Abstract
The ongoing worldwide obesity epidemic is paralleled by an elevated incidence of the metabolic syndrome, a disorder referred to as a clustering of metabolic abnormalities that increase the risk for cardiovascular disease and type 2 diabetes. Considered as a multifunctional molecule, the pineal gland hormone melatonin is also involved in body fat mass and energy metabolism regulation. A large body of evidence supports the beneficial effects of melatonin on the cardiovascular function in normal and pathophysiological conditions. However, melatonin’s role in cardiovascular risk factors such as obesity and other related disorders including the metabolic syndrome needs further investigations, particularly in humans. This chapter will address the effects of melatonin on the metabolic syndrome focusing on obesity and insulin-resistant conditions. Since cardiovascular disease is the primary outcome of the metabolic syndrome, the effects of melatonin on cardiovascular function will be also described focusing on normal and pathological conditions. In view of the current knowledge, we aim to reveal the potential clinical relevance of melatonin or melatonin receptor agonists in the setting of obesity-induced metabolic syndrome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiovascular disease
- Insulin resistance
- Melatonin
- Melatonin agonist
- Metabolic syndrome
- Obesity disorders
Introduction
Excessive food intake and reduced physical activity associated with modern lifestyle have led to the dramatic increase in the prevalence of obesity [1–3] which is paralleled by an elevated incidence of other metabolic disorders including, among others, the metabolic syndrome (MetS), type 2 diabetes (T2D), cardiovascular diseases (CVD), and some cancers [4–7]. The concept of MetS refers to the clustering of common metabolic alterations that increase the risk for CVD and T2D [8]. The most widely recognized MetS components include abdominal obesity, insulin resistance, raised blood pressure, atherogenic dyslipidemia, glucose intolerance, and a proinflammatory state [8]. Apart from an increased risk for CVD and T2D, additional numerous comorbidities have been observed in MetS including nonalcoholic fatty liver disease [9], reproductive disorders [10], obstructive sleep apnea syndrome [11], chronic kidney disease [12], osteoarthritis [13], periodontal diseases [14], some cancers [7], sleep/wake disturbances, as well as other circadian alterations [15, 16]. Furthermore, patients with MetS were recently indicated to be at high risk for neurological disorders such as depression and Alzheimer’s disease [17].
Epidemiological studies have shown that the prevalence of MetS continues to rise in most developed and developing countries, comprising 20–30 % of the adult population. Although not all obese people have the syndrome, the major driving force behind MetS prevalence is the actual obesity epidemic [4]. In 2008, more than 1.46 billion of the global adult population were overweight or obese [body mass index (BMI) ≥25 kg/m2] with more than 500 million among them being identified as obese (BMI ≥30 kg/m2) [1, 3], and it is predicted that by 2030 up to 58 % of the worldwide adult population (3.3 billion) could be either overweight or obese [18]. This alarming prevalence is not only a concern among adults, overweight and obesity prevalence is also dramatically increasing in children. In 2010, 43 million children (35 million in developing countries) were estimated to be overweight and obese, while 92 million were at risk of overweight [2]. Therefore, obesity and related metabolic abnormalities present serious socioeconomic challenges for both government and society [19].
Clinical approaches to treat or prevent obesity-induced MetS are still a challenging task. The pathophysiological mechanisms involved in the progression of obesity to MetS and other associated comorbidities are complex and not well understood. It is well established that genetic background, lifestyle behaviors, age, and gender contribute significantly in the development of obesity as well as MetS [20]. Several pathophysiological mechanisms associated with increased fat accumulation and insulin resistance have been proposed: adipose tissue dysfunction, generation of lipid metabolites, inflammation, and cellular stress (oxidative and endoplasmic reticulum stress) [21–23]. In this regard, the significance of elevated oxidative stress in obesity and its potential role in the development of insulin resistance and MetS have largely been studied by many workers [24–28].
Patients with MetS were reported to have a low serum antioxidant status with a concomitant increased levels of inflammatory markers compared with those without MetS [29, 30]. Interestingly, a higher dietary antioxidant intake has been shown to be beneficial in patients with MetS by preventing the body weight and abdominal fat gain during a 3-year follow-up [31]. Therefore, although the overall clinical relevance of conventional antioxidants (e.g., vitamins C and E, beta-carotene, zinc, and selenium) in metabolic diseases is still challenging [32], it appears that along with other substantial interventions (e.g., sustained lifestyle modification, calorie restriction, physical activity), the potential use of nonclassical antioxidants for an effective therapy to reduce or prevent obesity-related metabolic disorders is attracting many investigators.
In this chapter we consider the potential therapeutic role of melatonin in MetS. Besides its powerful antioxidant activities [33], melatonin has been shown to play an important role in metabolic regulation [34, 35]. Membrane melatonin receptors (MT1 and MT2) have been identified in the central and peripheral organs/tissues involved in the regulation of energy metabolism (e.g., hypothalamus, adipose tissue, pancreas, liver, skeletal muscle, heart, vessels, kidney) [36, 37]. It is well known that MetS features (e.g., obesity, insulin resistance, dyslipidemia, and hypertension) are more prevalent in the elderly [38, 39] where melatonin production levels decrease [40]. In addition to this, a positive association between melatonin suppression, sleep deprivation, and circadian rhythms system alteration has recently been described in obesity and MetS [15]. As consequence, the potential clinical use of either melatonin or its analogs in several pathological conditions appears exciting, and it is being used in aging and several metabolic diseases [41, 42].
We aim to review the current literature on the effects of melatonin and other melatonergic drugs in obesity and insulin-resistant conditions and the potential mechanisms underlying these effects. To better understand the overall activities of melatonin, the recent view of melatonin as a multifunctional molecule is summarized. Since CVD are the primary clinical outcome of the MetS, the effects of melatonin on cardiovascular function are also addressed. Because of space limitations, the description of obesity-associated oxidative stress, inflammation, and eventual antioxidant interventions as well as the role of melatonin in other features of MetS such as nonalcoholic fatty liver disease is not included.
Melatonin: A Multifunctional Molecule
Melatonin or 5-methoxy-N-acetyltryptamine is the neurohormone mainly produced by the pineal gland upon the activation of the suprachiasmatic nucleus (SNC) of the hypothalamus during the night (for details on biosynthesis and metabolism of melatonin, see [43]). It is a highly conserved indolamine found in almost all organisms including bacteria, algae, plants, fungi, insects, nematodes, and vertebrates including mammals [44]. In humans, the normal circulating melatonin levels vary between 1–10 pM and 43–400 pM, during the day and the night, respectively [45, 46]. The use of high doses of melatonin as frequently found in in vitro (1 μM–100 mM) or in vivo (1–300 mg/day) studies has been supported as a requirement to obtain therapeutic effects in some conditions [45, 47, 48].
Viewed as multifunctional molecule [49], melatonin is a small compound able to cross all morphological barriers and acts within every subcellular compartment due to its highly lipophilic and hydrophilic properties [47]. Since its isolation by Lerner et al. [50], apart from its classical role as a chronobiotic or endogenous synchronizer participating in the regulation of seasonal as well as circadian rhythm along with its sleep-inducing effects [43], melatonin has been shown to play a role in all most physiological functions in animals and humans [41, 49]. Indeed, melatonin has anti-excitatory, antioxidant, immunomodulatory, anti-inflammatory, oncostatic, and vasomotor properties [49, 51, 52]. However, besides its universal availability and, presumably, its presence in our daily food consumption [53], its potential beneficial effects and their underlying mechanisms are still vast and not fully explored [54].
Melatonin has powerful antioxidant properties with strong cytoprotective activities [33, 55]. It has been proved to be more effective than other classical antioxidants [56, 57] due to its multiple free radical scavenger cascades and its ability to stimulate the natural antioxidant capacity [58]. In addition to this, its metabolites have also free radical scavenging activities, and, moreover, melatonin does not have a pro-oxidant action [33, 59]. Recently, the efficacy of melatonin to improve oxidative stress-mediated metabolic disorders via its gene regulation has been shown to be linked to epigenetic mechanisms [60].
Besides its pineal production, melatonin is also secreted by wide variety of tissues including retina, thymus, spleen, heart, muscle, liver, stomach, intestine, placenta, testis, cerebral cortex, and striatum [47, 61–63]. Melatonin content in these tissues varies and decreases with age to a similar extent as the pineal melatonin production [61, 63]. Commonly, nocturnal melatonin production as shown by its circulating levels as well as its primary urinary metabolite, 6-sulfatoxymelatonin (aMT6s), is lowered in various pathologies characterized by increased oxidative stress such as neurological disorders, CVD, and T2D as well as obstructive sleep apnea syndrome [41, 64]. Importantly, circulating melatonin levels can be influenced by the diet [65, 66]. Melatonin was recently identified in common ingredients of the traditional Mediterranean diet [67] which has been reported to be efficient in improving MetS features such as waist circumference, the levels of triglycerides (TGs), high-density lipoprotein (HDL)-cholesterol (HDL-c), and blood pressure (BP) as well as glucose metabolism [68]. Interestingly, along with resveratrol, the minute amounts of melatonin present in red wine protect the heart against myocardial ischemia/reperfusion damage [69], indicating its therapeutic potential in CVD.
Melatonin and Cardiovascular Function
The Link Between Cardiovascular Function and Melatonin
The link between cardiovascular function and melatonin as well as the influence of endogenous melatonin on the cardiovascular function is well established [70]. Diurnal variations can be seen in BP, heart rate, cardiac output, and endothelial dilatory capacity of peripheral and coronary arteries, sympathetic activity, cardiac electrical stability, and platelet aggregation [70, 71]. In pathological conditions, adverse cardiovascular events including myocardial infarction [72], sudden cardiac death [73], and arrhythmias [74] have also been linked to the circadian rhythm in humans, having a higher incidence in the early morning hours, where circulating melatonin levels are considerably low [75].
The most important evidence linking melatonin to the cardiovascular function was the identification of membrane MT1 and MT2 receptors along with other intracellular binding sites such as the cytosolic quinone reductase 2 enzyme [also called the putative melatonin receptor 3 (QR2/MT3) and melatonin nuclear receptors (e.g., retinoic acid subfamily of orphan receptors (RORx)] in the heart and the arteries [76–78]. These receptors offer possibilities for melatonin signaling to interact with the cardiovascular function. Melatonin may indirectly affect cardiovascular function via MT receptors in the SCN which is known to modulate the cardiovascular function via multisynaptic autonomic neurons [79]. Melatonin may also influence the cardiovascular physiology via its direct actions on the peripheral intrinsic circadian clocks identified in both cardiomyocytes [78, 80] and vessels [81]. More surprisingly, melatonin has been shown to be also secreted within the heart [61, 63]. However, the exact role for this cardiac melatonin remains unknown.
Effects of Melatonin on the Heart
Animal studies showed that prolonged melatonin consumption under normal conditions affects cardiac metabolism, reduces the absolute and relative heart weights [82, 83], and increases its glycogen content [84] with no effects on heart function in vivo [85] and ex vivo [82]. Similarly, in healthy male volunteers, administration of 3 mg of melatonin had no effect on their heart rate [86].
Although raising circulating melatonin concentration by administration of exogenous melatonin does not appear to be harmful to the heart, the presence of very low circulating concentrations (as occurring during daytime) is essential and pinealectomy has profound effects on the heart. Surgical removal of pineal gland followed by 2 months of stabilization caused increase in serum cholesterol and cardiac malondialdehyde (MDA) levels as well as the heart weight. Other morphological changes such as increased myocardial fibrosis, myxomatous degeneration of the valves, and thickening of left atrial endocardium were also observed in the hearts isolated from these pinealectomized rats [87]. Importantly, despite failure to improve morphological alterations (presumably due to the short treatment time), melatonin administration (4 mg/kg/day) for 2 days reversed the changes in circulating cholesterol and cardiac MDA levels [87].
In pathological conditions, patients with coronary heart disease have impaired nocturnal melatonin secretion [75, 88] and subjects with myocardial infarction were reported to have reduced circulating melatonin levels [72]. Concordantly, a significant association between single nucleotide polymorphisms (SNPs) (rs28383653) of melatonin receptor type 1A (MT1A) and coronary artery disease has been demonstrated in a recent case–control study [89]. In a cohort of survivors of acute myocardial infarction, reduction of circulating melatonin was also associated with greater adverse remodeling [90]. As a consequence, reduced serum melatonin concentrations measured at admission were considered as an independent predictor of left ventricular remodeling [90]. Importantly, in a double-blind randomized clinical trial, melatonin supplementation (3 mg/day for 2 months) was able to improve left ventricular ejection fraction in patients with heart failure [91].
Melatonin and Experimental Myocardial Infarction
In view of the above, it is expected that melatonin treatment could play a clinically relevant role in the pharmacotherapy of ischemic heart disease. The role of oxidative stress and excessive free radical production in the pathogenesis of myocardial infarction as well as experimental ischemia/reperfusion damage is well established [92]. The ability of melatonin to attenuate ischemia/reperfusion damage in rodent hearts has been demonstrated in isolated hearts (in vitro) [69, 93–96], isolated cardiomyocytes [97], as well as in situ heart (in vivo) [98–100]. These beneficial actions were undiscovered by the observations that hearts from pinealectomized animals exhibited a bigger post-ischemic myocardial infarction compared to those from non-pinealectomized rats [101]. Melatonin was able to attenuate ventricular fibrillation and reduce infarct size and mortality rate of the pinealectomized rats [101]. Additional investigations in our own laboratory have shown that long-term effects of melatonin evaluated 1 day after melatonin administration (2.5 or 5.0 mg/kg, i.p.) or after oral administration for 7 days (20 or 40 μg/ml) were also cardioprotective, and this cardioprotection persisted for 2–4 days after withdrawal of treatment [102]. Finally, melatonin at either physiological or pharmacological doses, given before or after ischemia period, was able to protect the heart against myocardial ischemia/reperfusion damage (for review, see [103]).
Mechanism of Melatonin-Induced Cardioprotection
The mechanism underlying the beneficial effects of melatonin on the ischemic heart is complex and not yet fully explored. Several investigations agreed that melatonin protects the heart against ischemia/reperfusion injury directly via its antioxidant properties and indirectly via its free radical scavenging actions and stimulatory effects on antioxidant capacity activities, respectively [85, 104]. Other melatonin’s effects such as antiadrenergic, anti-inflammatory, and anti-excitatory [85, 105, 106] as well as the MT receptors may also be involved [102, 106, 107].
Although it was reported that patients with myocardial infarction have reduced circulating melatonin levels [72], experimental myocardial infarction was shown to increase circulating melatonin levels, followed by enhancement of MT receptors expression [107]. The observation that luzindole, a melatonin receptor antagonist, was able to suppress the cardioprotection induced by melatonin [102] stressed the importance of these receptors in cardioprotection. These events may affect the probability of the opening of mitochondrial permeability transition pore (MPTP): Petrosillo and co-workers [108] reported that melatonin protected the hearts against reperfusion injury by inhibiting the opening of the MPTP probably via prevention of cardiolipin peroxidation. Downstream signaling events include activation of the reperfusion injury salvage kinase (RISK) pathway (PI-3K, PKB/AKT, ERK1/2) and the protective survivor activating factor enhancement (SAFE) pathway (JAK/STAT-3) [103].
Additional studies have documented the beneficial effects of exogenous melatonin on the heart in physiological conditions such as aging [109] and in other pathophysiological conditions such as hyperthyroidism [110], cadmium-induced oxidative damage [111], and myocardial hypertrophy [112]. However, more investigations using melatonin agonists are warranted. To the best of our knowledge, only one study investigated a melatonin agonist on myocardial ischemia/reperfusion injury in mice: the melatonin receptor agonist 8-methoxy-2-propionamidotetralin which has no antioxidant activity was not found to be cardioprotective [113].
Melatonin and the Blood Vessels
Melatonin and Blood Pressure
Hypertension is more frequent in overweight or obese than in lean subjects [114, 115]. Melatonin’s ability to modulate and regulate BP and its potential therapeutic use in patients with hypertension have been a subject of interest for many investigators for several years [104, 116–119]. Early animal studies showed that pinealectomy caused a gradual and sustained elevation in arterial BP [120, 121], while chronic melatonin administration was able to reverse hypertension in the pinealectomized animals [122]. Additional animal investigations using spontaneously hypertensive rats confirmed this BP reduction following melatonin supplementation [104, 119]. Furthermore, the BP lowering effect of melatonin was demonstrated in healthy [119] as well as in hypertensive individuals [123]. A double-blind controlled clinical trial found that the bedtime melatonin (5 mg/day) ingestion for 4 weeks effectively reduced the BP in normotensive young subjects [124]. Acute oral melatonin (1–3 mg) was able to reduce BP of healthy male volunteers [86, 125] with a concomitant reduction of the aortic pulse wave velocity (PWV) which is considered as an important indicator of total cardiovascular risk estimation [125]. The PWV negatively correlated with diurnal levels of melatonin in young healthy men and women [126]. Importantly, low nocturnal melatonin production was suggested to be an independent pathophysiologic risk factor in the development of hypertension among young women [127]. A decrease in nocturnal melatonin levels indicating impairment of pineal melatonin secretion has consistently been observed in non-dipper hypertensive patients [128, 129].
In view of the above observations, melatonin supplementation is currently considered as a potential pharmacological agent in non-dippers or individuals with nocturnal hypertension and hypertensive heart disease [104]. Melatonin could also be administered in the elderly in order to attenuate the development of hypertension [130]. A recent meta-analysis of randomized controlled trials by Grossman et al. [117] indicated that melatonin administration (at 2–3 mg, controlled-release preparation, but not 5 mg, fast release) was effective to reduce nocturnal systolic and diastolic BP in patients with nocturnal hypertension. However, in patients with coronary artery disease, caution must be taken to monitor the circadian BP profile, before and during melatonin treatment, because of the danger of induction of arterial hypertension during daytime, hereby indicating a contraindication for melatonin in patients with “high normal” BP values [118]. The role of melatonin in the pathogenesis of hypertension has recently been demonstrated in MetS subjects [48, 131–133]. This is described in the section of obesity.
Mechanisms of BP Regulation by Melatonin
Unfortunately, early studies aimed at determining the effects of melatonin on vascular reactivity yielded controversial results [119]. In addition, involvement of intracellular signaling pathways further complicated matters. Melatonin causes a receptor-mediated reduction in cAMP and phosphatidylinositol-4,5-biphosphate (PIP2) hydrolysis, leading to vasoconstriction [134, 135]. However, improved nitric oxide (NO) signaling via enhancement of NOS activity and cGMP levels also appears to play an important role in melatonin-induced vasodilation [136, 137].
Other factors independent of NO pathway are also involved: for a 6-week treatment, only melatonin (10 mg/kg/day) but not antioxidant N-acetylcysteine (1.5 g/kg/day) was able to reduce the blood pressure of adult spontaneously hypertensive rats [138]. These results demonstrated thereby a possible involvement of additional mechanisms. This was later confirmed in renovascular hypertensive rats where hypertension caused a significant decrease in tissue antioxidant capacity and Na+, K+-ATPase activities, while MDA levels and myeloperoxidase (MPO) activity were increased [139]. In these rats, early or late administration of melatonin (10 mg/kg/day/i.p. for 9 or 6 weeks) not only lowered blood pressure but also improved the left ventricular function as well as the hypertensive profile by alleviating oxidative injury and increasing antioxidant capacity [139]. From these findings, it appears that melatonin might exhibit these effects presumably via both its direct antioxidant and receptor-mediated activities.
The overall regulation and modulation of BP is a complex mechanism with multifactorial aspects involving sympathetic neural mechanisms and central and peripheral nonneural factors including vasoactive substances and hormones. In this regard, while the role of endogenous melatonin on the BP is being recognized, the implication of neural vasomotor and alteration in renin-angiotensin-aldosterone system (RAAS) in the effects of melatonin reversing pinealectomy-induced hypertension is still hypothetic [140]. The potential antagonistic activities of angiotensin and melatonin in cardiovascular and metabolic diseases have been recently supported [141].
Furthermore, exogenous melatonin has been shown to differently affect the blood flow in humans depending on the vascular bed type or region [116]. For example, in healthy men and women, melatonin administration (3 mg) caused a reduction in renal blood flow and an increase in forearm blood flow with no effect on the cerebral blood flow [116, 142]. Consistently, this lack of effect on hemodynamic parameters (arterial and cerebral blood flows) was also reported in healthy men after acute melatonin premedication (0.2 mg/kg), suggesting that melatonin premedication may be safe under clinical conditions, such as postural changes, hemorrhage, and other operative stimuli in which arterial pressure decreases temporally [143].
Studies have also shown that these vascular effects could also differ depending on the type of experimental conditions. For example, in vitro melatonin caused vasoconstriction in porcine isolated coronary arteries [144], while in vivo intracoronary infusion of melatonin (70 pg/ml/min of coronary blood flow) in anesthetized pigs increased coronary blood flow and cardiac function through the beta-adrenoreceptors and NO pathways [137]. Similarly, intravenous infusion of melatonin (0.5 μg/kg/min) caused vasodilation in the umbilical vascular bed in pregnant sheep [145]. These controversial observations could be due to differences between the expression of MT1 and MT2 receptors in some vascular regions with eventual vasoconstriction for MT1 [144], vasodilation for MT2 [137], and involvement of the autonomic nervous system in in vivo experiments [137].
Although few clinical investigations on small number of patients have been done so far [123] and despite the controversies reported in experimental data, melatonin appears to be a suitable candidate for effective treatment for CVD and hypertension, in particular. It is well known that obesity increases the risk for the development of cardiovascular disorders [114]. These have been linked to derangement of melatonin’s circadian rhythm [70–75, 140]. As a consequence, the eventual role of melatonin in obesity and other cardiovascular risk factors is currently receiving much attention.
Melatonin and Obesity
Obesity, Circadian Rhythm, and Melatonin
Convincing evidence supports a link between the development of obesity (increased body fat accumulation) and a disrupted circadian system [15, 16, 146, 147]. Epidemiological and experimental studies have shown that the alteration of the circadian rhythm in obesity as shown by the decrease of the amplitude of daily pineal melatonin rhythm in shift workers [148], T2D patients [149], as well as in rats fed with a high-fat diet [150] is accompanied by alterations of other circulating metabolic factors including, among others, glucose, insulin, leptin, corticosterone, thyroid-stimulating hormone, prolactin, luteinizing hormone, and testosterone [148, 150]. Although the causal relationship between chronodisruption and obesity can be somehow considered as bidirectional [146], the supplementation of melatonin as well as melatonin agonists has been beneficial in resetting the circadian rhythm [43] and improving the obesity-related abnormalities [41, 151–153]. In addition to this, obesity was found to be associated with various comorbidities including sleep disorders, and melatonin or melatonergic drugs have been proved to be effective in treating sleep disorders [151].
Animal Studies
Body Weight and Fat Mass Regulation
Melatonin may play an important role in body weight regulation and energy metabolism. The involvement of melatonin in the regulation of body fat mass and energy metabolism was first observed in seasonal animals [154] and attributed to its role as regulator of seasonal and circadian rhythms [155]. In these seasonal animals, any increase in circulating melatonin levels due to photoperiodic changes or exogenous melatonin administration, depending on the animal species, was eventually associated with a reduction or an increase in body fat mass [154, 156]. Interestingly, nonseasonal animals like the obese Zucker rats exposed to long photoperiod conditions (characterized by a low nocturnal melatonin production) had an increased body mass gain compared to those exposed to short photoperiods [157]. In line with this, surgical removal of the pineal gland caused a reduction in circulating melatonin levels and an increase in body weight after 3 weeks in obese but not in normal rats [158]. When the postoperative period was extended to 2 months, the normal rats had also increased their body and heart weight [159]. These pinealectomy-induced changes could be prevented by melatonin (30 mg/kg/day, i.p. at 1 h before lights-out) for 3 weeks [158]. The same workers also showed that melatonin administered in the same manner was able to reduce high-fat-diet-induced body weight gain without affecting the total food intake [158]. In young normal rats a decrease in body weight and visceral fat mass was also noticed following 3 or 6 months of prolonged melatonin consumption in drinking water (4 μg/ml) [84, 160]. Similar effects have also been reported in normal middle-aged rats [161, 162] without altering food consumption [163]. These metabolic effects were independent of gonadal function [164].
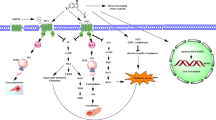
Several animal investigations have consistently demonstrated the efficacy for melatonin to prevent the development of obesity or reduce obesity-related metabolic features [46, 82, 152, 158, 165–168] (see Fig. 6.1a), and the potential therapeutic value of melatonin treatment in obesity and MetS has recently been summarized [169]. It was shown that long-term melatonin administration significantly reduced body weight and visceral fat mass as well as circulating glucose, insulin, leptin, TG, free fatty acid (FFA), and total cholesterol levels in young Zucker diabetic fatty (ZDF) rats [165, 170], in middle-aged rats fed with a high-fat diet [166, 171], in young rats with high-fat/high-sucrose diet [152], as well as rats drinking 10 % fructose solution [48]. In these studies, daily melatonin was administered in drinking water at 0.2–4 μg/ml [172] and 25 μg/ml [48, 166] or via intraperitoneal injection at 4 mg/kg [152] for a period of 8–12 weeks and did not affect the total food intake. A study in rabbits fed with a high-fat diet has however reported a reduction in food intake after 4 weeks of melatonin treatment (1 mg/kg/day subcutaneously at 2–3 h before lights-off) [167]. This weight-loss-inducing effect of melatonin was also confirmed in other animal models of obesity, for example, a rat model of ovariectomized-induced obesity [172, 173] and female rats treated with olanzapine [174].
(a) Obesity-induced metabolic abnormalities without melatonin treatment and (b) potential metabolic effects of melatonin in obesity and insulin resistance. Obesity induces systemic metabolic abnormalities associated with adipose tissue dysfunction and insulin resistance. Melatonin treatment reduces body weight and visceral fat gain of obese subjects. The overall melatonin’s effect in obesity is a combination of direct effects in peripheral organs involved in metabolism and indirect effects via systemic regulation. Melatonin reduces dyslipidemia, hyperglycemia, hyperinsulinemia, hyperleptinemia, oxidative stress, and inflammatory markers and increases adiponectinemia and antioxidants status. It increases insulin sensitivity in peripheral organs including liver, skeletal muscle, heart, vessels, and adipose tissue. Melatonin may affect indirectly the cardiovascular function (heart and vessels) via its effects on central nervous system reducing sympathetic activities. Melatonin may also affect indirectly the adipose tissue function and plasticity via sympathetic effects. The adipose tissue in turn may influence other peripheral organs via its secreted hormones (adipokines) such as adiponectin which has cardiovascular protective activities behind its insulin sensitizing properties. The overall effects will lead to body weight reduction and insulin sensitivity in peripheral organs and the improvement of cardiovascular functions. ?, no available data in obesity/MetS; ↓ or ↑, decrease or increase; solid lines (arrow), indirect systemic effects (from adipose tissue and other secretory organs, e.g., liver, pancreas); square dots (arrow), indirect sympathetic effects; BW body weight, MEL melatonin, IR insulin resistance, FFA free fatty acids, FA fatty acids, TG triglycerides, LDL-c low-density lipoprotein cholesterol, HDL-c high-density lipoprotein cholesterol, VLDL very low-density lipoprotein, TNF-α tumor necrosis factor alpha, IL-6 interleukin-6, NO nitric oxide, MI myocardial infarction, CHD coronary heart disease, ApoA-I,A-II apolipoprotein A-I and A-II, CRP C-reactive protein, HbA1c hemoglobin A1c, LPO lipid peroxidation, TBARS thiobarbituric acid reactive substances
Obesity-Induced Dyslipidemia
Melatonin has been shown to improve obesity-induced dyslipidemia. This was first documented in nonobese hypercholesterolemic rats [175, 176] and thereafter confirmed in various rat models of obesity [82, 158, 165, 169, 177]. For example, oral melatonin (4–10 mg/kg/day for 6–12 weeks) raised the HDL-c in both obese and lean Zucker rats [165] or in high-fat-/high-sucrose-diet-induced obese rats [152]. This has also been confirmed in obese rabbits [167], associated with a concomitant reduction in circulating TGs, FFA, and low-density lipoprotein cholesterol (LDL-c) with [152, 167] or without [165, 166] effect on total cholesterol levels. Similar results were recently confirmed in ApoE knockout C57BL/6 J male mice fed with a high-fat diet [177] and in rats fed with a high-fructose diet for 4 weeks [178]. Although the latter model did not gain weight, 2 weeks of coadministration of oral melatonin (10 mg/kg/day) reduced the intra-abdominal fat mass and circulating FFA levels as well as hepatic TG and cholesterol contents [178]. These studies strongly support the suggestion that melatonin supplement may ameliorate overweight and lipid metabolism in humans.
Obesity-Induced Low Antioxidant Status
Obesity is associated with elevated oxidative stress and low antioxidant status [179, 180]. The antioxidant as well as anti-inflammatory activities of melatonin have been well established [33, 60]. Daily melatonin administration to obese rabbits (1 mg/kg subcutaneously for 4 weeks) [167] or rats (4 mg/kg i.p. for 8 weeks) [152] increased the HDL-c levels, glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) activities and reduced oxidative stress as indicated by low plasma MDA levels. Furthermore, in young obese ZDF rats, chronic oral melatonin administration (10 mg/kg/day) for 6 weeks attenuated circulating biomarkers of systemic oxidative stress (basal plasma lipid peroxidation and Fe2+/H2O2-induced lipid oxidation) and low-grade inflammation [plasma interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) values] without affecting their profile in non-obese animals [181]. It is well established that low-grade chronic inflammation contributes to the pathogenesis of insulin resistance and diabetes as well as cardiovascular complications [182]. The antioxidant properties of melatonin have been reported along with the improvement of metabolic profile in diabetes [165, 181, 183] and diet-induced obesity [152, 167]. However, whether the improvement of insulin resistance by melatonin precedes or follows its suppressive effects on oxidative stress and inflammation is still not yet known.
Cardiovascular Effects of Melatonin in Obesity
Melatonin supplementation was able to improve cardiovascular function in obesity. A decrease in melatonin secretion was associated with hypertension in fructose-fed rats [131]. Administration of melatonin to these fructose-induced MetS rats reduced the BP rise and other metabolic abnormalities [48, 131]. Similarly, in animals receiving a high-fat diet, intake of melatonin was associated with a lowering of BP, heart rate, and sciatic nerve activity [167]. Melatonin prevented the appearance of fatty streaks produced by a mass of foam cells covered by the endothelium and a thin layer of mononucleated cells in the carotid artery intima of hypercholesterolemic rats [184]. It was also able to prevent deposition of fat in the liver and subintimal lipid in the blood vessels, kidney, and heart [167], indicating melatonin’s potential anti-atherosclerotic activities. In spontaneously hypertensive Wistar-Kyoto male rats, chronic (but not acute) administration of the selective melatonin receptor agonist, ramelteon, in drinking water (8 mg/kg/day, from 4 to 12 weeks of age) attenuated the age-associated increase of systolic BP by 45 % [153].
We recently showed that long-term oral melatonin consumption (4 mg/kg/day for 16 weeks) starting before the establishment of obesity prevented the increase in the heart weight and protected the hearts against obesity-induced increased susceptibility to myocardial ischemia/reperfusion damage [82]. We also found that melatonin treatment prevented the development of obesity-induced metabolic alterations (elevated visceral fat, serum insulin, leptin, and TG and reduced HDL-c) [82]. This finding was of particular significance since to date there is no effective cardioprotective strategy available in obesity, diabetes, as well as aging conditions [185–187]. However, how melatonin protected the heart in obesity remains unknown. We hypothesized that the direct effects of melatonin to the heart via its receptor-mediated effects may be involved, but this requires further investigation.
The melatonin-induced improvement of the cardiovascular function in MetS could also be linked to the body weight loss and improvement of dyslipidemia with eventual reduction of oxidative stress, inflammation, insulin resistance, and hyperglycemia [48, 82, 153, 167]. These two latter states are reviewed in the section of insulin resistance.
Human Studies
The overall circulating melatonin levels in obese humans are not consistent. For example, the mean nocturnal serum melatonin levels were reported to be reduced in patients with severe obesity [188] as well as with T2D [149], autonomic neuropathy [189], retinopathy [190], and coronary heart disease [191] as well as obese craniopharyngioma [192]. Surprisingly, despite a lack of difference in BMI or waist circumference between the obese nondiabetic and T2D subjects, nocturnal plasma melatonin levels were significantly higher in obese nondiabetic subjects compared to weight-matched T2D subjects [193] who failed to produce any detectable melatonin [193]. However, in young male and female obese MetS patients [194, 195] and in obese girls [196], circulating melatonin or urinary 6-sulfatoxymelatonin levels were not different from controls subjects, confirming the early observation that obesity had no effect on melatonin secretion and excretion [197]. Furthermore, it has been noticed that in MetS patients, the levels of melatonin per se are not as important as the melatonin/insulin ratio which correlates negatively with the lipid profile [194].
The reasons for these inconsistencies in melatonin secretion in obese subjects remain complex and not well understood. It is possible that the increased sympathetic tone in obesity and a consecutive alteration in sympathetic innervation of the pineal gland increased melatonin concentration in obese nondiabetic subjects [193]. Furthermore, low circulating melatonin levels have been linked to many factors including elevated oxidative stress and inflammation [198], but not by low testosterone levels in young men with MetS [195]. It appears therefore that melatonin circulating levels could vary depending on age of patients and severity of obesity [188].
Few clinical investigations on melatonin or melatonergic drugs have considered body weight change and adiposity mass in their aims. However, as discussed above, these parameters are affected by the long-term melatonin treatment [82]. A pilot study investigating the role of melatonin in obese craniopharyngioma survivors reported that low nocturnal and early morning melatonin levels were associated with increased daytime sleepiness and BMI, suggesting potential involvement of hypothalamic lesions [199]. Melatonin substitution (6 mg/day) in these subjects increased circulating melatonin and improved the sleep rhythms with no clear effect on BMI due probably to small sample size [199]. Interestingly, melatonin treatment (5 mg/day, 2 h before bedtime) in MetS patients significantly reduced their BMI, systolic BP, and plasma fibrinogen as well as lipid peroxidation levels after 1 month [132]. After 2 months, these patients had a further amelioration of BP and improved antioxidative capacity (e.g., catalase activity) and lipid profile (reduced LDL-c) [132].
Mechanism of Actions of Melatonin in Obesity
The mechanism of action of melatonin in obesity is complex and not well understood. As mentioned above, melatonin is a small pleiotropic molecule able to cross each membrane layer and enter each cellular compartment to exert its various activities with and/or without receptor-mediated pathways [47, 49]. Melatonin receptors as well as adipose tissue function and plasticity may be involved.
Melatonin Receptors
Involvement of the melatonin receptors in body fat mass regulation has been known for many years [200]. Administration of a melatonin receptor agonist or antagonist to seasonal animals (before night) affected the body weight and adiposity mass regulation as well as the onset of seasonal obesity: a melatonin agonist and the short day (6 h light/18 h dark) had a same effect, whereas an antagonist and the long day (18 h light/6 h dark) had also similar effects [200]. This involvement of MT receptors in the body weight and fat mass regulation was recently demonstrated in spontaneously hypertensive rats, using ramelteon, a potent selective MT1/MT2 receptors agonist [153], and in obese rats, using the melatonin agonist NEU-P11 [152].
MT receptors have been identified in the major organs involved in metabolism regulation: liver, pancreas, and skeletal muscle [201] as well as adipose tissue [202]. They have been involved in the regulation of insulin secretion and may play an active role in the glucose regulation [201]. Recently, it was reported that variation in MTNR1B, the gene encoding for MT2, was associated with increased risk of T2D, increased fasting plasma glucose, and impaired insulin secretion in populations of European ancestry [203, 204]. Similar observations were also made in Chinese [205] as well as in Japanese [206] populations. Thus, the overall effects of melatonin in obesity appear to be partly mediated through these receptors in addition to activation of the sympathetic nervous system via hypothalamic receptors and subsequent effects on lipolysis and adipose tissue plasticity [156, 207].
Adipose Tissue
The exact mechanisms whereby melatonin reduces body fat mass and the role of adipose tissue are complex and not clear. In vivo melatonin treatment prevents the increase in circulating TG and eventually body fat accumulation and weight gain in overweight and obese subjects [82, 170]. In vitro melatonin treatment of adipocytes inhibits differentiation and limits adipose tissue hypertrophy [208] by inhibiting fatty acid-induced TG accumulation in cells exposed to physiological levels of oleic acid [209]. The reduction in body weight gain might be due to a significant decrease in fat content as opposed to lean body mass [163] and could be related to melatonin-induced improvements in the compromised insulin and leptin signaling associated with obesity [210] accompanied by modulation of plasma levels of insulin, glucose, TG, cholesterol, and leptin [166] (see following section on “Leptin Resistance”).
The involvement of brown adipose tissue has also been suggested [35]. While white adipose tissue is specialized for energy storage, brown adipose tissue has a high concentration of mitochondria and uniquely expresses uncoupling protein 1(UCP-1), enabling it to be specialized for energy expenditure and thermogenesis [211]. Brown adipose tissue has been suggested to be the factor whereby animals lose weight in response to melatonin administration (and gain weight when there is a deficiency of melatonin) independently of food intake [35]. The exploitation of the functional role of brown adipose tissue could be of great interest in obesity management. Clearly, more research is required to elucidate the role of melatonin in weight loss.
Leptin Resistance
Leptin is one of adipose tissue-secreted hormones that plays a central role in modulation of food intake, body weight, and energy expenditure [212]. Leptin resistance is an essential feature of human obesity and refers to the inability of elevated circulating leptin levels to reduce common obesity [213]. It is associated with insulin resistance and an increased proinflammatory state [214]. Pinealectomy increases circulating leptin [215], while exogenous melatonin decreases serum leptin levels in both pinealectomized [216] and intact rat models of diet-induced obesity [163] before decreasing plasma insulin levels [171]. These observations suggest a secondary modulatory effect of leptin on insulin in body weight reduction [217]. However, increased leptin levels have also been observed following melatonin administration to normal and pinealectomized rats (3 mg/kg/day i.p. for 6 months) [218] and male C57BL/6 adult mice (10 μg/ml in drinking water for 1 month) [219]. In this regard, surprisingly, Baltaci and Mogulkoc [218] reported that pinealectomy decreased body weight gain and leptin levels. To further complicate matters, it has also been observed that melatonin had no effect on leptin levels in ovariectomized rats [172], obese horse [220], and menopausal women [221]. However, as expected, in a rat model of high-fructose-diet-induced MetS [178] and in young ZDF rats [165], melatonin administration reduced serum leptin levels. Apart from differences in experimental protocols and animal models, the causes of these controversial results remain unclear.
At a molecular level, the mechanism of leptin resistance and impaired leptin signaling has been associated with increased activity of suppressor of cytokine signaling 3 (SOCS3) [222, 223], which is a member of a family of proteins which inhibits the JAK/STAT signaling cascade [224]. It has been found that melatonin, leptin, and insulin activated the same intracellular signaling pathways, namely, PI-3K and STAT-3 [225–227]. Therefore, melatonin may attenuate or reverse the insulin resistance in obesity by mimicking the actions of insulin and leptin signaling via cross talk between these pathways. In this regard, insulin has been shown to modulate leptin-induced STAT3 activation in rat hypothalamus [225]. Thus, melatonin may act initially on hypothalamic insulin and leptin receptor sensitivity (as these hormones do under normal conditions) and eventually relay information about peripheral fat stores to central effectors in the hypothalamus to modify food intake and energy expenditure [207, 212]. It appears that an intricate relationship exists between leptin, melatonin, and insulin, synchronized in circadian fashion with profound effects on metabolism. However, this has not yet been studied in diet-induced obesity setting.
Melatonin and Insulin Resistance
Insulin resistance is the most important pathophysiological feature in the development of the MetS as well as T2D [228] and is referred to as a decrease or inhibition of cellular sensitivity to the effect of normal circulating insulin on glucose uptake, metabolism, and storage in peripheral tissues [21, 228]. Insulin resistance results in increased postprandial and fasting circulating insulin levels in order to normalize glycemia in prediabetic subjects and is closely associated with dyslipidemia and other metabolic abnormalities [228, 229]. It has recently been shown to be the best predictor of the metabolic syndrome in subjects with a first-degree relative with T2D [230]. Although not all forms of obesity result in insulin resistance [231], obesity (particularly abdominal obesity) is currently accepted as the major factor in the incidence and etiology of insulin resistance [21, 232], a condition which is generally considered as the common links between obesity and its vascular complications [229].
Effects of Melatonin on Insulin Resistance
Melatonin has been shown to play a role in the regulation of insulin secretion and glucose/lipid metabolism [183, 233, 234]. Studies have shown that in normal rats, pinealectomy-induced insulin resistance and glucose intolerance [235, 236] and increased serum cholesterol [87]. To demonstrate the role of endogenous melatonin on insulin secretion, the study done by Nishida et al. [237] using T2D rats found that after 21 weeks of pinealectomy, there was a significant increase in plasma insulin and accumulation of TG. The same study found also that when the post-pinealectomy period was extended to 35 weeks, circulating insulin levels were significantly decreased. This decrease is a clear indicator of impairment of insulin release from pancreatic β-cells as seen in patients at an advanced stage of T2D [238]. Additionally, it was found that pineal gland melatonin synthesis is decreased in T2D Goto-Kakizaki (GK) rats [239].
Since insulin resistance precedes the establishment of T2D, the possibility that melatonin replacement could reverse insulin resistance has been a subject of numerous investigators in the field of obesity and diabetes as well as MetS. In this regard, it was found that long-term melatonin consumption (2.5 mg/kg/day for 9 weeks) increased plasma melatonin levels with a concomitant reduction in insulin levels in T2D GK rats [240]. In mice fed with a high-fat diet, 8-week oral melatonin (100 mg/kg/day) markedly improved insulin sensitivity and glucose tolerance [210]. Using the same model, 2 weeks of melatonin administration (10 mg/kg/day i.p), attenuated insulin resistance, and glucose intolerance associated with an increase in hepatic glycogen and improvement in liver steatosis [168]. Furthermore, in high-fat-/high-sucrose-fed rats, 8-week treatment with melatonin or its agonist NEU-P11 increased insulin sensitivity [152]. In rats with T2D, 30 weeks of melatonin treatment (1.1 mg/kg/day, subcutaneously via implanted melatonin-releasing pellets) reduced circulating insulin, leptin, and TG levels [241]. These findings were also confirmed by additional studies in young ZDF rats [165, 170] and fructose-fed rats [48, 178]. In the latter model, administration of melatonin (1 or 10 mg/kg/day for 2 weeks) improved the abnormal serum insulin response curve in oral glucose tolerance test [178], indicating potential insulin sensitizing effects of melatonin.
Mechanism of Actions of Melatonin in Insulin Resistance
The mechanism of actions of melatonin on obesity-induced insulin-resistant state is complex and not fully explored. The reduction of circulating insulin levels in these obese animals may be linked to a reduced body weight and improved lipid metabolism as it was recently demonstrated in young ZDF rats [165] or rats drinking 10 % fructose solution [48]. In these rat models, the amelioration of insulin resistance was also characterized by improvement in glucose tolerance [48, 170]. In addition, in young ZDF rats melatonin treatment reduced fasting blood glucose, plasma insulin, hemoglobin A1c (HbA1c), HOMA-IR, and FFA levels and increased index of beta-cell function [170].
The improvement in lipid and glucose regulation could be also linked to amelioration of the proinflammatory state and oxidative stress [181]. It is well established that oxidative stress and proinflammatory states are important pathological features that underlie the development of insulin resistance, MetS, diabetes, and CVD. Therefore, in insulin-resistant condition, the reduction of oxidative stress and proinflammatory state may lead to the avoidance of lipid peroxidation resulting from free radical generation due to the continuous hyperglycemia and hyperlipidemia. As expected, melatonin administration for 2 or 6 weeks (1 or 10 mg/kg/day) attenuated the levels of circulating IL-6 and TNF-α [178, 181] accompanied by a reduction in serum and hepatic lipid peroxidation concentrations and increase in hepatic GSH concentration [178]. Interestingly, melatonin treatment was associated with increase in serum adiponectin levels and reduction in leptin levels with [170] or without [178] effects on body weight. Adiponectin (which is reduced in MetS subjects) has been shown to have insulin sensitizing actions in the liver and peripheral tissues and other beneficial properties associated with cardiovascular protection (antiapoptotic, anti-inflammatory, and antiatherogenic properties) [242]. Therefore, increased circulating adiponectin levels may play important role in melatonin’s effects.
On a molecular level, insulin resistance is associated with abnormal or compromised intracellular insulin signaling cascade in peripheral tissues/organs that are more involved in the glucose metabolism regulation (skeletal muscle, liver, and adipose tissue). This cascade principally includes binding of insulin to insulin receptor (IR), tyrosine phosphorylation of insulin receptor substrate (IRS) proteins, and activation of phosphotidylinositol-3-kinase (PI-3K), protein kinase B (PKB/Akt), and protein kinase C (PKC) isoforms (for details see [228]). Melatonin (1nM) treatment has been shown to stimulate glucose transport in skeletal muscle via the phosphorylation and activation of IRS-1 and PI-3K, respectively [243]. It was further demonstrated that melatonin improves glucose homeostasis by restoring the vascular actions of insulin which were characterized by increased phosphorylation of Akt and endothelial nitric oxide synthase (eNOS) in aortic tissue [210]. In addition to the phosphorylation of Akt and PKC-ζ, melatonin (1 nM) stimulated glycogen synthesis and increased the phosphorylation of glycogen synthase kinase 3 β (GSK3-β) in hepatic cells [168]. More interestingly, these effects of melatonin could be blocked by using the nonselective MT1/MT2 antagonist, luzindole, or the MT2 selective antagonist, 4-phenyl-2-propionamidotetralin (4P-PDOT) [168, 243], suggesting possible MT receptor involvement. However, it is not clear how activation of the high affinity MT receptors which are G-protein linked leads to stimulation of the IRS-1/PI-3K pathway and the role of PKC-ζ in this regard. In addition, the role of PKB/Akt is not clear in view of the different results that have been reported showing its activation in skeletal muscle cells [243] as opposed to its inactivation in hepatic cells [168].
Melatonin treatment (100 ng/ml and 500 pg/ml) enhanced the insulin-stimulated glucose uptake of adipocytes obtained from female fruit bat (Cynopterus sphinx) [244]. There was however no correlation between glucose uptake and the protein expression of glucose transporter 4 (GLUT-4) in these cells [244]. In this regard, investigation of GLUT4-translocation could give more insight in the results obtained. Pinealectomy was shown to reduce the expression of GLUT-4 protein translocation in adipose tissue [235, 236]. Although a decrease in GLUT-4 gene expression was reported following melatonin treatment (1 μM for 14 days) in human brown adipocyte cell lines (PAZ6) [202], Zanquetta et al. [235] found that 30 days of calorie restriction or melatonin replacement (50 μg/100 g/day i.p.) to pinealectomized rats was accompanied by improvement of insulin resistance and increased plasma membrane GLUT-4 protein content in white adipose tissue. Importantly, in the hyperthyroid rat heart, melatonin administration was able to protect the heart against oxidative damage and restored expression of GLUT-4 gene, establishing the ability of antioxidants to reverse oxidative stress-mediated metabolic alterations [110]. However, whether melatonin affects glucose regulation in the normal or obese heart is still not yet explored.
Melatonin receptors may play an important role in regulation of glucose metabolism. An important support for the role of melatonin in the regulation of energy metabolism came from the finding that removal of the MT1 receptor significantly impairs the ability of mice to metabolize glucose and probably induces insulin resistance in these animals [245]. Epidemiological studies have also revealed that variants near/in the MTNR1B (or MT2) receptor are associated with impaired pancreatic beta-cell function as shown by impaired early insulin secretion and concomitant elevated plasma fasting glucose levels [246, 247]. Indeed, MT1/MT2 receptors are expressed in pancreatic islets [248] and as insulin levels exhibit a nocturnal drop, its production has been suggested to be controlled, at least in part, by melatonin [249]. Melatonin reduced the fasting insulin levels [152, 171] probably via its inhibitory effects on insulin secretion in rat pancreatic islets [233, 250]. Catecholamines have been indicated as a key feature to understand the biological relevance of insulin-melatonin antagonisms in type 1 and T2D [251]. It was found that catecholamines (noradrenaline and adrenaline) and melatonin levels were reduced in T2D GK rats (characterized by high insulin levels) and elevated in T1D rats (associated with reduced insulin levels) [251], assuming that elevated catecholamines decrease insulin secretion via stimulation of melatonin synthesis [251].
Clinical Implications
The exploitation of melatonin’s inhibitory effect on insulin secretion by phototherapy as a potential therapy to increase insulin secretion has been effective in treating an insulin-dependent diabetes mellitus (IDDM) patient [252]. However, in the case of T2D, the exploitation of melatonin-insulin interaction as a potential therapy to reduce hyperinsulinemia is not currently suggested [234] before further large clinical studies.
Conclusions
The effects of melatonin in obesity and the MetS have been largely studied in experimental animals, particularly in rodents. Few clinical studies have considered the role of melatonin in obesity and MetS. However, available data show that melatonin treatment may influence and improve all metabolic abnormalities found in MetS patients (Fig. 6.1b). Similar effects have also been found following administration of melatonin agonists (ramelteon, NEU-P1). Behind its antioxidant properties, the overall metabolic action of melatonin is a combined result from its various pleiotropic activities associated with multiple signaling in areas of the central nervous system and in peripheral organs [49]. The current findings suggested melatonin treatment as a suitable candidate for effective therapy of CVD at both preventive and curative levels especially when circulating melatonin levels are decreased. In this regard, a randomized controlled trial of melatonin supplementation in men and women with the MetS has been recently designed to determine the feasibility, efficacy, and safety of melatonin supplementation in humans [253]. The use of high doses of melatonin compared to the physiological concentration has been explained as a requirement to obtain therapeutic effects in some conditions [45, 47, 48]. Melatonin is an affordable molecule having exceptional beneficial effects without toxicity [45].
References
Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14.
de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–64.
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–67.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36.
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang Y, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40.
Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJV. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15000 middle-aged men and women (the Renfrew–Paisley study). Eur Heart J. 2006;27:96–106.
Pothiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metab Syndr Relat Disord. 2009;7:279–88.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome. Circulation. 2009;120:1640–5.
Uchil D, Pipalia D, Chawla M, Patel R, Maniar S, Narayani, Juneja A. Non-alcoholic fatty liver disease (NAFLD)–the hepatic component of metabolic syndrome. J Assoc Physicians India. 2009;57:201–4.
Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism. 2013;62:457–78.
Wolk R, Somers VK. Sleep and the metabolic syndrome. Exp Physiol. 2007;92:67–78.
Ruan X, Guan Y. Metabolic syndrome and chronic kidney disease. J Diabetes. 2009;1:236–45.
Katz JD, Agrawal S, Velasquez M. Getting to the heart of the matter: osteoarthritis takes its place as part of the metabolic syndrome. Curr Opin Rheumatol. 2010;22:512–9.
Pietropaoli D, Monaco A, Del Pinto R, Cifone MG, Marzo G, Giannoni M. Advanced glycation end products: possible link between metabolic syndrome and periodontal diseases. Int J Immunopathol Pharmacol. 2012;25:9–17.
Reiter RJ, Tan D, Korkmaz A, Ma S. Obesity and metabolic syndrome: association with chronodisruption, sleep deprivation, and melatonin suppression. Ann Med. 2012;44:564–77.
Gomez-Abellan P, Madrid JA, Ordovas JM, Garaulet M. Chronobiological aspects of obesity and metabolic syndrome. Endocrinol Nutr. 2012;59:50–61.
Farooqui AA, Farooqui T, Panza F, Frisardi V. Metabolic syndrome as a risk factor for neurological disorders. Cell Mol Life Sci. 2012;69:741–62.
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431–7.
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25.
Naukkarinen J, Rissanen A, Kaprio J, Pietiläinen KH. Causes and consequences of obesity: the contribution of recent twin studies. Int J Obes. 2012;36:1017–24.
Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43.
Rizvi AA. Cytokine biomarkers, endothelial inflammation, and atherosclerosis in the metabolic syndrome: emerging concepts. Am J Med Sci. 2009;338:310–8.
de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–55.
Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15:1911–26.
Ozgen IT, Tascilar ME, Bilir P, Boyraz M, Guncikan MN, Akay C, Dundaroz R. Oxidative stress in obese children and its relation with insulin resistance. J Pediatr Endocrinol Metab. 2012;25:261–6.
Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radic Biol Med. 2009;47:213–8.
Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang Y, Zonderman AB. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr. 2011;141:903–13.
Beydoun MA, Canas JA, Beydoun HA, Chen X, Shroff MR, Zonderman AB. Serum antioxidant concentrations and metabolic syndrome are associated among U.S. adolescents in recent national surveys. J Nutr. 2012;142:1693–704.
Bahadoran Z, Golzarand M, Mirmiran P, Shiva N, Azizi F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr Metab. 2012;9:70.
Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol Sci. 2011;32:82–9.
Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43–50.
Korkmaz A, Topal T, Tan DX, Reiter RJ. Role of melatonin in metabolic regulation. Rev Endocr Metab Disord. 2009;10:261–70.
Tan DX, Manchester LC, FuentesBroto L, Paredes SD, Reiter RJ. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 2011;12:167–88.
Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10.
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–66.
Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11.
Mendes KG, Theodoro H, Rodrigues AD, Olinto MT. Prevalence of metabolic syndrome and its components in the menopausal transition: a systematic review. Cad Saude Publica. 2012;28:1423–37.
Reiter RJ. The ageing pineal gland and its physiological consequences. Bioessays. 1992;14:169–75.
Hardeland R. Melatonin in aging and disease – multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3:194–225.
Shatilo VB, Bondarenko EV, Antoniuk-Shcheglova IA. Metabolic disorders in elderly patients with hypertension and their correction with melatonin. Adv Gerontol. 2012;25:84–9.
Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61:383–410.
Hardeland R, Fuhrberg B. Ubiquitous melatonin—presence and effects in unicells, plants and animals. Trends Comp Biochem Physiol. 1996;2:25–45.
Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology. 2010;278:55–67.
Barrenetxe J, Delagrange P, Martinez JA. Physiological and metabolic functions of melatonin. J Physiol Biochem. 2004;60:61–72.
Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, GarciaCorzo L, Lopez LC, Reiter RJ, AcunaCastroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–27.
Cardinali DP, Bernasconi PA, Reynoso R, Toso CF, Scacchi P. Melatonin may curtail the metabolic syndrome: studies on initial and fully established fructose-induced metabolic syndrome in rats. Int J Mol Sci. 2013;14:2502–14.
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin–a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–84.
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587.
Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–6.
Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS J. 2006;273:2813–38.
Tan D, Hardeland R, Manchester LC, Korkmaz A, Ma S, RosalesCorral S, Reiter RJ. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 2012;63:577–97.
Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139–66.
Reiter RJ, Tan DX, Jou MJ, Korkmaz A, Manchester LC, Paredes SD. Biogenic amines in the reduction of oxidative stress: melatonin and its metabolites. Neuro Endocrinol Lett. 2008;29:391–8.
Sener G, Sehirli AÖ, Ayanoğlu-Dülger G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res. 2003;35:61–8.
Montilla-Lopez P, Munoz-Agueda MC, Feijoo Lopez M, Munoz-Castaneda JR, Bujalance-Arenas I, Tunez-Finana I. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer’s disease induced by okadaic acid in neuroblastoma cells. Eur J Pharmacol. 2002;451:237–43.
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42.
Tan DX, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: hypothesis and significance. J Pineal Res. 2007;43:317–20.
Korkmaz A, Rosales-Corral S, Reiter RJ. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012;503:1–11.
Sanchez-Hidalgo M, Montavez JMG, Carrascosa Salmoral MP, Gutierrez MCN, Lardone PJ, Lardone PJ, de la Lastra Romero CA. Decreased MT1 and MT2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging. J Pineal Res. 2009;46:29–35.
Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, Semmler J, Liebmann PM. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J Pineal Res. 2001;30:243–7.
Sanchez-Hidalgo M, de la Lastra CA, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, Guerrero JM. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp Gerontol. 2009;44:328–34.
Hernandez C, Abreu J, Abreu P, Castro A, Jimenez A. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J. 2007;30:496–500.
Sae-Teaw M, Johns J, Johns NP, Subongkot S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J Pineal Res. 2013;55:58–64.
Johns NP, Johns J, Porasuphatana S, Plaimee P, Sae-Teaw M. Dietary intake of melatonin from tropical fruit altered urinary excretion of 6-sulfatoxymelatonin in healthy volunteers. J Agric Food Chem. 2013;61:913–9.
Iriti M, Varoni EM, Vitalini S. Melatonin in traditional Mediterranean diets. J Pineal Res. 2010;49:101–5.
Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–313.
Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S. Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J Pineal Res. 2011;50:374–80.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res. 2010;49:14–22.
Takeda N, Maemura K. Circadian clock and cardiovascular disease. J Cardiol. 2011;57:249–56.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia MJ, Sanchez J, Marrero F, de Armas-Trujillo D. Decreased nocturnal melatonin levels during acute myocardial infarction. J Pineal Res. 2002;33:248–52.
Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–8.
Siegel D, Black DM, Seeley DG, Hulley SB. Circadian variation in ventricular arrhythmias in hypertensive men. Am J Cardiol. 1992;69:344–7.
Altun A, Yaprak M, Aktoz M, Vardar A, Betul UA, Ozbay G. Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X. Neurosci Lett. 2002;327:143–5.
Schepelmann M, Molcan L, Uhrova H, Zeman M, Ellinger I. The presence and localization of melatonin receptors in the rat aorta. Cell Mol Neurobiol. 2011;31:1257–65.
Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Holzenbein T, Markovic O, Leibetseder VJ, Strauss-Blasche G, Marktl W. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003;35:40–4.
Peliciari-Garcia RA, Zanquetta MM, Andrade-Silva J, Gomes DA, Barreto-Chaves ML, Cipolla-Neto J. Expression of circadian clock and melatonin receptors within cultured rat cardiomyocytes. Chronobiol Int. 2011;28:21–30.
Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709.
Durgan DJ, Young ME. The cardiomyocyte circadian clock. Circ Res. 2010;106:647–58.
Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1694–705.
Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res. 2011;50:171–82.
Bojkova B, Orendas P, Friedmanova L, Kassayova M, Datelinka I, Ahlersova E, Ahlers I. Prolonged melatonin administration in 6-month-old Sprague–Dawley rats: metabolic alterations. Acta Physiol Hung. 2008;95:65–76.
Kassayova M, Markova M, Bojkova B, Adamekova E, Kubatka P, Ahlersova E, Ahlers I. Influence of long-term melatonin administration on basic physiological and metabolic variables of young Wistar:Han rats. Biologia. 2006;61:313–20.
Patel V, Upaganlawar A, Zalawadia R, Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur J Pharmacol. 2010;644:160–8.
Kitajima T, Kanbayashi T, Saitoh Y, Ogawa Y, Sugiyama T, Kaneko Y, Sasaki Y, Aizawa R, Shimisu T. The effects of oral melatonin on the autonomic function in healthy subjects. Psychiatry Clin Neurosci. 2001;55:299–300.
Mizrak B, Parlakpinar H, Acet A, Turkoz Y. Effects of pinealectomy and exogenous melatonin on rat hearts. Acta Histochem. 2004;106:29–36.
Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345:1408.
SamimiFard S, AbreuGonzalez P, DominguezRodriguez A, JimenezSosa A. A case–control study of melatonin receptor type 1A polymorphism and acute myocardial infarction in a Spanish population. J Pineal Res. 2011;51:400–4.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Arroyo-Ucar E, Reiter RJ. Decreased level of melatonin in serum predicts left ventricular remodelling after acute myocardial infarction. J Pineal Res. 2012;53:319–23.
Garakyaraghi M, Siavash M, Alizadeh MK. Effects of melatonin on left ventricular ejection fraction and functional class of heart failure patients: a randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2012;17:S13–6.
Opie LH. Heart physiology from cell to circulation. Philadelphia: Williams & Wilkens; 2004.
Kaneko S, Okumura K, Numaguchi Y, Matsui H, Murase K, Mokuno S, Morishima I, Hira K, Toki Y, Ito T, Hayakawa T. Melatonin scavenges hydroxyl radical and protects isolated rat hearts from ischemic reperfusion injury. Life Sci. 2000;67:101–12.
Lagneux C, Joyeux M, Demenge P, Ribuot C, Godin-Ribuot D. Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 2000;66:503–9.
Szarszoi O, Asemu G, Vanecek J, Ost’adal B, Kolar F. Effects of melatonin on ischemia and reperfusion injury of the rat heart. Cardiovasc Drugs Ther. 2001;15:251–7.
Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El-Sokkary GH. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. J Pineal Res. 1998;25:184–91.
Salie R, Harper I, Cillie C, Genade S, Huisamen B, Moolman J, Lochner A. Melatonin protects against ischaemic-reperfusion myocardial damage. J Mol Cell Cardiol. 2001;33:343–57.
Lee YM, Chen HR, Hsiao G, Sheu JR, Wang JJ, Yen MH. Protective effects of melatonin on myocardial ischemia/reperfusion injury in vivo. J Pineal Res. 2002;33:72–80.
Sahna E, Acet A, Ozer MK, Olmez E. Myocardial ischemia-reperfusion in rats: reduction of infarct size by either supplemental physiological or pharmacological doses of melatonin. J Pineal Res. 2002;33:234–8.
Sahna E, Parlakpinar H, Turkoz Y, Acet A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res. 2005;54:491–5.
Sahna E, Olmez E, Acet A. Effects of physiological and pharmacological concentrations of melatonin on ischemia-reperfusion arrhythmias in rats: can the incidence of sudden cardiac death be reduced? J Pineal Res. 2002;32:194–8.
Lochner A, Genade S, Davids A, Ytrehus K, Moolman JA. Short- and long-term effects of melatonin on myocardial post-ischemic recovery. J Pineal Res. 2006;40:56–63.
Lochner A, Huisamen B, Nduhirabandi F. Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed). 2013;5:305–15.
Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann Med. 2010;42:276–85.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Clinical aspects of melatonin in the acute coronary syndrome. Curr Vasc Pharmacol. 2009;7:367–73.
Genade S, Genis A, Ytrehus K, Huisamen B, Lochner A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J Pineal Res. 2008;45:449–58.
Sallinen P, Manttari S, Leskinen H, Ilves M, Vakkuri O, Ruskoaho H, Saarela S. The effect of myocardial infarction on the synthesis, concentration and receptor expression of endogenous melatonin. J Pineal Res. 2007;42:254–60.
Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, Fiore T, Paradies G. Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2009;297:H1487–93.
Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res. 2010;48:340–6.
Ghosh G, De K, Maity S, Bandyopadhyay D, Bhattacharya S, Reiter RJ, Bandyopadhyay A. Melatonin protects against oxidative damage and restores expression of GLUT4 gene in the hyperthyroid rat heart. J Pineal Res. 2007;42:71–82.
Mukherjee R, Banerjee S, Joshi N, Singh PK, Baxi D, Ramachandran AV. A combination of melatonin and alpha lipoic acid has greater cardioprotective effect than either of them singly against cadmium-induced oxidative damage. Cardiovasc Toxicol. 2011;11:78–88.
Reiter RJ, Manchester LC, FuentesBroto L, Tan D. Cardiac hypertrophy and remodelling: pathophysiological consequences and protective effects of melatonin. J Hypertens. 2010;28:S7–12.
Chen Z, Chua CC, Gao J, Hamdy RC, Chua BH. Protective effect of melatonin on myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1618–24.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. American Heart Association, Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918.
Qin X, Zhang Y, Cai Y, He M, Sun L, Fu J, Li J, Wang B, Xing H, Tang G, Wang X, Xu X, Xu X, Huo Y. Prevalence of obesity, abdominal obesity and associated factors in hypertensive adults aged 45–75 years. Clin Nutr. 2013;32(3):361–7.
Cook JS, Sauder CL, Ray CA. Melatonin differentially affects vascular blood flow in humans. Am J Physiol Heart Circ Physiol. 2011;300:H670–4.
Grossman E, Laudon M, Zisapel N. Effect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trials. Vasc Health Risk Manag. 2011;7:577–84.
Rechcinski T, Trzos E, Wierzbowska-Drabik K, Krzeminska-Pakula M, Kurpesa M. Melatonin for nondippers with coronary artery disease: assessment of blood pressure profile and heart rate variability. Hypertens Res. 2010;33:56–61.
Paulis L, Simko F. Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol Res. 2007;56:671–84.
Karppanen H, Lahovaara S, Mannisto P, Vapaatalo H. Plasma renin activity and in vitro synthesis of aldosterone by the adrenal glands of rats with spontaneous, renal, or pinealectomy-induced hypertension. Acta Physiol Scand. 1975;94:184–8.
Zanoboni A, Zanoboni-Muciaccia W. Experimental hypertension in pinealectomized rats. Life Sci. 1967;6:2327–31.
Holmes SW, Sugden D. Proceedings: the effect of melatonin on pinealectomy-induced hypertension in the rat. Br J Pharmacol. 1976;56:360P–1.
Katsi V, Karagiorgi I, Makris T, Papavasileiou M, Androulakis AE, Tsioufis C, Tousoulis D, Stefanadis C, Kallikazaros IE. The role of melatonin in hypertension: a brief review. Cardiovasc Endocrinol. 2012;1:13–8.
Lusardi P, Preti P, Savino S, Piazza E, Zoppi A, Fogari R. Effect of bedtime melatonin ingestion on blood pressure of normotensive subjects. Blood Press Monit. 1997;2:99–103.
Yildiz M, Sahin B, Sahin A. Acute effects of oral melatonin administration on arterial distensibility, as determined by carotid-femoral pulse wave velocity, in healthy young men. Exp Clin Cardiol. 2006;11:311–3.
Yildiz M, Akdemir O. Assessment of the effects of physiological release of melatonin on arterial distensibility and blood pressure. Cardiol Young. 2009;19:198–203.
Forman JP, Curhan GC, Schernhammer ES. Urinary melatonin and risk of incident hypertension among young women. J Hypertens. 2010;28:446–51.
Zeman M, Dulková K, Bada V, Herichová I. Plasma melatonin concentrations in hypertensive patients with the dipping and non-dipping blood pressure profile. Life Sci. 2005;76:1795–803.
Jonas M, Garfinkel D, Zisapel N, Laudon M, Grossman E. Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press. 2003;12:19–24.
Simko F. Chronobiology of blood pressure: emerging implications of melatonin. Eur J Clin Invest. 2012;42:1252–4.
Leibowitz A, Peleg E, Sharabi Y, Shabtai Z, Shamiss A, Grossman E. The role of melatonin in the pathogenesis of hypertension in rats with metabolic syndrome. Am J Hypertens. 2008;21:348–51.
Kozirog M, Poliwczak AR, Duchnowicz P, KoterMichalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50:261–6.
Pechanova O, Parohova J, Vrankova S, Barta A, Kovacsova M, Janega P. 604 Effect of melatonin on blood pressure and fibrosis enlargement in the heart and aorta in experimental metabolic syndrome. J Hypertens. 2012;30:e177.
Witt-Enderby PA, Dubocovich ML. Characterization and regulation of the human ML1A melatonin receptor stably expressed in Chinese hamster ovary cells. Mol Pharmacol. 1996;50:166–74.
Dubocovich ML. Melatonin receptors: are there multiple subtypes? Trends Pharmacol Sci. 1995;16:50–6.
Anwar MM, Meki AR, Rahma HH. Inhibitory effects of melatonin on vascular reactivity: possible role of vasoactive mediators. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:357–67.
Grossini E, Molinari C, Uberti F, Mary DA, Vacca G, Caimmi PP. Intracoronary melatonin increases coronary blood flow and cardiac function through beta-adrenoreceptors, MT1/MT2 receptors, and nitric oxide in anesthetized pigs. J Pineal Res. 2011;51:246–57.
Pechanova O, Zicha J, Paulis L, Zenebe W, Dobesova Z, Kojsova S, Jendekova L, Sladkova M, Dovinova I, Simko F, Kunes J. The effect of N-acetylcysteine and melatonin in adult spontaneously hypertensive rats with established hypertension. Eur J Pharmacol. 2007;561:129–36.
Ersahin M, Sehirli O, Toklu HZ, Suleymanoglu S, EmekliAlturfan E, Yarat A, Tatldede E, Yegen BC, Sener G. Melatonin improves cardiovascular function and ameliorates renal, cardiac and cerebral damage in rats with renovascular hypertension. J Pineal Res. 2009;47:97–106.
Reiter RJ, Tan D, Korkmaz A. The circadian melatonin rhythm and its modulation: possible impact on hypertension. J Hypertens. 2009;27:S17–20.
Campos LA, Cipolla-Neto J, Amaral FG, Michelini LC, Bader M, Baltatu OC. The Angiotensin-melatonin axis. Int J Hypertens. 2013;2013:521783.
van der Helm-van Mil Annette HM, van Someren EJW, van den Boom R, van Buchem MA, de Craen AJM, Blauw GJ. No influence of melatonin on cerebral blood flow in humans. J Clin Endocrinol Metab. 2003;88:5989–94.
Bang J, Park YS, Jeong S, Song J, Kim Y, Hwang G. Melatonin does not attenuate dynamic cardiovascular and cerebrovascular reflex responses to acute hypotension in healthy men. Korean J Anesthesiol. 2012;63:245–52.
Tunstall RR, Shukla P, Grazul-Bilska A, Sun C, O’Rourke ST. MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries. J Pharmacol Exp Ther. 2011;336:127–33.
Thakor AS, Herrera EA, SeronFerre M, Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010;49:399–406.
Bray MS, Young ME. Chronobiological effects on obesity. Curr Obes Rep. 2012;1:9–15.
Boer-Martins L, Figueiredo VN, Demacq C, Martins LC, Consolin-Colombo F, Figueiredo MJ, Cannavan FP, Moreno Jr H. Relationship of autonomic imbalance and circadian disruption with obesity and type 2 diabetes in resistant hypertensive patients. Cardiovasc Diabetol. 2011;10:24.
Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171:557–64.
Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Muhlbauer E. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006;40:135–43.
Cano P, Jimenez-Ortega V, Larrad A, Reyes Toso CF, Cardinali DP, Esquifino AI. Effect of a high-fat diet on 24-h pattern of circulating levels of prolactin, luteinizing hormone, testosterone, corticosterone, thyroid-stimulating hormone and glucose, and pineal melatonin content, in rats. Endocrine. 2008;33:118–25.
Cardinali DP, Pagano ES, Scacchi Bernasconi PA, Reynoso R, Scacchi P. Disrupted chronobiology of sleep and cytoprotection in obesity: possible therapeutic value of melatonin. Neuro Endocrinol Lett. 2011;32:588–606.
She M, Deng X, Guo Z, Laudon M, Hu Z, Liao D, Hu X, Luo Y, Shen Q, Su Z, Yin W. NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res. 2009;59:248–53.
Oxenkrug GF, Summergrad P. Ramelteon attenuates age-associated hypertension and weight gain in spontaneously hypertensive rats. Ann N Y Acad Sci. 2010;1199:114–20.
Bartness TJ, Wade GN. Body weight, food intake and energy regulation in exercising and melatonin-treated siberian hamsters. Physiol Behav. 1985;35:805–8.
Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37.
Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med (Maywood). 2002;227:363–76.
Larkin LM, Moore BJ, Stern JS, Horwitz BA. Effect of photoperiod on body weight and food intake of obese and lean Zucker rats. Life Sci. 1991;49:735–45.
Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, Casteilla L, Penicaud L. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003;144:5347–52.
Kurcer Z, Sahna E, Olmez E. Vascular reactivity to various vasoconstrictor agents and endothelium-dependent relaxations of rat thoracic aorta in the long-term period of pinealectomy. J Pharmacol Sci. 2006;101:329–34.
Bojkova B, Markova M, Ahlersova E, Ahlers I, Adamekova E, Kubatka P, Kassayova M. Metabolic effects of prolonged melatonin administration and short-term fasting in laboratory rats. Acta Vet Brno. 2006;75:21–32.
Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999;140:1009–12.
Rasmussen DD, Mitton DR, Larsen SA, Yellon SM. Aging-dependent changes in the effect of daily melatonin supplementation on rat metabolic and behavioral responses. J Pineal Res. 2001;31:89–94.
Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141:487–97.
Puchalski SS, Green JN, Rasmussen DD. Melatonin effects on metabolism independent of gonad function. Endocrine. 2003;21:169–73.
Agil A, Navarro-Alarcon M, Ruiz R, Abuhamadah S, El-Mir MY, Vazquez GF. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 2011;50:207–12.
Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat? Fed rats. J Pineal Res. 2010;49:342–8.
Hussein MR, Ahmed OG, Hassan AF, Ahmed MA. Intake of melatonin is associated with amelioration of physiological changes, both metabolic and morphological pathologies associated with obesity: an animal model. Int J Exp Pathol. 2007;88:19–29.
Shieh JM, Wu HT, Cheng KC, Cheng JT. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J Pineal Res. 2009;47:339–44.
Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205:209–23.
Agil A, Rosado I, Ruiz R, Figueroa A, Zen N, Fernandez-Vazquez G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J Pineal Res. 2012;52:203–10.
Puchalski SS, Green JN, Rasmussen DD. Melatonin effect on rat body weight regulation in response to high-fat diet at middle age. Endocrine. 2003;21:163–7.
Sanchez-Mateos S, Alonso-Gonzalez C, Gonzalez A, Martinez-Campa CM, Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Melatonin and estradiol effects on food intake, body weight, and leptin in ovariectomized rats. Maturitas. 2007;58:91–101.
Baxi D, Singh PK, Vachhrajani K, Ramachandran AV. Melatonin supplementation therapy as a potent alternative to ERT in ovariectomized rats. Climacteric. 2012;15:382–92.
Raskind MA, Burke BL, Crites NJ, Tapp AM, Rasmussen DD. Olanzapine-induced weight gain and increased visceral adiposity is blocked by melatonin replacement therapy in rats. Neuropsychopharmacology. 2007;32:284–8.
Hoyos M, Guerrero JM, Perez-Cano R, Olivan J, Fabiani F, Garcia-Perganeda A, Osuna C. Serum cholesterol and lipid peroxidation are decreased by melatonin in diet-induced hypercholesterolemic rats. J Pineal Res. 2000;28:150–5.
Hussain SA. Effect of melatonin on cholesterol absorption in rats. J Pineal Res. 2007;42:267–71.
Zhang K, Lv Z, Jia X, Huang D. Melatonin prevents testicular damage in hyperlipidaemic mice. Andrologia. 2012;44:230–6.
Kitagawa A, Ohta Y, Ohashi K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J Pineal Res. 2012;52:403–13.
D’Archivio M, Annuzzi G, Vari R, Filesi C, Giacco R, Scazzocchio B, Santangelo C, Giovannini C, Rivellese AA, Masella R. Predominant role of obesity/insulin resistance in oxidative stress development. Eur J Clin Invest. 2012;42:70–78.
Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress In humans. Int J Obes (Lond). 2006;30:400–18.
Agil A, Reiter RJ, Jimenez-Aranda A, Iban-Arias R, Navarro-Alarcon M, Marchal JA, Adem A, Fernandez-Vazquez G. Melatonin ameliorates low-grade inflammation and oxidative stress in young zucker diabetic fatty rats. J Pineal Res. 2013;54:381–88.
de Rooij SR, Nijpels G, Nilsson PM, Nolan JJ, Gabriel R, Bobbioni-Harsch E, Mingrone G, Dekker JM, Relationship Between Insulin Sensitivity and Cardiovascular Disease (RISC) Investigators. Low-grade chronic inflammation in the relationship between insulin sensitivity and cardiovascular disease (RISC) population: associations with insulin resistance and cardiometabolic risk profile. Diabetes Care. 2009;32:1295–301.
Nishida S. Metabolic effects of melatonin on oxidative stress and diabetes mellitus. Endocrine. 2005;27:131–6.
Pita ML, Hoyos M, Martin-Lacave I, Osuna C, Fernández-Santos JM, Guerrero JM. Long-term melatonin administration increases polyunsaturated fatty acid percentage in plasma lipids of hypercholesterolemic rats. J Pineal Res. 2002;32:179–86.
Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–61.
Kloner RA, Schwartz LL. State of the science of cardioprotection: challenges and opportunities – proceedings of the 2010 NHLBI Workshop on Cardioprotection. J Cardiovasc Pharmacol Ther. 2011;16:223–32.
Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–7.
Shafii M, MacMillan DR, Key MP, Kaufman N, Nahinsky ID. Case study: melatonin in severe obesity. J Am Acad Child Adolesc Psychiatry. 1997;36:412–6.
Tutuncu NB, Batur MK, Yildirir A, Tutuncu T, Deger A, Koray Z, Erbas B, Kabakci G, Aksoyek S, Erbas T. Melatonin levels decrease in type 2 diabetic patients with cardiac autonomic neuropathy. J Pineal Res. 2005;39:43–9.
Hikichi T, Tateda N, Miura T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:655–60.
Cardinali DP, Cano P, Jimenez-Ortega V, Esquifino AI. Melatonin and the metabolic syndrome: physiopathologic and therapeutical implications. Neuroendocrinology. 2011;93:133–42.
Lipton J, Megerian JT, Kothare SV, Cho YJ, Shanahan T, Chart H, Ferber R, Adler-Golden L, Cohen LE, Czeisler CA, Pomeroy SL. Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology. 2009;73:323–5.
Mantele S, Otway DT, Middleton B, Bretschneider S, Wright J, Robertson MD, Skene DJ, Johnston JD. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS One. 2012;7:e37123.
Robeva R, Kirilov G, Tomova A, Kumanov P. Melatonin-insulin interactions in patients with metabolic syndrome. J Pineal Res. 2008;44:52–6.
Robeva R, Kirilov G, Tomova A, Kumanov P. Low testosterone levels and unimpaired melatonin secretion in young males with metabolic syndrome. Andrologia. 2006;38:216–20.
Lee J, Yoon J, Lee JA, Lee SY, Shin CH, Yang SW. Urinary 6-sulfatoxymelatonin level in girls and its relationship with obesity. Korean J Pediatr. 2012;55:344–9.
Rojdmark S, Berg A, Rossner S, Wetterberg L. Nocturnal melatonin secretion in thyroid disease and in obesity. Clin Endocrinol (Oxf). 1991;35:61–5.
Ozguner F, Koyu A, Cesur G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health. 2005;21:21–6.
Muller HL, Handwerker G, Gebhardt U, Faldum A, Emser A, Kolb R, Sorensen N. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control. 2006;17:583–9.
LeGouic S, Delagrange P, Atgie C, Nibbelink M, Hanoun N, Casteilla L, Renard P, Lesieur D, GuardiolaLemaitre B, Ambid L. Effects of both a melatonin agonist and antagonist on seasonal changes in body mass and energy intake in the garden dormouse. Int J Obes. 1996;20:661–7.
Muhlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71.
Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology. 2001;142:4264–71.
Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81.
Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94.
Ling Y, Li X, Gu Q, Chen H, Lu D, Gao X. A common polymorphism rs3781637 in MTNR1B is associated with type 2 diabetes and lipids levels in Han Chinese individuals. Cardiovasc Diabetol. 2011;10:27.
Tabara Y, Osawa H, Kawamoto R, Onuma H, Shimizu I, Makino H, Kohara K, Miki T. Genotype risk score of common susceptible variants for prediction of type 2 diabetes mellitus in Japanese: the Shimanami Health Promoting Program (J-SHIPP study). Development of type 2 diabetes mellitus and genotype risk score. Metabolism. 2011;60:1634–40.
Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol. 2001;281:R666–72.
Alonso-Vale MI, Peres SB, Vernochet C, Farmer SR, Lima FB. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBPbeta transcriptional activity. J Pineal Res. 2009;47:221–7.
Sanchez-Hidalgo M, Lu Z, Tan DX, Maldonado MD, Reiter RJ, Gregerman RI. Melatonin inhibits fatty acid-induced triglyceride accumulation in ROS17/2.8 cells: implications for osteoblast differentiation and osteoporosis. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2208–15.
Sartori C, Dessen P, Mathieu C, Monney A, Bloch J, Nicod P, Scherrer U, Duplain H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology. 2009;150:5311–7.
Townsend K, Tseng Y. Brown adipose tissue: recent insights into development, metabolic function and therapeutic potential. Adipocyte. 2012;1:13–24.
Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–85.
Scarpace PJ, Zhang Y. Leptin resistance: a predisposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–500.
Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation–current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–45.
Baydas G, Gursu F, Canpolat S, Konar V, Yasar A, Canatan H, Kelestimur H. Effects of pinealectomy on the circadian release pattern of leptin in male rat. Neuro Endocrinol Lett. 2001;22:449–52.
Canpolat S, Sandal S, Yilmaz B, Yasar A, Kutlu S, Baydas G, Kelestimur H. Effects of pinealectomy and exogenous melatonin on serum leptin levels in male rat. Eur J Pharmacol. 2001;428:145–8.
Morrison CD, Huypens P, Stewart LK, Gettys TW. Implications of crosstalk between leptin and insulin signaling during the development of diet-induced obesity. Biochim Biophys Acta. 2009;1792:409–16.
Baltaci AK, Mogulkoc R. Pinealectomy and melatonin administration in rats: their effects on plasma leptin levels and relationship with zinc. Acta Biol Hung. 2007;58:335–43.
Song YM, Chen MD. Effects of melatonin administration on plasma leptin concentration and adipose tissue leptin secretion in mice. Acta Biol Hung. 2009;60:399–407.
Buff PR, Morrison CD, Ganjam VK, Keisler DH. Effects of short-term feed deprivation and melatonin implants on circadian patterns of leptin in the horse. J Anim Sci. 2005;83:1023–32.
Cagnacci A, Malmusi S, Zanni A, Arangino S, Cagnacci P, Volpe A. Acute modifications in the levels of daytime melatonin do not influence leptin in postmenopausal women. J Pineal Res. 2002;33:57–60.
Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65.
Emilsson V, Arch JR, de Groot RP, Lister CA, Cawthorne MA. Leptin treatment increases suppressors of cytokine signaling in central and peripheral tissues. FEBS Lett. 1999;455:170–4.
Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56.
Carvalheira JB, Siloto RM, Ignacchitti I, Brenelli SL, Carvalho CR, Leite A, Velloso LA, Gontijo JA, Saad MJ. Insulin modulates leptin-induced STAT3 activation in rat hypothalamus. FEBS Lett. 2001;500:119–24.
Anhe GF, Caperuto LC, Pereira-Da-Silva M, Souza LC, Hirata AE, Velloso LA, Cipolla-Neto J, Carvalho CR. In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J Neurochem. 2004;90:559–66.
Picinato MC, Hirata AE, Cipolla-Neto J, Curi R, Carvalho CR, Anhe GF, Carpinelli AR. Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J Pineal Res. 2008;44:88–94.
Benito M. Tissue specificity on insulin action and resistance: past to recent mechanisms. Acta Physiol (Oxf). 2011;201:297–312.
Tesauro M, Cardillo C. Obesity, blood vessels and metabolic syndrome. Acta Physiol (Oxf). 2011;203:279–86.
Utzschneider KM, Van de Lagemaat A, Faulenbach MV, Goedecke JH, Carr DB, Boyko EJ, Fujimoto WY, Kahn SE. Insulin resistance is the best predictor of the metabolic syndrome in subjects with a first-degree relative with type 2 diabetes. Obesity (Silver Spring). 2010;18:1781–7.
McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53:495–9.
Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19:81–7.
Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40.
Peschke E, Muhlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab. 2010;24:829–41.
Zanquetta MM, Seraphim PM, Sumida DH, Cipolla-Neto J, Machado UF. Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J Pineal Res. 2003;35:141–8.
Lima FB, Machado UF, Bartol I, Seraphim PM, Sumida DH, Moraes SM, Hell NS, Okamoto MM, Saad MJ, Carvalho CR, Cipolla-Neto J. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol. 1998;275:E934–41.
Nishida S, Sato R, Murai I, Nakagawa S. Effect of pinealectomy on plasma levels of insulin and leptin and on hepatic lipids in type 2 diabetic rats. J Pineal Res. 2003;35:251–6.
Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T, Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85.
Frese T, Bach AG, Muhlbauer E, Ponicke K, Bromme HJ, Welp A, Peschke E. Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2009;85:526–33.
Peschke E, Schucht H, Muhlbauer E. Long-term enteral administration of melatonin reduces plasma insulin and increases expression of pineal insulin receptors in both Wistar and type 2-diabetic Goto-Kakizaki rats. J Pineal Res. 2010;49:373–81.
Nishida S, Segawa T, Murai I, Nakagawa S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Delta-5 desaturase activity. J Pineal Res. 2002;32:26–33.
Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–90.
Ha E, Yim SV, Chung JH, Yoon KS, Kang I, Cho YH, Baik HH. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res. 2006;41:67–72.
Banerjee A, Udin S, Krishna A. Regulation of leptin synthesis in white adipose tissue of the female fruit bat, Cynopterus sphinx: role of melatonin with or without insulin. Exp Physiol. 2011;96:216–25.
Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring). 2010;18(9):1861–3.
Kan MY, Zhou DZ, Zhang D, Zhang Z, Chen Z, Yang YF, Guo XZ, Xu H, He L, Liu Y. Two susceptible diabetogenic variants near/in MTNR1B are associated with fasting plasma glucose in a Han Chinese cohort. Diabet Med. 2010;27:598–602.
Tam CH, Ho JS, Wang Y, Lee HM, Lam VK, Germer S, Martin M, So WY, Ma RC, Chan JC, Ng MC. Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta cell function in Chinese subjects. PLoS One. 2010;5:e11428.
Peschke E, Fauteck JD, Musshoff U, Schmidt F, Beckmann A, Peschke D. Evidence for a melatonin receptor within pancreatic islets of neonate rats: functional, autoradiographic, and molecular investigations. J Pineal Res. 2000;28:156–64.
Mulder H, Nagorny CL, Lyssenko V, Groop L. Melatonin receptors in pancreatic islets: good morning to a novel type 2 diabetes gene. Diabetologia. 2009;52:1240–9.
Picinato MC, Haber EP, Carpinelli AR, Cipolla-Neto J. Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. J Pineal Res. 2002;33:172–7.
Peschke E, Hofmann K, Ponicke K, Wedekind D, Muhlbauer E. Catecholamines are the key for explaining the biological relevance of insulin-melatonin antagonisms in type 1 and type 2 diabetes. J Pineal Res. 2012;52:389–96.
Nieuwenhuis RF, Spooren PF, Tilanus JJ. Less need for insulin, a surprising effect of phototherapy in insulin-dependent diabetes mellitus. Tijdschr Psychiatr. 2009;51:693–7.
Terry PD, Goyal A, Phillips LS, Superak HM, Kutner MH. Design and rationale of a randomized controlled trial of melatonin supplementation in men and women with the metabolic syndrome. Open Access J Clin Trials. 2013;5:51–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Nduhirabandi, F., Lochner, A. (2014). Melatonin and the Metabolic Syndrome. In: Srinivasan, V., Brzezinski, A., Oter, S., Shillcutt, S. (eds) Melatonin and Melatonergic Drugs in Clinical Practice. Springer, New Delhi. https://doi.org/10.1007/978-81-322-0825-9_6
Download citation
DOI: https://doi.org/10.1007/978-81-322-0825-9_6
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-0824-2
Online ISBN: 978-81-322-0825-9
eBook Packages: MedicineMedicine (R0)