Abstract
Background
Cancer results from a series of molecular changes that alter the normal function of cells. Breast cancer is the second leading cause of cancer death in women. To develop novel anticancer agents, new series of chromen derivatives were synthesized and evaluated for their cytotoxic activity against human breast cancer cell lines.
Method
The growth inhibitory activities of synthesized hexahydrobenzo chromen-4-one were screened against six human cancer cell lines using an in vitro cell culture system (MTT assay). Fluorochrome staining (acridine orange/ethidium bromide double staining) and DNA fragmentation by the diphenylamine method were used to investigate the effects of most potent compounds on the process of apoptosis in breast cancer cell lines. To determine the mechanism of apoptosis, ROS and NOX production in treated breast cancer cells with compounds was evaluated.
Results
The cytotoxicity data of tested compounds demonstrate these compounds had varying degree of toxicity. Compound 7h was the most potent compound with IC50 = 1.8 ± 0.6 µg/mL against T-47D cell line. Analyses of the compounds treated (MCF-7, MDA-MB-231, and T-47D) cells by acridine orange/ethidium bromide double staining and DNA fragmentation by the diphenylamine method showed that the synthetic compounds induce apoptosis in the cells. A significant increase in ROS production was observed in T-47D cells treated with IC50 value of compound 7g. Incubation with IC50 value of synthetic compounds increased the NOX production in cell lines, especially T-47D cells.
Conclusion
Our results show that most compounds have a significant anti-proliferative activity against six human cancer cell lines. The observations confirm that chromen derivatives have induced the cell death through apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is an extremely heterogeneous disease which is leading to uncontrolled cell growth and leading cause of death worldwide. Due to cancer progression, drug resistance and side effects of current drugs, researchers are seeking new synthetic drugs with fewer side effects for the treatment of cancer. During the past years, many studies have been done on the biological activity of chromen derivatives. Recently, the antibacterial, anti-fungal, and antitumor properties of chromen have been studied [1, 2].
Previously, Liu et al. have reported cytotoxicity of phenyl-4H-chromen-4-one as derivatives of chromens [3]. In addition, a series of chromen derivatives previously were synthesized and were considered for their activity against MCF-7 and MDA-MB-468 breast cancer cell lines [4].
Whereas apoptosis or programmed cell death contributes to the antitumor activity of many chemotherapeutic drugs; therefore, evaluation of induction of apoptosis by the compounds is a key factor. Apoptosis is a normal process and one kind of cell death which plays a crucial role to maintain the integrity and tissue homeostasis of multi-cellular organisms. It has been known that inadequate or abnormal inhibition of apoptotic pathways are intimately involved in the development of cancer [5].
In recent years, a number of studies have shown that oxidative stress plays a pivotal role in apoptosis [6, 7]. Reactive oxygen species (ROS) and nitric oxide (NO) generation are important mediators that can induce DNA damage. DNA damage activates p53, a transcription factor, which is transported to the nucleus and bound to DNA and mediated transcriptional activation of many genes whose products trigger cell-cycle arrest, apoptosis, or DNA repair.
Actually, ROS is a collective term that is used to describe a number of reactive molecules and free radicals derived from the incomplete reduction of oxygen. These molecules are generated as by-products of normal cellular metabolism primarily in the mitochondria. In addition, ROS are generated by xenobiotic exposure and as essential mediators of metal catalyzed oxidation reactions. ROS can act as essential biomolecules in the regulation of cellular functions and as toxic by-products of metabolism in according to differences in the concentrations of ROS produced. ROS is useful to the cell, because they play roles in cell signaling, including; apoptosis; gene expression; and the activation of cell-signaling cascades [8].
NO, an important mediator of both physiological and pathophysiological processes, has wide-ranging and varied effects on cellular physiology, signal transduction, and cell survival [9]. The results from many studies have demonstrated that NO is a Doubled-Edged Sword in cancer and the role of NO in the carcinogenic process depends on its concentration. Low levels of NO can be pro-angiogenic and pro-tumor formations, whereas its high concentration acts cytotoxically on tumor cells [10, 11].
NO producers has been reported to inhibit HIF-1α accumulation and activation in hypoxic malignant cells and considered to develop a novel cancer therapy. NO producers were also reported to cause DNA damage via the generation of peroxynitrite (ONOO–) and N2O3. Peroxynitrite can attack on the sugar-phosphate backbone on the DNA and lead to single-strand DNA breaks. N2O3 can alkylate DNA through nitrosation of amines and formation of N-nitrosamines ions and subsequent DNA-crosslinks [11, 12]. While NO-releasing drugs are under development, NOS-encoding cDNA sequence could be transfer into cancer cells for the gene therapy purposes [11].To find novel potent derivatives with improved cytotoxicity than current drugs on cancer cells, a series of chromen derivatives (Table 1) prepared and were examined for their cytotoxic effect on a panel of six human cancer cell lines: breast cancer cells (MCF-7, MDA-MB231, and T-47D), hepatocarcinoma (HepG2), neuroblastoma cell line (SK-N-MC), and mouth Epidermal Carcinoma Cells (KB). Then, we assessed apoptosis as pathway for cell-death induction by the compounds on breast cancer cell lines. Increased production of ROS and NOX has been recognized as a critical factor in cancer [13]. In addition, a key feature of apoptosis in many cell types is the induction of genomic DNA fragmentation [14]. Thus, the ROS and NOX production and DNA fragmentation on three breast cancer cell lines were investigated.
Materials and methods
Materials
The cell-culture medium (RPMI 1640), fetal bovine serum (FBS), streptomycin, and penicillin were purchased from Gibco BRL (life technologies, Paisley, Scotland). MTT [3-(4, 5-dimethyl tiazol-2, 5-diphenyl tetrazolium bromide], ethidium bromide, acridine orange, VCL3, Griess reagent, 2, 7 dichlorofluorescein diacetate (DCFH-DA), trypsin–EDTA solution, and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Company (Germany). The culture plates were purchased from Nunc (Brand products, Denmark).
General procedure for the preparation of compounds 7a–k
Dry hydrogen chloride gas was passed through an ice-cold solution of 2, 3, 6, 7, 8, 9-Hexahydro-6, 6, 9, 9-tetramethylbenzo[g]chromen-4-one (5) (0.03 mol) and substituted benzaldehyde (0. 03 mol) in ethanol (10 mL) for 5 min. The reaction mixture was allowed to stand at room temperature for 24 h. The precipitate was filtered, dried, and crystallized from ethanol (scheme 1).
Synthesis of compounds 7a–k. a HCl, b phenol, dichloromethane, aluminum chloride, reflux, 45 min then HCl 25 %, extracted with CHCl3 and dried over Na2SO4. c Trifluoromethanesulfonic acid, 30 min < 5 °C, reflux, 40 min, 65°C, extracted with CHCl3 and dried over Na2SO4. d NaOH, 4 h, rt, HCl 10 %, extracted with ethyl acetate and dried Na2SO4. e Dry HCl gas, substituted benzaldehyde, 5 min, 24 h, rt
Selected spectroscopic data for the most potent synthetic compounds (7e, 7g, 7h)
(E)-3-(3-hydroxy-4-methoxybenzylidene)-6,6,9,9-tetramethyl-2,3,6,7,8,9-hexahydrobenzo[g]chromen-4-one (7e).
Yield 35 %; mp 205–208 °C; 1HNMR (CDCl3, 500 MHz) δ: 7.98 (s, 1H, H-ethylene), 7.77 (s, 1H, H5-Chroman), 6.94–6.86 (m, 4H, H2′, H5′, H6′-phenyl and H10-Chroman), 5.71 (s, 1H, OH), 5.33 (s, 2H, H2-chroman), 3.96 (s, 3H, OMe), 1.69 (s, 4H, H7 and H8-chroman), 1.32 (s, 6H, Me), and 1.29 (s, 6H, Me).
IR (KBr) ν: 3339, 1657.
(E)-3-(3-chloro-4,5-dimethoxybenzylidene)-6,6,9,9-tetramethyl-2,3,6,7,8,9-hexahydrobenzo[g]chromen-4-one (7g).
Yield 12 %;mp 112–114 °C; 1HNMR (CDCl3, 400 MHz) δ: 7.96 (s, 1H, H-ethylene), 7.70 (s, 1H, H5-Chroman), 6.98–6.82 (m, 2H, H2′-phenyl and H10-Chroman), 6.77 (s, 1H, H6′-phenyl), 5.28 (s, 2H, H2-chroman), 3.92 (s, 3H, OMe), 3.91 (s, 3H, OMe), 1.69 (s, 4H, H7 and H8-chroman), 1.31 (s, 6H, Me), and 1.29 (s, 6H, Me).
IR (KBr) ν: 1676.
(E)-3-(3-chlorobenzylidene)-6,6,9,9-tetramethyl-2,3,6,7,8,9-hexahydrobenzo[g]chromen-4-one (7h).
Yield 40 %; mp: 138–139 °C; 1HNMR (CDCl3, 500 MHz) δ: 7.98 (s, 1H, H-ethylene), 7.77 (s, 1H, H5-Chroman), 7.4–7.38 (m, 2H, H5′ and H6′-phenyl), 7.31–7.29 (m, 1H, H4′-phenyl), 7.21–7.16 (m, 1H, H2′-phenyl), 6.91 (s, 1H, H10-Chroman), 5.27 (s, 2H, H2-chroman), 1.70 (s, 4H, H7 and H8-chroman), 1.33 (s, 6H, Me), and 1.30 (s, 6H, Me).
IR (KBr) ν:1660.
Cell lines and culture
The six cell lines, including breast cancer cells (MCF-7, MDA-MB231 and T-47D), hepatocarcinoma cell line (HepG2), Neuroblastoma cell line (SK-N-MC), and mouth epidermal carcinoma cell line (KB) were purchased from the National Cell Bank of Iran (Pastor Institute, Tehran, Iran). The cells were grown at 37 °C in humidified air with 5 % CO2 in RPMI 1640 supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), 1 %l-Glutamine, and 10 % heat-inactivated fetal calf serum.
MTT cytotoxicity assay
A colorimetric assay using the tetrazolium salt MTT was used to assess the cytotoxicity of synthesized compounds and etoposide and doxorubicin [15]. MTT assay is based on the cleavage of the yellow tetrazolium salt which turned to purple formozan crystals by mitochondrial dehydrogenases in viable cells. The cells (1 × 104) were treated with the chromen-4-one compounds in a 96-well plate and incubated overnight. Negative control wells contained the same number of cells with DMSO %0.1 and without any test compounds, while positive control wells contained etoposide and doxorubicin. After overnight incubation at 37 °C, the growth medium was removed and 200 μL of medium supplemented with 4 different final concentrations (1, 5, 10, 20 µg/mL) of synthetic compounds were added in triplicate. The test compounds were all first dissolved in DMSO and then diluted in the growth medium. To perform the MTT assay, after 24 h treatment, the culture medium was removed and 200-μL phenol red-free medium containing MTT (0.5 mg/mL) was added to wells. After 4-h incubation under standard conditions, the MTT solution was removed and 100 μL of DMSO were added into each well to solubilize the formazan crystals. The absorbance was measured at 492 nm with a microplate reader (Gen5, Powerwave xs2, BioTek, America). The IC50 value was defined as the drug concentration that produced a 50 % reduction of absorbance at 492 nm in drug-treated cells as compared with untreated controls containing DMSO 0.1 %.
Fluorescence microscopic analysis of cell death
Morphological changes in cells were studied by acridine orange/ethidium bromide (AO/EB) double staining assay [16]. Acridine orange is taken up by both viable and nonviable cells and would fluoresce green if intercalated into double stranded DNA or fluoresce red when bound to single-stranded nucleic acids RNA and DNA which single-stranded DNA dominates in dead cells. Ethidium bromide was excluded from living cells, but is taken up only by cells with ruptured membrane that allows the entrance of ethidium bromide to intercalate into DNA and emits red fluorescence. MCF-7, T-47D, and MDA-MB-231 cell lines were grown in 6-well plates (5 × 105 cells/well) and after overnight incubation treated with IC50 concentration of selected compounds (7e, 7g and 7h) for 24 h. After treatment, cells were harvested and washed twice with phosphate buffer saline (PBS). 1 µL of dye mixture (100-μg/mL AO and 100-μg/mL EB) in PBS was mixed with 25 μL of cell suspension (5 × 105 cells/well) on a clean microscope slide. The suspension was immediately examined by a Axoscope 2 plus fluorescence microscope from Zeiss (Germany) at 40× magnification.
Detection of reactive oxygen species
Intracellular ROS generation was assessed using cell permeable fluorogenic probe 2′, 7′-dichlorofluorescin diacetate (DCFH-DA). This nonpolar compound is deacetylated to the membrane-impermeant polar derivative 2′, 7′-Dichlorodihydrofluorescin (DCFH) by cellular esterases when it is taken up by the cell. DCFH is nonfluorescent, but is rapidly oxidized to the highly fluorescent DCF by intracellular H2O2 and other peroxides. In the cells, superoxide dismutase (SOD), as a mechanism of defense against the harmful effects of ROS, catalyzes the conversion of two superoxide anions into a molecule of hydrogen peroxide (H2O2) and oxygen (O2). Therefore, the oxidation of DCFH to DCF has been used quite extensively for the quantitation of H2O2 [17, 18]. In this study, breast cancer cells were seeded in 6-well plates at a density of, 5 × 105 cells/well and cultured at 37 °C overnight. Cells were treated with IC50 dose of the compounds (7e, 7g and 7h) for 24 h. After that, cells were incubated with DCFH-DA (10 mM) for 1 h and then they were washed with PBS and suspended in 500-μL PBS, and the fluorescence intensity was measured using a spectrofluorometer at excitation and emission wavelength of 495 and 525 nm, respectively. DCF standard curve was prepared by diluting the 1-mM DCF stock in cell culture media, and the amount of ROS was subsequently estimated from DCF production.
Detection of nitric oxide
Measurement NO in biological systems needs careful considerations. NO can rapidly oxidize to Nitrite (NO2–) and nitrate (NO3–) by oxygen. NO has a very short half-life in biological matrix (t 1/2 < 5 s) [19, 20]; therefore, a direct measurement of its production is difficult and the determination of total nitrate and nitrite concentration (NO x ) is routinely used as an index of NO production in biological fluids and cell-culture medium [21]. In this study, the nitrite concentration was measured in the culture medium as an indicator of NO production using the Griess reaction method. Briefly, 5 × 105 cells/well of three breast cancer cell lines were grown in 6-well plates and after overnight incubation, the cells treated with IC50 concentration of compounds (7e, 7g and 7h) for 4 h. After this time, 100 μL of each supernatant was mixed with 50-μL VCl3 as a reductant for the reduction of nitrate-to-nitrite.
The same volume of Griess reagent which contains 1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphthylethylenediamine dihydrochloride in water was added. The Griess Reagent System is based on the chemical diazotization reaction that was originally described by Griess in 1879 [22], which uses sulfanilamide under acidic conditions to form diazonium salt which readily couples with N-(1-naphthalenediamine) to develop a highly colored azo dye that can be detected at 543 nm [23].
Evaluation of DNA fragmentation
A simple spectrophotometric method for measuring DNA in proliferating cells is described. The method is an adaptation of the widely used diphenylamine (DPA) reaction to examine DNA in cells grown in a 96-well plate. This assay was capable of detecting as little as 10-ng DNA and could be used to measure DNA in stored as well as viable tissue samples [24]. Cells treated with test compounds for 24 h were harvested and centrifuged at 2000 RPM, for 10 min (4 °C) to obtain a cell pellet; then, 0.5-mL Tris Triton EDTA (TTE) buffer was added to the pellet and centrifuged at 13000g for 10 min. Then, 0.5 mL of 25 % trichloro acetic acid (TCA) was added to both tubes and vortexed vigorously. Both tubes were kept overnight at 4 °C followed by centrifugation at 13000g. The supernatant was removed and the pallet obtained was hydrolyzed by the addition of 80 μL of 5 % TCA and heating at 90 °C for 15 min. This was followed by addition of 160 μL of freshly prepared DPA and incubation at 37 °C for 24 h. Finally, optical density of the solution was read at 620 nm in an ELISA reader, and the percentage of DNA fragmentation was expressed by the formula:
Statistical analysis
The experimental results are presented as mean values plus or minus the standard deviation (SD) of three independent experiments. The test value of p < 0.05 was considered highly significant by Student’s t-test.
Results
Growth inhibition and cell viability
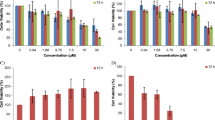
To measure the cytotoxic activity of the synthesized compounds in human cancer cells, such as MCF-7, MDA-MB-231,T-47D, HEPG-2, SK-NMC, and KB cells were treated with (1, 5, 10, and 20 µg/mL) of synthetic compounds for 24 h. Cell viability and cell growth were evaluated by MTT reduction assay. The IC50 values of compounds 7a–7k in comparison to etoposide and doxorubicin as positive control are showed in Table 2. The dose-dependent cytotoxicity effects of most potent compounds (7e, 7g, and 7h) and etoposide against three breast cancer cell lines are presented in Fig. 1. Results revealed that most compounds showed more potent cytotoxic activity than etoposide. The most potent compound is 7h with the IC50 value of 1.8 ± 0.6 µg/mL which is five-fold more active than etoposide against T-47D. In addition, this compound showed desirable growth inhibitory activity against MDA-MB-231 (IC50 = 2.4 ± 0.6 µg/mL). After that, compound 7e with IC50 = 2.5 ± 0.8 µg/mL against MDA-MB-231 and compound 7g with IC50 = 2.9 ± 0.9 µg/mL against the T-47D were the potent compounds. Since breast cancer is the most common cancer and second leading cause of cancer death in women, the effects and mechanisms of action of the most potent compounds on breast cancer cells were investigated.
Acridine orange/ethidium bromide doubles staining
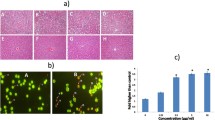
Acridine orange/ethidium bromide double staining was applied to observe the morphological changes in cell death. Three potent compounds, such as 7e, 7g, and 7h, which showed a better cytotoxic activity against all three cancer cell lines, were nominated for the next stages. As shown in Fig. 2 the non-apoptotic control cells were stained green and the apoptotic cells had orange particles in their nuclei due to nuclear DNA fragmentation. According to the results, the synthetic compounds induced apoptosis in three cancer cell lines. According to Fig. 3, the highest percentage of apoptotic cells in this assay was 52 %, resulting from treatment T-47D cell lines with the compound 7h. In addition, Compounds 7e and 7g induced apoptosis in 33 and 48 % of T-47D cell lines. Compound 7e, 7g, and 7h could cause 37, 35.5, and 30 % apoptosis against MCF-7 cells, respectively. Exposure of MDA-MB- 231 cells to IC50 concentration of tested compounds could induce apoptosis in 39, 33, and 40 % of the cells by 7e, 7g, and 7h, respectively.
Morphological changes of apoptosis cell in three cell lines. White arrow indicates live cells, dashed arrow shows apoptosis. a DMSO 0.1 % as control, b cells after exposure to Etoposide for 24 h, c cells treated with compound 7e for 24 h, d cells treated with compound 7g for 24 h, and e cells treated with compound 7h for 24 h
The compounds treatment causes ROS production in human breast cancer cells
Because ROS are implicated in apoptosis induction by a variety of natural anticancer agents [25], we questioned if pro-apoptotic response to compounds was mediated by ROS generation. The ROS generation was measured as one of the indicators of apoptosis in breast cancer cells. As shown in Fig. 4, our data demonstrate that all compounds have induced considerably production of ROS in three breast cancer cell lines. The generation of ROS in T-47D cells had the highest percentage among the other breast cancer cell lines, and 7g was the powerful compound in production of ROS.
Effect of compounds on ROS generation in breast cancer cell lines. Cells were cultured in RPMI 1640 medium in the present of IC50 concentration of selected compounds and compared with untreated cells (control). The bars are mean ± SD for three experiments. *Significantly different from control cells (P < 0.05)
The compounds treatment causes NOX production in human breast cancer cells
NO is an important chemical mediator generated by endothelial cells, macrophages, neurons, etc., and involved in the regulation of various physiological processes. NO is used in various types of disorders like AIDS, cancer, Alzheimer’s, and arthritis by cytotoxic effects. As shown in Fig. 5, all compounds could induce NO generation in three cancer cell lines. The most percentage of NO production was related to compound 7e in T-47D cells. In addition, the highest amount of NO induction was demonstrated against T-47D cells.
Effect of compounds on NOX production in breast cancer cell lines. Cells were cultured in RPMI 1640 medium in the present of IC50 concentration of selected compounds and compared with untreated cells (control). The bars are mean ± SD for three times study which is significantly different from control. *Significantly different from control cells (P < 0.05)
DNA fragmentation
DNA fragmentation is a marker of apoptosis, and cells exhibiting the morphological characteristics associated with DNA fragmentation are referred to as “apoptotic cells” [26]. Figure 6 demonstrates the mean percentage of DNA fragmentation induced in breast cancer cell lines by synthesized compounds. According to the results, compound 7h was the most potent in DNA fragmentation against T-47D and MDA-MB-231.
Discussion
In the present study, the cytotoxic activities of hexahydrobenzo [g]chromen-4-one derivatives 7a–k are reported against a panel of human cancer cell lines, including MCF-7, MDA-MB-231, T-47D, HepG2, SK-N-MC, and KB. Our results show that most compounds have a significant anti-proliferative activity against six human cancer cell lines. Many chemotherapeutic drugs have been shown to induce apoptosis in various cancer types. It is, therefore, important to understand how each drug acts to induce apoptosis on certain cancers [27]. Global statistics show that the annual incidence of breast cancer is increasing, and there is an essential need to develop potent cytotoxic drugs for invasive breast cancer; Therefore, in this study, we decided to investigate the effects and mechanisms of action of the most potent compounds on breast cancer cells.
The apoptotic effects of the compounds (7a–k) were investigated on breast cancer cell lines using acridine orange in combination with ethidium bromide staining, and the percentage of apoptotic cells in the early stages of cell death was investigated. According to the results, Compounds 7g and 7h induced 48 and 52 % apoptosis in T-47D cells, respectively.
DNA fragmentation is an endpoint characteristic of apoptosis occurring after caspase activation. Recent studies have suggested that caspase-3 and caspase-7 are essential for DNA fragmentation [28]. According to the results, DNA fragmentation showed in three cell lines. However, MCF-7 cell line used in this study did not express caspase-3. Therefore, we suggested that is another mechanism for DNA fragmentation in MCF-7 cell line that is a caspase-3 independent pathway [27]. Our results show that the compounds 7g and 7h caused 38.5 and 45 % DNA fragmentation in T-47D cells, respectively, which was much higher than DNA fragmentation in other cells treated with compounds.
Chemotherapic agents have been shown to produce oxidative stress in cells, because during apoptosis, cytochrome c is released from mitochondria to the cytosol [29, 30]. ROS are essential for life, because low levels of ROS regulate cellular signaling and play an important role in normal cell proliferation [31]. Many cellular processes result in the generation of ROS and excess ROS induce oxidative DNA damage, genomic instability, and activation of the mitochondrially mediated intrinsic apoptotic pathway [32]. According to the results of this study, synthetic compounds produced oxidative stress in cancer cells which treated with these compounds. Because ROS attack DNA, resulting in structural changes and DNA dysfunction, we hypothesized that our compounds may induce DNA damage. DNA fragmentation assay confirmed that compounds caused DNA damage in three breast cancer cell lines.
One critical factor involved in apoptosis is Bcl-2, which is highly expressed in 40–80 % of breast cancer patients, and is also common in other tumors [33]. MCF-7 cell line normally expresses higher level of Bcl-2 in comparison with two other breast cancer cell lines [34]. Our results showed that compounds increase ROS production in three breast cancer cell lines compare with control, especially in T-47D. These results suggest that as regards MCF-7, cell line showed lower level of ROS, it can be related to Bcl-2. It has been proposed that Bcl-2 protects against apoptosis by inhibiting the generation or action of ROS [35, 36]. Indeed, p53 effectively regulate the basal levels of ROS and there is a crosstalk between the basal levels of cellular ROS and p53 [37]. Our results showed that ROS formation in the untreated and treated MCF-7 cells with wild-type P53 is lower than two other untreated and treated breast cancer cell lines with mutant-type p53.
NO induces the intrinsic apoptotic pathway to induce cell death. NO activates BAX and BAK, integral members of the intrinsic pathway, which results in cytochrome c release from the mitochondria and activation of Caspase-9. In addition, NO increases superoxide generation and peroxynitrite formation through mitochondrial electron transport chain inhibition [38].
Our result indicates that MCF-7 cells (wild-type p53) produced lower amount of NO than cell lines T-47D (mutant-type p53) and MDA-MB-231 (mutant-type p53). In addition, it has been reported previously that there may be a negative feedback loop existing between NO production and wild-type p53 tumor suppressor gene [37]. NO induces wild-type p53, which is itself a negative regulator of iNOS that binds to its promoter, resulting in the down-regulation of iNOS expression [39, 40]. The possible mechanisms associated with the reduction in NO production in MCF-7 cells may be the presence of wild-type p53 in the feedback loop which suppresses the expression of NO in comparison with two other cancer cell lines with mutant-type p53. P53 controls overproduction of NO, and, therefore, the potential for NO-induced DNA damage, through repression of iNOS gene promoter [37].
Recent results have shown that NO can stimulate p53 expression and apoptosis in breast cancer cell lines [39, 40]. NO from NOS or NO donors have been demonstrated to trigger p53 in cancer cells via DNA damage by peroxynitrite (ONOO-), which can induce apoptosis [11, 12]. This is a possible process by which NO may induce death of tumor MCF-7 cells.
Results from the present study suggested that ROS and NO might act as the signal molecules for inducing cell death, but it might be through different mechanisms. T-47D cell line treated with compounds showed higher levels of ROS and NO production in comparison to other two breast cancer cell lines. In addition, DNA fragmentation and apoptosis percent in T-47D cells treated with 7g and 7h were higher than the other cells, which confirmed the involvement of oxidative stress in inducing apoptosis T-47D. All of these observations confirm that the cell death caused by chromen derivatives is through apoptosis.
References
Lago JH, Ramos CS, Casanova DC, Morandim Ade A, Bergamo DC, Cavalheiro AJ, et al. Benzoic acid derivatives from Piper species and their fungitoxic activity against Cladosporium cladosporioides and C. sphaerospermum. J Nat Prod. 2004;67:1783–8.
Baldoqui DC, Kato MJ, Cavalheiro AJ, Bolzani VS, Young MC, Furlan M. A chromene and prenylated benzoic acid from Piper aduncum. Phytochemistry. 1999;51:899–902.
Liu XH, Li J, Wu FR, Song BA, Bhadury PS, Shi L. Novel 3-(2-(3-methyl-5-substituted-phenyl-4, 5-dihydropyrazol-1-yl)-2-oxoethoxy)-2-subst ituted-phenyl-4H-chromen-4-one: synthesis and anticancer activity, medicinal chemistry. Med Chem. 2011;7:605–10.
Aiello S, Wells G, Stone EL, Kadri H, Bazzi R, Bell DR, et al. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648). J Med Chem. 2008;51:5135–9.
Dey SK, Bose D, Hazra A, Naskar S, Nandy A, Munda RN, et al. Cytotoxic activity and apoptosis-inducing potential of di-spiropyrrolidino and di-spiropyrrolizidino oxindole andrographolide derivatives. PLoS One. 2013;8(3):e58055.
Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–63.
Sinha K, Das J, Pal PB, et al. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87(7):1157–80.
Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356(2–3):295–8.
Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16(6):1834–44.
Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12(5–6):311–20.
Yasuda H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: Nitric oxide donor as a therapeutic enhancer. Nitric Oxide. 2008;19(2):205–16.
Ostrakhovitch EA, Cherian MG. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis. 2005;10:111–21.
Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–68.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Baskić D, Popović S, Ristić P, Arsenijević NN. Analysis of cyclohexamide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32.
Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci U S A. 1999;96(14):8271–6.
LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31.
Feldman PL, Griffith OW, Hong H, Stuehr DJ. Irreversible inactivation of macrophage and brain nitric oxide synthase by L-NG-methylarginine requires NADPH-dependent hydroxylation. J Med Chem. 1993;36:491–6.
Gharavi N, El-Kadi AO. Measurement of nitric oxide in murine Hepatoma Hepa1c1c7 cells by reversed phase HPLC with fluorescence detection. J Pharm Pharmaceut Sci. 2003;6(2):302–7.
Griess P. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt “Ueber einige azoverbindungen”. EurJIC. 1879;12:426–8.
Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Mol Divers Preserv Int. 2003;3(8):276–84.
Natarajan N, Shambaugh GE, Elseth KM, Haines GK, Radosevich JA. Adaptation of the diphenylamine (DPA) assay to a 96-well plate tissue culture format and comparison with the MTT assay. Biotechniques. 1994;17:166–71.
Antosiewicz J, Ziolkowski W, Kar S, Powolny AA, Singh SV. Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 2008;74(13):1570–9.
Widlak P, Garrard WT. Discovery, regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J Cell Biochem. 2005;94:1078–87.
Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PRM. Apoptotic mechanisms in T-47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87(8):909–17.
Liu X, Zou H, Widlak P, Garrard W, Wang X. Activation of the apoptotic endonuclease DFF40 (Caspase activated Dnase or Nuclease). Oligomerisation and direct interaction with histone H1. J Biol Chem. 1999;274:13836–40.
Kheirollahi A, Pordeli M, Safavi M, Mashkouri S, Naimi-Jamal MR, Ardestani SK. Cytotoxic and apoptotic effects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn-Schmiedeberg’s Arch Pharmacol. 2014;387(12):1199–208.
Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300.
Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18(4):775–94.
Marchi S, Giorgi C, Suski JM, Agnoletto Chiara, Bononi Angela, Bonora Massimo, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635.
Oh S, Xiaofei E, Ni D, Pirooz SD, Lee JY, Lee D, et al. Down regulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011;18(3):452–64.
Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54(8):2095–7.
Jacobson Michael D, Raff Martin C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374(6525):814–6.
Amstad PA, Liu H, Ichimiya M, Berezesky IK, Trump BF, Buhimschi IA, et al. BCL-2 is involved in preventing oxidant-induced cell death and in decreasing oxygen radical production. Redox Rep. 2001;6(6):351–62.
Liu Bin, Chen Yumin. Daret K Clair. ROS and p53: versatile partnership. Free Radic Biol Med. 2008;44(8):1529–35.
Synder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS ONE. 2009;4(9):e7059.
Chen YK, Hsue SS, Lin LM. Correlation between inducible nitric oxide synthase and p53 expression for DMBA-induced hamster buccal-pouch carcinomas. Oral Dis. 2003;9(5):227–34.
Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front Physiol. 2013;4:144.
Messmer UK, Ankarcrona M, Nicotera P. Brune B.P53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355:23–6.
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Natl Acad Sci USA. 1996;93(6):2442–7.
Acknowledgments
This research has been supported by a grant from Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
About this article
Cite this article
Pordeli, M., Nakhjiri, M., Safavi, M. et al. Anticancer effects of synthetic hexahydrobenzo [g]chromen-4-one derivatives on human breast cancer cell lines. Breast Cancer 24, 299–311 (2017). https://doi.org/10.1007/s12282-016-0704-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0704-5