension and cardiovascular remodelling in rats exposed to continuous light: protection by ACE-inhibition and melatonin. Mediators Inflamm 2014:703175
Simko F, Baka T, Paulis L, Reiter RJ (2016) Elevated heart rate and nondipping heart rate as potential targets for melatonin: a review. J Pineal Res 61:127–137
Simko F, Baka T, Krajcirovicova K, Repova K, Aziriova S, Zorad S et al (2018) Effect of melatonin on the renin-angiotensin-aldosterone system in L-NAME-induced hypertension. Molecules (Basel, Switzerland) 23:E265
Simões D, Riva P, Peliciari-Garcia RA, Cruzat VF, Graciano MF, Munhoz AC, Taneda M, Cipolla-Neto J, Carpinelli AR (2016) Melatonin modifies basal and stimulated insulin secretion via NADPH oxidase. J Endocrinol 231:235–244
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351:152–166
Slominski AT, Semak I, Fischer TW, Kim TK, Kleszczynski K, Hardeland R et al (2017) Metabolism of melatonin in the skin: why is it important? Exp Dermatol 26:563–568
Stankov B, Capsoni S, Lucini V, Fauteck J, Gatti S, Gridelli B, Biella G, Cozzi B, Fraschini F (1993) Autoradiographic localization of putative melatonin receptors in the brains of two Old World primates: Cercopithecus aethiops and Papio ursinus. Neuroscience 52:459–468
Su H, Chen T, Li J, Xiao J, Wang S, Guo X, Bu P (2017) Correlations of serum cyclophilin a and melatonin concentrations with hypertension-induced left ventricular hypertrophy. Arch Med Res 48:526–534
Sun H, Wang X (2018) Melatonin treatment improves insulin resistance and pigmentation in obese patients with Acanthosis nigricans. Int J Endocrinol 2018:2304746
Sun H, Wang X, Chen J, Gusdon AM, Song K, Li L et al (2018) Melatonin treatment improves insulin resistance and pigmentation in obese patients with acanthosis nigricans. Int J Endocrinol 2018:2304746
Tain YL, Huang LT, Lin IC, Lau YT, Lin CY (2010) Melatonin prevents hypertension and increased asymmetric dimethylarginine in young spontaneous hypertensive rats. J Pineal Res 49:390–398
Tain YL, Lee CT, Chan JYH, Hsu CN (2016) Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal NG-nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am J Obstet Gynecol 215:636.e631–636.e672
Tain YL, Chan JYH, Lee CT, Hsu CN (2018a) Maternal melatonin therapy attenuates methyl-donor diet-induced programmed hypertension in male adult rat offspring. Nutrients 10:E1407
Tain YL, Leu S, Lee WC, Wu KLH, Chan JYH (2018b) Maternal melatonin therapy attenuated maternal high-fructose combined with post-weaning high-salt diets-induced hypertension in adult male rat offspring. Molecules (Basel, Switzerland) 23:E886
Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev 12:167–188
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20:18886–18906
Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ, Zhou Q, Wei W, Zhu HQ, Wang Y (2016) Melatonin attenuates aortic endothelial permeability and arteriosclerosis in streptozotocin-induced diabetic rats: possible role of MLCK- and MLCP-dependent MLC phosphorylation. J Cardiovasc Pharmacol Ther 21:82–92
Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, Fischer KM, Sussman MA, Miyamoto S, Gustafsson ÅB (2013) Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev 27:1365–1377
Torres F, Gonzalez-Candia A, Montt C, Ebensperger G, Chubretovic M, Seron-Ferre M et al (2015) Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J Pineal Res 58:362–373
van der Zwan LP, Scheffer PG, Teerlink T (2010) Reduction of myeloperoxidase activity by melatonin and pycnogenol may contribute to their blood pressure lowering effect. Hypertension 56:e34 author reply e35
Virto L, Cano P, Jiménez-Ortega V, Fernández-Mateos P, González J, Haugen HJ, Esquifino AI, Sanz M (2018) Melatonin as adjunctive therapy in the treatment of periodontitis associated with obesity. J Clin Periodontol 45:1336–1346
Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM (2013) Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62:D22–D33
Wang Z, Ni L, Wang J, Lu C, Ren M, Han W, Liu C (2016) The protective effect of melatonin on smoke-induced vascular injury in rats and humans: a randomized controlled trial. J Pineal Res 60:217–227
Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B et al (2006) Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res 41:313–323
Xiong FY, Tang ST, Su H, Tang HQ, Jiang P, Zhou Q et al (2018) Melatonin ameliorates myocardial apoptosis by suppressing endoplasmic reticulum stress in rats with long-term diabetic cardiomyopathy. Mol Med Rep 17:374–381
Xu J, Huang L (2017) Urinary 6-sulfatoxymelatonin level and breast cancer risk: systematic review and meta-analysis. Sci Rep 7:e5353
Xu P, Wang J, Hong F, Wang S, Jin X, Xue T et al (2017) Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res 62:e12399
Yang GH, Li YC, Wang ZQ, Liu B, Ye W, Ni L, Zeng R, Miao SY, Wang LF, Liu CW (2014) Protective effect of melatonin on cigarette smoke-induced restenosis in rat carotid arteries after balloon injury. J Pineal Res 57:451–458
Yaprak M, Altun A, Vardar A, Aktoz M, Ciftci S, Ozbay G (2003) Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int J Cardiol 89:103–107
Yin J, Li Y, Han H, Chen S, Gao J, Liu G et al (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res 65:e12524
Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai M, Yang Y, Chen W, Liu J, Yi W, Yang J, Yi D, Duan W, Yu S (2015a) Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res 59:376–390
Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang Y, Yang J, Yi D, Chen W, Wang X, Duan W, Jin Z, Yu S (2015b) Membrane receptor-dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: in vivo and in vitro studies. J Pineal Res 59:420–433
Yu L, Fan C, Li Z, Zhang J, Xue X, Xu Y et al (2017) Melatonin rescues cardiac thioredoxin system during ischemia-reperfusion injury in acute hyperglycemic state by restoring Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner. J Pineal Res 62:e12375
Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N, Carpi A, Carullo P, Pesce P, Sacerdoti D, Sarais C, Catalucci D, Krüger M, Mongillo M, Sandri M (2014) Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Investig 124:2410–2424
Zanoboni A, Forni A, Zanoboni-Muciaccia W, Zanussi C (1978) Effect of pinealectomy on arterial blood pressure and food and water intake in the rat. J Endocrinol Investig 1:125–130
Zanuto R, Siqueira-Filho MA, Caperuto LC, Bacurau RFP, Hirata E, Peliciari-Garcia RA, do Amaral FG, Marçal AC, Ribeiro LM, Camporez JP, Carpinelli AR, Bordin S, Cipolla-Neto J, Carvalho CR (2013) Melatonin improves insulin sensitivity independently of weight loss in old obese rats. J Pineal Res 55:156–165
Zeman M, Dulkova K, Bada V, Herichova I (2005) Plasma melatonin concentrations in hypertensive patients with the dipping and non-dipping blood pressure profile. Life Sci 76:1795–1803
Zhang M, Lin J, Wang S, Cheng Z, Hu J, Wang T et al (2017) Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res 63:e12418
Zhao YW, Zhao J, Pang QH (2017) Effect of melatonin on insulin and Gαi/o expression in rat insulinoma cell line. Sci Agric Sin 50:3429–3438
Zhou J, Wang D, Luo X, Jia X, Li MX, Laudon M, Zhang R, Jia Z (2018a) Melatonin receptor agonist piromelatine ameliorates impaired glucose metabolism in chronically stressed rats fed a high-fat diet. J Pharmacol Exp Ther 364:55–69
Zhou H, Yue Y, Wang J, Ma Q, Chen Y (2018b) Melatonin therapy for diabetic cardiomyopathy: a mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal 47:88–100
Zhu HQ, li Q, Dong LY, Zhou Q, Wang H, Wang Y (2014) MicroRNA-29b promotes high-fat diet-stimulated endothelial permeability and apoptosis in apoE knock-out mice by down-regulating MT1 expression. Int J Cardiol 176:764–770
Zhu P, Liu J, Shi J, Zhou Q, Liu J, Zhang X, du Z, Liu Q, Guo Y (2015) Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. J Cell Mol Med 19:2232–2243
Acknowledgments
The authors thank the Vice Chancellor of the Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Contributions
HH suggested the idea for the article; HH and MI provided the overall concept and framework of the manuscript; MI researched and identified appropriate articles, and wrote the manuscript; HH and GK revised the manuscript; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imenshahidi, M., Karimi, G. & Hosseinzadeh, H. Effects of melatonin on cardiovascular risk factors and metabolic syndrome: a comprehensive review. Naunyn-Schmiedeberg's Arch Pharmacol 393, 521–536 (2020). https://doi.org/10.1007/s00210-020-01822-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01822-4