Abstract
With the aim of discovering potential cytotoxic agents, a series of benzochromene derivatives were screened for their cytotoxic activity against seven human cancer cell lines by standard 3-(4, 5-dimethyl thiazol)-2,5-diphenyl tetrazolium bromide (MTT) assay. Apoptosis, as the mechanism of cell death, was investigated morphologically by acridine orange/ethidium bromide staining and cell surface expression assay of phosphatidylserine by Annexin V-PE/7-AAD technique. The effects of compounds on reactive oxygen species (ROS) and nitric oxide (NO) generations in three human breast cancer cell lines were also studied. All compounds showed significant cytotoxic activity with inhibitory concentration (IC50) values in the micromolar range (4.6–21.5 μM). The results of apoptosis evaluation suggested that the cytotoxic activity of these compounds in breast cancer cells occurs via apoptosis. MCF-7 cell line showed higher levels of ROS and NO production after treatment with compounds. The increase in ROS production after 4 and 24 h indicated that one of the ways that these compounds can induce apoptosis is by increasing ROS generation. Cytotoxic and apoptotic effects of these compounds in human cancer cells indicated that they can be a good candidate for further pharmacological studies to discover effective anticancer agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery and development of new anticancer agents are required due to problems with currently available medicines, like toxicities and drug resistance (Zhang et al. 2005). Traditional anticancer drug discovery has focused on the identification of cytotoxic chemotherapeutic agents that could be of natural or synthetic origin (Lu et al. 2009). In the recent years, the biological activities of chromene derivatives were the subject of many research studies. Chromenes constitute the basic structural framework of many tannins and polyphenols widely found in green tea, fruits, vegetables, and red wine (Van Rensburg et al. 1997). The presence of the chromene-containing structure has been related with the capability to prevent various diseases (O'Kennedy et al. 1997). Synthetic analogues of chromenes have attracted considerable attention due to their diverse and broad spectrum of biological activity including antimicrobial (Khafagy et al. 2002), antitumoral (Mohr et al. 1975), antiviral (Dell et al. 1993), antihypertensive (Bedair et al. 2000), local anesthetic (Longobardi 1990), and antiallergic activities (Augstein et al. 1976). Chromenes have been identified to inhibit proliferation of human cancer cell lines, whereas less or no toxic to human normal cells (Musa et al. 2010).
The cytotoxic activity of many chemotherapeutic drugs is a result of their potential in apoptosis induction (Huang et al. 1995; Safavi et al. 2012). Apoptosis is defined as programmed cell death that occurs during development, differentiation, in tumor cell deletion and in response to a variety of insults such as cytotoxic molecules or compounds (Lu et al. 2009; Xie et al. 2003). Cytotoxic drug-induced damage to the cells, especially to the DNA, triggers apoptosis through either the extrinsic pathway or the intrinsic pathway (Lu et al. 2009). The extrinsic pathway is initiated at the plasma membrane by the interaction of cell-surface death receptors with their ligands (Lu et al. 2009; Mahdavi et al. 2011). In contrast, intrinsic pathway, also known as the mitochondrial pathway, is triggered through the release of cytochrome c by mitochondria within the cell (Lu et al. 2009; Elmore 2007).
Reactive oxygen species (ROS) generation is one of the signs observed in cells subjected to anticancer drug treatment (Meshkini et al. 2012). In contrast to their role on promoting cell growth under non-stress conditions, ROS are powerful inducers of apoptosis when cells are under stress (Polyak et al. 1997; Benhar et al. 2002; Brodska et al. 2011). Along with ROS generation, DNA damage has usually been observed during the process of cell death. These two phenomena, the increase of ROS levels and DNA damage, can be found either independent or one being caused by the other one (Brodska et al. 2011).
Nitric oxide, which is one of the smallest biological products of mammalian cells, plays various roles such as an intracellular or transcellular messenger and apoptosis inducer in cells (Thomsen et al. 1998). Induction of apoptosis by nitric oxide (NO) was first observed in normal and tumor cells, which have been confirmed by numerous laboratories using diverse cell systems (Bonfoco et al. 1995; Sarih et al. 1993; Xie et al. 1993). Apoptosis induction by cytotoxic compounds that increase endogenous NO can be considered as a therapeutic strategy (Xie et al. 2003). Experimental evidence suggests that NO induces apoptosis by altering the expression and function of p53 and Bcl-2 proteins (Xie et al. 2003).
In this study, a series of synthetic benzochromene derivatives have been screened for their cytotoxicity activity against a panel of seven human cancer cell lines. Since apoptosis is described as the main mode of cell death induced by a variety of stimuli such as drugs, so, we evaluated the ability of synthetic compounds to induce apoptosis in human cancer cell lines. The ROS and NO generations on three human breast cancer cell lines including MDA-MB-231, MCF-7, and T-47D were also studied after treatment with synthetic compounds.
Methods
Materials
The cell culture medium (RPMI 1640), fetal bovine serum (FBS), and penicillin–streptomycin were purchased from Gibco BRL (Life Technologies, Paisley, Scotland). The culture plates were purchased from Nunc (Brand products, Denmark). 3-(4, 5-dimethyl thiazol)-2,5-diphenyl tetrazolium bromide (MTT), ethidium bromide, acridine orange, VCl3, Griess reagent, and 2',7'-dichlorofluorescein diacetate (DCFH-DA) were obtained from Sigma Chemical Company (Germany). Annexin V-PE kit was purchased from BD Pharmingen.
Compound synthesis
Previously, we reported synthesis of 2-amino-4H-2 chromene scaffold in solvent-free condition (Naimi-Jamal et al. 2010). 2-Amino-4H-chromene derivatives were synthesized by condensation of divertive of benzaldehyde (1), malononitrile (2), and α-or β-naphthol (3) (1.0 mmol each) in the presence of sodium carbonate catalysts under solvent-free heating at 125 °C (Scheme 1).
Cell culture
The PC-3, HepG-2, KB, SK-N-MC, MDA-MB-231, MCF-7, and T-47D cell lines were obtained from National Cell Bank of Iran (Pastor Institute, Tehran, Iran). The cells were cultured in RPMI 1640 supplemented with fetal bovine serum (10 %, v/v), streptomycin (100 μg/ml), and penicillin (100 U/ml) in 5 % CO2-humidified atmosphere at 37 °C. The number of viable cells was estimated by trypan blue exclusion test.
Measurement of cell viability
MTT assay, which is based on reduction of the tetrazolium salt to blue colored formazone by mitochondrial dehydrogenases in viable cells, was used to determine cell viability (Mosmann 1983). To evaluate the cytotoxic activity of each synthesized benzochromene derivatives 4a–4e, the cells (1.5 × 104 cells/well) were seeded in 96-well plates and incubated overnight. The cells were treated with various concentrations of test compounds for 48 h. Etoposide and DMSO were used as positive and negative controls, respectively, and the final concentration of DMSO in the highest concentration of the applied compounds was 1 %. After 48 h, cells were incubated with MTT solution (final concentration 0.5 mg/ml) for 4 h. Then, the supernatant was removed, and the formazone crystals were dissolved using DMSO. The absorbance was read at 492 nm with an ELISA plate reader (BioTek Instruments, Winooski, VT). The inhibition percent was expressed as the mean percentage of viable cells comparing to DMSO-treated cells, and the half maximal growth inhibitory concentration (IC50) values were calculated from the concentration-response curves by non-linear regression analysis.
Morphological evaluation by fluorescence microscopy
Morphological changes in cells were studied by acridine orange/ethidium bromide double staining as described previously in Current Protocols of Immunology (Cohen 1993). Using this technique, the cells undergoing apoptosis distinguished from the viable cells by the morphological changes of apoptotic nuclei. Ethidium bromide and acridine orange are DNA intercalating dyes. Acridine orange is taken up by both viable and dead cells and giving a green nucleus of viable cells. Ethidium bromide is taken up only by dead cells and stains DNA orange in non-viable cells. Briefly, cells were cultured in six-well plates (5 × 105 cells/well) and incubated overnight. Then, cells were treated with IC50 of compounds 4a–4e. After treatment, cells were harvested and washed twice with phosphate buffer saline (PBS). Finally, ethidium bromide/acridin orange solution was added to the cell suspension and the nuclear morphology was evaluated by fluorescence microscopy (Zeiss, Germany).
Flow cytometry analysis of the apoptotic cells with Annexin V-PE and 7-AAD double staining
Annexin V-PE/7-AAD double staining was used to determine the percentage of cells within a population that are undergoing apoptosis. Annexin V-PE/7-AAD double staining was performed using Annexin V-PE Apoptosis Detection Kit (BD Pharmingen) as described in protocol. Using this technique, living cells (annexin V-PE−/7-AAD−), early apoptotic cells (Annexin V-PE+/7-AAD−), late apoptotic or secondary apoptotic cells (Annexin V-PE+/7-AAD+), and necrotic cells (Annexin V-PE−/7-AAD+) were distinguished (Koopman et al. 1994). In brief, the breast cancer cells were treated with IC50 of compounds 4a–4e and etoposide as reference drug. After incubation, the cells were harvested and washed twice with cold PBS and resuspended in the binding buffer (100 μl of calcium buffer containing 10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Finally, the cells were double stained with 5 μl of Annexin V-PE and 5 μl of 7-AAD solution and the samples were gently mixed and incubated for 15 min at room temperature in the dark before flow cytometry.
Measurement of intracellular ROS
The net intracellular levels of ROS generated by synthesized compounds were measured by a non-fluorescence dye, DCFH-DA, which is permeable in cells and interacts with intracellular ROS, to generate fluorescent 2′,7′-dichlorofluorescein (DCF) (LeBel et al. 1992). In this experiment, cells were treated with IC50 of compounds 4a–4e for 4 and 24 h. After treatment, the cells were incubated with 10 μM DCFH-DA for 1 h, and then, the cells were washed twice with phosphate buffer saline and suspended in 500 μl PBS. Finally, the fluorescent intensity was detected by a spectrofluorometer at excitation and emission wavelength of 495 and 525 nm, respectively. Furthermore, the relationship between fluorescence intensity and the various concentrations of DCF was determined, and the amount of ROS was subsequently estimated from DCF production.
Measurement of nitric oxide
Direct measurement of nitric oxide in biological systems is difficult because it has very short half-life and rapidly oxidized to nitrite and/or nitrate by oxygen. We measured the nitrite concentration in the culture medium as an indicator of NO production using the Griess reaction method (Sun et al. 2003; Tsai et al. 1999). Briefly, cells were treated with IC50 of compounds 4a–4e for 4 and 24 h. After this time, 100 μl of each supernatant was mixed with 50 μl VCl3 as a reductant for the reduction of nitrate to nitrite and then added the same volume of Griess reagents (1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphthylethylenediamine dihydrochloride in water) to mixture. Finally, the absorbance of the mixture was read at 543 nm.
Statistical evaluation
Values were presented as mean values plus or minus the standard deviation (SD) of three independent experiments. Statistical differences between the means of the treated and untreated cells were determined by Student’s t test, and P < 0.05 was accepted as being statistically significant.
Results
Growth inhibition and cell viability
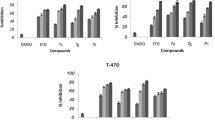
The in vitro cytotoxic activity of compounds 4a–4e was assessed against a panel of human cancer cell lines including MCF-7, MDA-MB-231, T-47D, SK-N-MC, KB, HepG-2, and PC3 after 48 h by MTT assay. Etoposide was used as a positive control. As shown in Table 1, all compounds decreased cell viability and showed significant cytotoxic activity with IC50 values in micromolar range (4.6–21.5 μM). Results revealed that all compounds showed more potent cytotoxic activity than etoposide.
Evaluation of apoptosis
Since breast cancer is the most common cancer and second leading cause of cancer death in women, we decided to investigate the apoptotic effects of these compounds against breast cancer cell lines. For evaluating the apoptosis, cells were treated with indicated concentrations (IC50 values) of test compounds and cellular apoptosis was inspected by flow cytometry and acridine orange/ethidium bromide staining methods. Acridine orange/ethidium bromide staining was used to visualize nuclear changes and apoptotic body formation that are characteristic of apoptosis. Cells were viewed under a fluorescence microscope, and the images of apoptotic and control cells after acridine orange and ethidium bromide staining are presented in Fig. 1. The apoptotic cells had orange particles in their nuclei, whereas the viable cells were observed green. The cells treated with test compounds showed the extend apoptosis relative to control and even etoposide-treated cells.
Fluorescent microscopy of the MCF-7 (I), MDA-MB-231 (II), and T-47D (III) cells stained with acridine orange/ethidium bromide with characteristic symptoms of apoptosis: A untreated control, B DMSO 1 % as negative control, C, D, E, F, G, and H cells treated with IC50 of etoposide and compounds 4a, 4b, 4c, 4d, and 4e, respectively for 24 h
Apoptosis induction for synthetic compounds was further confirmed by flow cytometry analysis. Annexin V/7-AAD double staining followed by flow cytometric analysis revealed that cells undergo apoptosis after treatment with IC50 of test compounds. As shown in Fig. 2, test compounds induced apoptosis in all three malignant breast cancer cell lines. The results indicated that exposure of MDA-MB-231 cells to IC50 of 4a and 4b caused apoptosis in 58.1 and 40.22 % of the cells, respectively. Test compounds induced apoptosis in the range of 18.7 to 38.7 % in T-47D cells, and 4a and 4c induced 37.71 and 52.1 % apoptosis in the MCF-7 cell line, respectively. Therefore, the evaluation of apoptosis in cells treated with the test compounds confirmed the cytotoxicity activity of these compounds was due to inducing apoptosis in cancer cell lines.
Flow cytometric analysis of MDA-MB231 (a), T-47D (b), and MCF-7 (c) cells treated with synthetic compounds. Cells were stained with Annexin V/7-AAD and quantitated by flow cytometry. The cells treated with DMSO 1 % (negative control) or with IC50 values of etoposide (positive control) and compounds 4a–4e (MDA-MB231 and T-47D) and 4a and 4c (MCF-7). Percentage of PE-positive events (% apoptotic cells) was calculated
Intracellular ROS generation
The level of ROS generation in breast cancer cell lines (MCF-7, MDA-MB-231, and T-47D) treated with compounds 4a–4e at 4 and 24 h was obtained from the fluorescence of DCF using spectrofluorometer. As shown in Fig. 3, all compounds in IC50 concentration significantly induced ROS production in all three breast cancer cell lines. The level of ROS production in MCF-7 cells was higher than that of the other two cell lines, and 4a and 4e were more potent than other compounds in the induction of ROS generation.
Effect of compounds 4a–4e on intracellular ROS level of MCF-7 (a), MDA-MB-231 (b), and T-47D (c) cell lines. Cells were treated with compounds for 4 and 24 h, and ROS levels were monitored using DCFH-DA staining. The fluorescence intensity was monitored on spectrofluorometer with excitation and emission wavelengths of 495 and 525 nm, respectively. The level of ROS generation in all three breast cancer cell lines was increased, and MCF-7 cells showed the higher level of ROS production than other two cell lines. Values correspond to mean ± SD of three independent experiments. *Significantly different from control cells treated with DMSO (P < 0.05)
NO generation
The nitric oxide level in all three breast cancer cell lines treated at IC50 of compounds for 4 and 24 h was measured by Griess reaction method. None of the cell lines produced NO after 4 h (data not shown). As shown in Fig. 4, NO generation by MCF-7 cell line treated for 24 h was increased and the most NO generation was induced by 4a and 4d. Also, 4a and 4e induced NO generation in MDA-MB-231, whereas a significant increase in the concentration of NO only was observed in the T-47D cells treated with 4e.
NO production by MCF-7, MDA-MB-231, and T-47D cells exposed to test compounds. NO2 − levels were determined by the Griess assay of culture supernatants from cancer cells that were treated for 24 h with compounds 4a–4e. Results are shown as the mean NO2 − levels and SD. *Significantly different from control cells treated with DMSO (P < 0.05)
Discussion
In the present study, we report the cytotoxic activities of benzochromene derivatives 4a–4e against a panel of human cancer cell lines including MCF-7, MDA-MB-231, T-47D, SK-N-MC, KB, HepG-2, and PC3 for the first time. Our results show that all of the compounds have a significant antiproliferative activity against seven human cancer cell lines. The IC50 value for each compound was less than of etoposide as a standard drug.
It has been repeatedly demonstrated that most chemotherapeutic agents induce apoptosis as a mode of cell death in cancer cells (Hannun 1997). The advantage of apoptosis over necrosis is the lack of a systemic inflammatory response after cell death. For this reason, several studies have suggested that induction of apoptosis by chemotherapeutic agents reserves a physiological advantage in the cancer treatment (Satchell et al. 2003; Tolcher 2002). On the other hand, global statistics show that the annual incidence of breast cancer is increasing and there is an essential need to develop potent cytotoxic drugs with less side effects for invasive breast cancer (Parkin et al. 2005). Thus, we decided to investigate the apoptotic effects of these compounds against breast cancer cell lines using flow cytometric analysis of Annexin V-PE/7-AAD and acridine orange in combination with ethidium bromide staining after 24 h. The IC50 value for each compound was evaluated after 48 h using MTT assay. Therefore, we investigated the percentage of apoptotic cells in the early stages of cell death. According to the flow cytometric analysis, all of the compounds induced apoptosis in breast cancer cells. Different rates of apoptosis were observed in various cancer cells after 24 h depending on treated compounds. Some compounds caused major apoptosis after 24-h treatment, whereas others may be induced apoptosis in longer time.
Reactive oxygen species, which are powerful inducers of apoptosis, can play a direct or indirect role in the cell death (Polyak et al. 1997; Lampiasi et al. 2009). ROS levels are increased in cells treated with various stress agents, such as anticancer drugs, and they induce apoptosis by stimulating pro-apoptotic signaling molecules (Benhar et al. 2002). Due to higher level of ROS in cancer cells than in normal cells, they are more sensitive to chemotherapeutic agents. Furthermore, ROS-stressing agents that increase the production of ROS have been proposed as therapeutic strategies to selectively target the destruction of cancer cells (Parkin et al. 2005).
ROS generation by cytotoxic drugs may result from cellular metabolism and direct action of enzymes on drugs to form reactive intermediate metabolites, or indirect actions of the drug resulting in an induction of ROS through activation of stress-related signaling pathways (England et al. 2006). It is difficult to predict the mechanism of ROS induction by benzochromene compounds but it is known that ROS generated by cytotoxic drugs in cells may lead to multiple adverse effects including energetic imbalance, mitochondrial dysfunction, p53 accumulation, and, ultimately, cell death. ROS promote apoptosis by stimulating pro-apoptotic signaling molecules, such as ASK1, JNK, and p38 and also can act directly on the apoptotic machinery, by accelerating mitochondrial depolarization and dysfunction. In addition, ROS plays a pivotal role in p53-induced apoptosis (Benhar et al. 2002). Indeed, there are many studies that suggest p53 induce apoptosis by the induction of redox-related gene expression and then the formation of ROS (Polyak et al. 1997). In the present study, we showed that benzochromene compounds 4a–4e increase ROS production in three breast cancer cell lines after 4 and 24 h. MCF-7 cell line showed higher levels of ROS after 4 or 24 h in comparison to other two breast cancer cell lines, and that may be due to its wild-type p53. On the other hand, ROS production may be mediated by the p53-inducible gene (PIG3) and may contribute to cytochrome c release from mitochondria. Damage to the mitochondrial membrane and release of cytochrome c still disrupts the electron transport chain and enhances ROS production. All chemotherapeutic agents generate some ROS as they induce apoptosis in cancer cells, because during apoptosis, cytochrome c is released from mitochondria to the cytosol (Conklin 2004). The results of this study showed that all of compounds induced ROS generation in MDA-MB-231 and T-47D with mutant p53, so in these cells, mitochondria could be a major source of ROS during the course of apoptosis.
In general, it has been suggested that NO at high concentrations causes programmed cell death whereas at low levels, it can be pro-angiogenic and pro-tumor formations. Low concentrations of NO cause tumor cell resistance by inducing p53 alterations or mutations; however, at high concentrations, the DNA damage induced by NO may trigger the increase of wild-type p53 proteins leading to programmed cell death (Huerta et al. 2008). So, NO-induced apoptosis is p53-dependent, and cells containing wild-type p53 are more sensitive to NO-induced apoptosis (Xie et al. 2003; Huerta et al. 2008; Geller et al. 1998). In this study, we observed that MCF-7 cells, which had wild-type p53, could produce higher amount of NO than other two cell lines after 24 h. NO either can be produced by a variety of NO donor complexes or can be formed by the enzyme nitric oxide synthase (NOS) (Wink 1997). Since NO production was induced after 24 h, so, a possible mechanism for this is the induction of NOS gene expression through signaling pathways by benzochromene compounds.
All of these observations confirm that the cell death caused by benzochromene derivatives is through apoptosis. This study indicated that one of the ways that these compounds can induce apoptosis is by increasing ROS. ROS can induce apoptosis through a variety of mechanisms, including direct modification of proteins, lipids, and DNA. Apoptosis observation in MCF-7 (wt p53), MDA-MB-231 (mutant p53), and T-47D (mutant p53) cell lines suggest that these compounds induce apoptosis by various targets in cells, and it seems in MCF-7 cells, that high generation of ROS and NO could be induced by p53; the apoptosis is p53 dependent.
References
Augstein J, Cairns H, Chambers A, Burns JW, Radziwonik H (1976) 6,8-Di-t-butyl-4-oxo-4H-1-benzopyran-2-carboxylic acid: a chromone derivative with anti-allergic, anti-inflammatory and uricosuric activity. J Pharm Pharmacol 28:919–920, PMID:12270
Bedair AH, El-Hady NA, El-Latif A, Fakery AH, El-Agrody AM (2000) 4-Hydroxycoumarin in heterocyclic synthesis. Part III. Synthesis of some new pyrano[2,3-d]pyrimidine, 2-substitute. Farmaco 55:708–714, PMID:11204946
Benhar M, Engelberg D, Levitzki A (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 3:420–425, PMID:11991946
Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA (1995) Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A 92:7162–7166, PMID:7638161
Brodská B, Holoubek A (2011) Generation of reactive oxygen species during apoptosis induced by DNA-damaging agents and/or histone deacetylase inhibitors. Oxid Med Cell Longev 2011:253529. doi:10.1155/2011/253529
Cohen JJ (1993) Apoptosis. Immunol Today 14:126–130, PMID:8466628
Conklin KA (2004) Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integ Cancer Ther 3:294–300
Dell CP, Smith CW (1993) European Patent Appl. EP 537949. Chem Abstr 119:139102d, PMID:15523100
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516, PMID:17562483
England K, Driscoll CO, Cotter TG (2006) ROS and protein oxidation in early stages of cytotoxic drug induced apoptosis. Free Radic Res 40:1124–1137, PMID:17050166
Geller DA, Billiar TR (1998) Molecular biology of nitric oxide synthases. Cancer Metastasis Rev 17:7–23, PMID:9544420
Hannun YA (1997) Apoptosis and the dilemma of cancer chemotherapy. Blood 89:1845–1853, PMID:9058703
Huang P, Robertson LE, Wright S, Plunkett W (1995) High molecular weight DNA fragmentation: a critical event in nucleoside analogue-induced apoptosis in leukemia cells. Clin Cancer Res 1:1005–1013, PMID:9816073
Huerta S, Chilka S, Bonavida B (2008) Nitric oxide donors: novel cancer therapeutics (review). Int J Oncol 33:909–927, PMID:18949354
Khafagy MM, Abd El-Wahab AHF, Eid FA, El-Agrody AM (2002) Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 57:715–722, PMID:12385521
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, Van Oers MH (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420, PMID:8068938
Lampiasi N, Azzolina A, D’Alessandro N et al (2009) Antitumor effects of dehydroxymethylepoxyquinomicin, a novel nuclear factor-kappaB inhibitor, in human liver cancer cells are mediated through a reactive oxygen species-dependent mechanism. Mol Pharmacol 76:290–300. doi:10.1124/mol.109.055418, PMID:19461054
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231, PMID:1322737
Longobardi M, Bargagna A, Mariani E et al (1990) 2H-[1] benzothiepino [5, 4-b] pyran derivatives with local anesthetic and antiarrhythmic activities. Farmaco 45:399–404, PMID:2400514
Lu Y, Mahato RI (2009) Pharmaceutical perspectives of cancer therapeutics. In: Narang AS, Desai DS (eds) Anticancer drug development. Springer, New York, pp 49–92
Mahdavi M, Davoodi J, Zali MR, Foroumadi A (2011) Concomitant activation of caspase-9 and down- regulation of IAP proteins as a mechanism of apoptotic death in HepG2, T47D and HCT-116 cells upon exposure to a derivative from 4-aryl-4H-chromenes family. Biomed Pharmacother 65:175–182. doi:10.1016/j.biopha.2011.03.001, PMID:21565459
Meshkini A, Yazdanparast R (2012) Involvement of oxidative stress in taxol-induced apoptosis in chronic myelogenous leukemia K562 cells. Exp Toxicol Pathol 64:357–365. doi:10.1016/j.etp.2010.09.010, PMID:21074392
Mohr SJ, Chirigos MA, Fuhrman FS, Pryor JW (1975) Pyran copolymer as an effective adjuvant to chemotherapy against a murine leukemia and solid tumor. Cancer Res 35:3750–3754, PMID:1192431
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63, PMID:6606682
Musa MA, Badisa VLD, Latinwo LM, Waryoba C, Ugochukwu N (2010) In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res 30:4613–4617, PMID:21115914
Naimi-Jamal MR, Mashkouri S, Sharifi A (2010) An efficient, multicomponent approach for solvent-free synthesis of 2-amino-4H-chromene scaffold. Mol Divers 14:473–477. doi:10.1007/s11030-010-9246-5, PMID:20373141
O'Kennedy R, Thornes RD (1997) Coumarins: biology, applications, and mode of action. Wiley, New York, Chichester
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108, PMID:15761078
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389:300–305, PMID:9305847
Sarih M, Souvannavong V, Adam A (1993) Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun 191:503–508, PMID:7681667
Safavi M, Esmati N, Ardestani SK, Emami S, Ajdari S, Davoodi J, Shafiee A, Foroumadi A (2012) Halogenated flavanones as potential apoptosis-inducing agents: synthesis and biological activity evaluation. Eur J Med Chem 58:573–580. doi:10.1016/j.ejmech.2012.10.043
Satchell PG, Gutmann JL, Witherspoon DE (2003) Apoptosis: an introduction for the endodontist. Int Endod J 36:237–245, PMID:12702117
Sun J, Zhang X, Broderick M, Fein H (2003) Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 3:276–284. doi:10.3390/s30800276
Thomsen LL, Miles DW (1998) Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev 17:107–118, PMID:9544426
Tolcher AW (2002) Regulators of apoptosis as anticancer targets. Hematol Oncol Clin North Am 16:1255–1267, PMID:12512391
Tsai SH, Lin-Shiau SY, Lin JK (1999) Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol 126:673–680, PMID:10188978
Van Rensburg H, Van Heerden PS, Bezuidenhoudt BCB, Ferreira D (1997) Enantioselective synthesis of the four catechin diastereomer derivatives. Tetrahedron Lett 38:3089–3092. doi:10.1016/S0040-4039(97)00552- 2
Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, DeGraff W, Gamson J, Vodovotz Y, Russo A, Mitchell JB (1997) Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide 1:88–94, PMID: 9701048
Xie K, Huang S (2003) Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency. Free Radic Biol Med 34:969–986, PMID:12684082
Xie KP, Huang SY, Dong ZY, Fidler IJ (1993) Cytokine induced apoptosis in transformed murine fibroblasts involves synthesis of endogenous nitric-oxide. Int J Oncol 3:1043–1048, PMID:21573470
Zhang H-Z, Kasibhatla S, Kuemmerle J et al (2005) Discovery and structure-activity relationship of 3-aryl-5-aryl-1, 2, 4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J Med Chem 48:5215–5223, PMID:16078840
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kheirollahi, A., Pordeli, M., Safavi, M. et al. Cytotoxic and apoptotic effects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn-Schmiedeberg's Arch Pharmacol 387, 1199–1208 (2014). https://doi.org/10.1007/s00210-014-1038-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1038-5