Abstract
Adenosine triphosphate (ATP) plays the role of an autocrine/paracrine signal molecule in a variety of cells. So far, however, the membrane machinery in the export of intracellular ATP remains poorly understood. Activation of B2-receptor with bradykinin-induced massive release of ATP from cultured taenia coli smooth muscle cells. The evoked release of ATP was unaffected by gap junction hemichannel blockers, such as 18α-glycyrrhetinic acid and Gap 26. Furthermore, the cystic fibrosis transmembrane regulator (CFTR) coupled Cl− channel blockers, CFTR(inh)172, 5-nitro-2-(3-phenylpropylamino)-benzoic acid, Gd3+ and glibenclamide, failed to suppress the export of ATP by bradykinin. On the other, the evoked release of ATP was greatly reduced by multidrug resistance protein (MRP) transporter inhibitors, MK-571, indomethacin, and benzbromarone. From western blotting analysis, blots of MRP 1 protein only, but not MRP 2 and MRP 3 protein, appeared at 190 kD. However, the MRP 1 protein expression was not enhanced after loading with 1 μM bradykinin for 5 min. Likewise, niflumic acid and fulfenamic acid, Ca2+-activated Cl− channel blockers, largely abated the evoked release of ATP. The possibility that the MRP transporter system couples with Ca2+-activated Cl− channel activities is discussed here. These findings suggest that MRP transporters, probably MRP 1, unlike CFTR-Cl− channels and gap junction hemichannels, may contribute as membrane machinery to the export of ATP induced by G-protein-coupled receptor stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been well-accepted that, in addition to an intracellular energy supplier, adenosine triphosphate (ATP) acts as an extracellular signal molecule via P2X and P2Y purinoceptors in modulating cellular functions (Ralevic and Burnstock 1998). Our studies with rodent smooth muscle cells (SMCs) have revealed that various agonists for G-protein coupled receptors, including ATP per se, are capable of releasing ATP from vas deferens and ileal SMCs (Katsuragi et al. 1996, 2008; Matsuo et al. 1997; Tamesue et al. 1998). Furthermore, we provided evidence that adenosine, a metabolite of ATP, also releases ATP from Madin-Darby canine kidney epithelial cells through a process regulated by a type of positive feedback system (Migita et al. 2005, 2007) and that the angiotensin AT1 receptor-mediated ATP secretion is triggered by Ins(1,4.5)P3-signaling (Katsuragi et al. 2002; Zhao et al. 2007). Aside from the SMCs, it has been shown that cardiac endothelial cells and glial cells release ATP in response to the stimulation of drug receptors with peptide and glutamate (Queiroz et al. 1997). So far, however, the mechanism underlying the autocrine/paracrine type of ATP release by receptor stimulation remains unknown. Recent studies have showed findings that ATP is released extracellularly by mechanical and hypotonic stress from epithelial (Hazama et al. 1999; Walsh et al. 2000), hepatic (Schlosser et al. 1996; Feranchak et al. 2000), and red blood cells (Sprague et al. 2001). In cultured epithelial cells, it has been revealed that the release of ATP induced by hypotonic stress is suppressed by 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) and 4,4'-diisothiocyanatostilbene-2,2'- disulphonic acid (DIDS), Cl− channel blockers, and Gd3+, a blocker of stretch-activated channel (Hazama et al. 1999; Mitchell, 2001; Braunstein et al. 2001). Therefore, it is presumed that ATP moves out of the cells through a type of anionic channels after ionization to ATP4− or [Mg ATP]2− (Roman and Fitz 1999; Dutta et al. 2004). There seems to be some candidates for the membrane machinery in ATP transport across the cell membrane. ATP binding cassette family, P-glycoprotein, multidrug resistance protein (MRP) and cystic fibrosis transmembrane regulator (CFTR) as well as gap junction hemichannels have been considered as ATP carriers. Accordingly, the present study was designed to clarify the membrane machinery that is responsible for the bradykinin-inducible release of ATP from cultured guinea-pig taenia coli (T. coli) SMCs.
Materials and methods
Materials
Alpha-GA, indomethacin and benzbromarone were purchased from Wako Pure Chemical (Osaka, Japan). MK-571, Gap 26, and CFTR(inh)172 were obtained from Tocris (Ellisville, MO, USA). All other reagents were obtained from Sigma (St. Louis, MO, USA).
Cell culture
The study protocols were approved by Fukuoka University, Animal Care Committee. For a successful cell culture, neonatal, immature male guinea pigs within one day of birth were purchased from KBT-Oriental (Tosu, Japan) and used here. The guinea pigs were stunned and bled. Then, taenia coli was removed and cut into segments in phosphate buffered saline (PBS) supplemented with 0.25 mg/mL of collagenase. The segments were then moved to a CO2 incubator (37°C) and maintained there for 40 min. Under a microscope, the longitudinal muscle layer was separated from the circular muscles and plexus with a pair of fine tweezers. After mincing, the small pieces were transferred to a PBS dish supplemented with 0.125% trypsin and placed in the incubator. Following trituration, the content was moved to a tube with 10% fetal bovine serum (FBS) and centrifuged at 180×g for 5 min. The resulting pellet was dispersed in a culture medium, M-199 (Life Technologies, Rockville, MD, USA) with 10% FBS and culturing in the incubator was started. On the third day, the cells were rinsed with a fresh medium. After 4 days of culturing, the cells were used for the release experiment. The purity of SMCs cultured in this manner was checked by staining them with anti-α-actin (mouse monoclonar anti-actin α-smooth muscle antibody, Sigma) under fluorescence microscopy (data not shown).
ATP release and luciferase assay
The cells collected from two dishes were trapped in a Millipore filter (pore size 3 µm) and superfused at 0.5 mL/min using a peristaltic pump with oxygenated Krebs solution (37°C) of the following composition (mM): NaCl 122, KCl 5.2, CaCl2 2.4, MgSO4 1.2, NaHCO3 25.6, D-glucose 11, EDTA 0.03, and ascorbic acid 0.1. After 20 min of equilibration, the superfusate was collected every 90 s for 15 min. The agonist was applied to the medium from the fifth to the seventh fractions and antagonists were present in the superfusion medium throughout the experiment. Superfusate (200 µL in each fraction) was transferred into microtubes and ATP measured with 100 µL of ATP reagent solution (Lucifel-LU; Kikkoman, Noda, Japan). The cells’ protein content was determined using Bio-Rad protein assay kit II following cell lysis after overnight incubation (4°C) in deionized water containing 0.1% Triton X-100. Values for ATP released are expressed as pmol/mL/mg protein in the cultured cells.
Western blotting
SMCs treated with and without bradykinin were collected in a sample tube containing an ice cold buffer [50 mM Tris-HCl (pH 7.5) containing a protease-inhibitor cocktail after being washed with PBS. Cells were then centrifuged at 3,000×g for 10 min at 4°C. Pellets were homogenized by a lysis buffer (50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 5 mM EGTA, 1% Triton X-100) supplemented with a protease-inhibitor cocktail on ice. Then, the crude membrane proteins were obtained by centrifugation at 20,000×g for 1 h at 4°C. Total protein was determined by the Bio-Rad protein assay kit II. Protein samples (10 and 20 µg per lane) were resolved by 7% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Millipore, Bedford, MA, USA) using 25 mM Tris base, 192 mM glycine, and 20% methanol buffer. Blocking was performed with 5% skim milk in 150 mM NaCl, 10 mM Tris, pH 7.5, 0.1% Tween 20 (TBST) buffer for 1 h. The membrane was then incubated for 1 h with the rat anti-MRP1 antibody at 1:500 (clone MRPr1, Alexis Biochemicals, CA, USA), anti-MRP2 monoclonal antibody at 1:100 (clone M2III-6 Chemicon), anti-MRP3 monoclonal antibody at 1:100 (clone M3II-9, Alexis Biochemicals, CA, USA) and anti-β-actin monoclonal antibody at 1:10,000 (Sigma) dilution with 1% skim milk in TBST. The blot was then incubated for 1 h with horseradish peroxidase conjugated goat anti-rat IgG and anti-mouse IgG (Jackson Immunoresearch, West Grove, PA, USA) diluted 1:4,000 in 1% skim milk-TBST, and developed using an ECL system (Amersham Biosciences, Piscataway, NJ, USA) and then exposed to Kodak films.
Statistics
Differences between multiple means were tested for statistical significance by one-way analysis of variance followed by Dunnett’s test. A value of P < 0.05 was considered to be significant.
Results
In the superfusing experiment with cultured taenia coli SMCs, 1 μM bradykinin caused a sizable release of ATP. The evoked release of ATP was clearly diminished by HOE 140, a B2 receptor antagonist, however, not by [des-Arg10] HOE 140, a B1 receptor antagonist. The basal and the peak releases of ATP with and without 1 μM bradykinin were 54.48 ± 5.60 pmol ml−1 mg protein−1 (n = 5) and 295.71 ± 40.55 pmol ml−1 mg protein−1 (n = 5), respectively.
In the presence of 3 μM HOE 140, these releases became 43.89 ± 10.34 pmol ml−1 mg protein−1 (N = 6) and 88.67 ± 16.19 pmol ml−1 mg protein−1 (*p < 0.01, N = 6), respectively. In the presence of 3 μM [des-Arg10] HOE 140, however, these releases amounted to 77.07 ± 7.91 pmol ml−1 mg protein−1 (N = 4) and 308.31 ± 8.91 pmol mg−1 mg protein−1 (N = 4), indicating no prevention of ATP release. Further experiments were arranged to reveal the membrane machinery underlying the export of ATP.
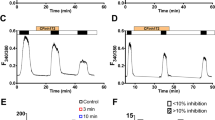
First, the role of the MRP transporters in the release of ATP were assessed by using MRP inhibitors. The bradykinin-induced release of ATP was markedly inhibited by MK-571, indomethacin, and benzbromarone, thus, suggesting that the activation of MRP transporters contributes to the outflux of ATP (Fig. 1). MRP transporter proteins in human consist of nine superfamilies (MRP 1-MRP 9). In the present western blot study, three superfamilies (MRP 1, MRP 2, and MRP 3), which have been reported to distribute to intestinal smooth muscles, were examined. Blots of MRP 1 protein only, but not MRP 2 and MRP 3 proteins, with and without bradykinin, appeared at 190 kD from the crude membrane proteins of guinea pig taenia coli SMCs (Fig. 2a). However, the enhancement in the protein level of the MRP 1 transporter in the presence of bradykinin was not shown after the 5-min loading with 1 μM bradykinin (Fig. 2b,c).
Effects of MRP tranporter inhibitors on the release of ATP evoked by 1 μM bradykinin. Cells were exposed to bradykinin during the fifth, sixth, and seventh fractions and to antagonist 15 min before bradykinin. The time interval of one fraction is 90 s. Values are expressed as mean ± SEM of pmol−1 ml−1 mg protein−1 of ATP (n = 3-5). *p < 0.05, **p < 0.01 compared with the corresponding evoked control
Western blot analysis for MRP protein expression. a Effects of bradykinin (1-3 µM) on expressions of MRP 1, MRP 2 and MRP 3(protein: 20 μg/lane). b The effects of exposures (5 and 10 min) to 1 μM bradykinin on MRP1 protein expression. c Mean density relative to MRP1 protein expression after a 5 and 10 min- exposure to 1 μM of the peptide (n = 5)
Second, the involvement of gap junction hemichannels in the bradykinin-inducible release of ATP was evaluated. The release of ATP was unaffected by 18α-glycyrrhetinic acid (α-GA) and Gap-26, both being hemichannel blockers. Gap-26 was likely to increase rather than decrease the release (Fig. 3a).
Effects of hemichannel blockers (a), CFTR-Cl− channel blockers (b) and Ca2+ activated Cl− channel blockers (c) on the release of ATP evoked by 1 μM bradykinin. In the fractional experiment as shown in Fig. 1, the forth fraction (empty square, basal ATP release) and the seventh fraction (filled square, evoked peak ATP release with and without antagonists) were extracted and illustrated here. The cells were exposed to antagonists 30 min before bradykinin. Values are expressed as mean ± SEM of pmol−1 ml−1 mg protein−1 of ATP (n = 3-10). *p < 0.05 compared with the evoked control
Third, the contribution of the CFTR coupled Cl− channels to the evoked release of ATP was elucidated. CFTR (inh) 172, NPPB, Gd3+ and glibenclamide failed to prevent the release of ATP (Fig. 3b). This casts doubt on the possibility that the transport of ATP is mediated by CFTR-Cl− channels.
Finally, however, niflumic acid and flufenamic acid, Ca2+-activated Cl− channel blockers, largely abolished the release of ATP (Fig. 3c).
The concentrations of all antagonists were used in a range of effective concentrations confirmed in a pile of studies.
Discussion
Bradykinin is capable of releasing the intracellular ATP via the activation of G-protein coupled B2 receptor and then, via the Ins(1,4,5)P3 sensitive- Ca2+-signaling as shown previously (Zhao et al. 2007). Similarly, a G-protein coupled NK-2 receptor agonist, neurokinin-A induced ATP release via Ins(1,4,5)P3-sensitive Ca2+ release from the endoplasmic reticulum, then as further signals, via activations of protein kinase C and Ca2+/calmodulin. The neurokinin A-inducible ATP release was also mediated by MRP transporters (unpubished observation). Accordingly, it is assumed that the bradykinin-inducible ATP release may also be mediated by activations of protein kinase C and Ca2+/calumodulin. However, so far, there is no information how ATP is transported across the cell membrane following the signal pathway. Therefore, the present work aims to clarify the membrane machinery involved in the bradykinin-inducible transport of ATP. Recent investigations provide much evidence that the activities of gap junction hemichannels enhance the release of ATP from rat brain endothelial cell lines (Braet et al. 2003a, b), rodent cultured astrocytes (Cotrina et al. 1998; Stout et al. 2002; Coco et al. 2003), and retinal pigment epithelium from chicken embryos (Pearson et al. 2005). In the present study, however, the bradykinin-evoked release of ATP was unaffected by the typical hemichannel blockers, α-GA and Gap 26, indicating a lesser role of gap junction hemichannels in the membrane transport of ATP. The relevance of CFTR as another ABC (ATP-binding cassette) transporter in ATP transport is a matter of dispute (Abraham et al. 1997). Cantiello et al. (1998), on the basis of their patch-clamp study, postulated that CFTR is a dual ATP and Cl− channel (Reisin et al. 1994; Lader et al. 2000). In wild-type CFTR-expressing cells, the basal and hypotonic stress-evoked release of ATP was attenuated by CFTR-Cl− channel blockers, Ga3+, and DIDS (Braunstein et al. 2001). Sprague et al. (2001) reported a deformation-induced, glibenclamide-sensitive release of ATP from the red blood cells of healthy humans but not from patients with cystic fibrosis. On the other hand, a number of objections to the hypothesis of the CFTR-related ATP release have been raised (Reddy et al. 1996; Grygorczyk and Hanrahan 1997; Watt et al. 1998). Besides, our current study showed that a series of CFTR-Cl− channel blockers, Gd3+, glibenclamide, CFTR (inh)-172, and NPPB failed to attenuate the bradykinin-induced outflow of ATP from the cells, thus, strongly indicating that the evoked release of ATP is not mediated by a CFTR-channel. As to the other ABC superfamilies, there is the possibility that the P-glycoprotein (P-gp) and MRP are able to regulate ATP release induced by hypotonic stress from hepatoma cells (Roman et al. 2001) and cultured astrocyte cells (Darby et al. 2003). Our study found that the extracellular release of ATP with bradykinin was markedly suppressed by MRP inhibitors, MK-571 (Prime-Chapman et al. 2004; Ji and Morris 2005), indomethacin (Darby et al., 2003), and benzbromarone (Hooijberg et al. 2004; Prime-Chapman et al. 2004). We failed to obtain antibodies for MRPs against guinea pig. Thus, in the present study, all antibodies were applied to against rat. As in western blot analysis, a clear band from MRP 1 protein appeared at 190 kD, MRP 1 protein was defined, at least, to exist on the smooth muscle cells in spite of the species mismatch. However, it is necessary to be considered that the negative expressions of MRP 2 and MRP 3 proteins may be due to a matter of specificity of antibodies. The release of ATP with bradykinin is a transient and instantaneous because the peak release occurred around 3 min after the addition. Nevertheless, the 5-min loading with bradykinin did not enhance the expression of MRP 1 protein. At present, the reason remains uncertain whether it comes from a transient response to the peptide or from the species mismatch of antibodies.
Our study provided evidence that niflumic acid and flufenamic acid, Ca2+-activated Cl− channel blockers, strongly suppressed the evoked release of ATP. There are several findings indicating that activities for MRP transporters and Ca2+-activated Cl− channels are involved in facilitating the outflux of ATP. Niflumic acid and flufenamic acid blocked the release of the nucleotide triggered by zero calcium from brain endothelial cells (Braet et al. 2003a, b). Similarly, niflumic acid significantly inhibited the release of ATP from multicellular tumor spheroids evoked by electrical field stimulation (Sauer et al. 2002). ATP release and Cl−-current induced by hypotonic stress was reversibly prevented by MRP transporter inhibitors, MK-571 and indomethacin in rat cultured astrocytes (Darby et al. 2003). These findings suggest that the MRP transport system may also couple with the Ca2+-activated Cl− channel activities in the release of ATP, by analogy that the CFTR transport is mediated by coupling with Cl− channels. Supportingly, it has been postulated that MRP and CFTR are closely related ABC proteins and both transporters may share a structurally similar binding site on the cytoplasmic membrane (Linsdell and Hanrahan 1999). The release of ATP was blocked by Ca2+-activated Cl− channel blockers such as niflumic acid, whereas a volume-sensitive outwardly rectifying (VSOR) Cl−channel blocker, NPPB, and a Maxi Cl− channel blocker, Gd3+ were incapable to inhibit the release. There may be a different nature in ATP release between G-protein-coupled receptor stimulation and hypotonic or mechanical stimulation. The meaning of the dissimilar effects shown with these Cl− channel blockers might be clarified by a patch-clamp analysis in future studies.
In conclusion, the membrane transport of ATP induced by B2 receptor-stimulation with bradykinin is enhanced by the activation of MRP, presumably MRP 1 transporter, in coupling with Cl− channels, and not by the activations of CFTR-Cl−channels and jap junction hemichannels, However, the possibility that P-gp as the member of MDR group, similar to MRP, may be involved in operating the membrane transport of ATP via receptor- stimulation remains to be clarified (Roman et al. 2001)
Abbreviations
- ABC:
-
ATP-binding cassette
- α-GA:
-
18-α-glycyrrhetinic acid
- CFTR:
-
cystic fibrosis transmembrane regulator
- CFTR(inh)172:
-
4-[[4-Oxo-2-thioxo-3-(3-trifluoromethyl)phenyl]-5-thiazo lidinylidene]methyl]benzoic acid
- Gap 26:
-
Val-Cys-Tyr-Asp-Lys-Ser-Phe- Pro-Ile-Ser-His-Val-Arg
- MK-571:
-
3-[[[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl] [[3-(dimethylamino)-3oxopropyl]thio]methyl]thio]propanoic acid
- MRP:
-
multidrug resistance protein
- NPPB:
-
5-nitro-2-(3-phenylpropylamino)-benzoic acid
- SMCs:
-
smooth muscle cells
References
Abraham EH, Okunieff P, Scala S, Vos P, Oosterveld MJ, Chen AY, Shrivastav B (1997) Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 275:1324–1326
Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PEM, Evans WH, Leybaert L (2003a) Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol 197:205–213
Braet K, Vandamme W, Martin PEM, Evans WH, Leybaert L (2003b) Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33:37–48
Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Schwiebert EM (2001) Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem 276:6621–6630
Cantiello HF, Jackson GR Jr, Grosman CF, Prat AG, Borkan SC, Wang Y, Reisin IL, O’Riordan CR, Ausiello DA (1998) Electrodiffusional ATP movement through the cystic fibrosis transmembrane conductance regulator. Am J Physiol 274:C799–C809
Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C (2003) Storage and release of ATP from astrocyte in culture. J Biol Chem 278:1354–1362
Cotrina ML, Lin JHC, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CCG, Nedergaard M (1998) Connexin regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95:15735–15740
Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA (2003) ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol 89:1870–1877
Dutta AK, Sabirov LM, Uramoto H, Okada Y (2004) Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischemic or hypotonic conditions. J Physiol 559:799–812
Feranchak AP, Fitz JG, Roman RM (2000) Volume-sensitive purinergic signaling in human hepatocytes. J Hepatol 33:174–182
Grygorczyk R, Hanrahan JW (1997) CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol 272:C1058–C1066
Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y (1999) Swelling-induced, CFTR-independent ATP release from a human epithelial cell line—lack of correlation with volume-sensitive Cl− channels. J Gen Physiol 114:525–533
Hooijberg JH, Jansen G, Assaraf YG, Kathmann I, Pieters R, Laan AC, Veeman AJP, Kaspers GJL, Peters GJ (2004) Folate concentration dependent transport actibity of the multidrug resistance protein 1 (ABCC1). Biochem Pharmacol 67:1541–1548
Ji Y, Morris ME (2005) Transport of dietary phenethyl isothiocyanate is mediated by multidrug resistance protein 2 but not P-glycoprotein. Biochem Pharmacol 70:640–647
Katsuragi T, Matsuo K, Sato C, Sato Y, Honda K, Kamiya H, Furukawa T (1996) Non-neuronal release ATP and inositol 1, 4, 5-trisphosphate accumulation evoked by P2- and M-receptor stimulation in guinea pig ileal segments. J Pharmacol Exp Ther 277:747–752
Katsuragi T, Sato C, Lou G, Honda K (2002) Inositol(1,4,5)trisphosphate signal triggers a receptor-mediated ATP release. Biochem Biophys Res Commun 293:686–690
Katsuragi T, Sato C, Usune S, Ueno S, Segawa M, Migita K (2008) Caffeine-inducible ATP release is mediated by Ca2+-signal transducing system from the endoplasmic leticulum to mitochondria. Naunyn-Schmiedeb Arch Pharmacol 378:93–101
Lader AS, Xiao Y-F, O’Riordan CR, Prat AG, Jackson GR Jr, Cantiello HF (2000) C-AMP activates an ATP-permeable pathway in neonatal rat cardiac myocytes. Am J Physiol 27(9):C173–C187
Linsdell P, Hanrahan JW (1999) Substrates of multidrug resistance-associated proteins block the cystic fibrosis transmembrane conductance regulator chloride channel. Br J Pharmacol 126:1471–1477
Matsuo K, Katsuragi T, Fujiki S, Sato C (1997) ATP release and contraction mediated by different P2-receptor subtypes in guinea-pig ileal smooth muscle. Br J Pharmacol 121:1744–1748
Migita K, Lu L, Zhao Y, Honda K, Iwamoto T, Kita S, Katsuragi T (2005) Adenosine induces ATP release via an inositol 1,4,5-trisphosphate signaling pathway in MDCK cells. Biochem Biophys Res Commun 328:1211–1215
Migita K, Zhao Y, Katsuragi T (2007) Mitochondria play an important role in adenosine-induced ATP release from Madin-Darby canine kidney cells. Biochem Phamacol 73:1676–1682
Mitchell CH (2001) Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol 534:193–202
Pearson RA, Dale N, Liaudet E, Mobbs P (2005) ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46:731–744
Prime-Chapman HM, Fearn RA, Cooper AE, Moore V, Hirst BH (2004) Differential multidrug resistance-associated protein 1 through 6 isoform expression and function in human intestinal epithelial caco-2 cells. J Pharmacol Exp Ther 311:476–484
Queiroz G, Geibicke-Haerter PJ, Schobert A, Starke K, von Kügelgen I (1997) Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience 78:1203–1208
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR (1996) Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science 271:1876–1879
Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF (1994) The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem 269:20584–20591
Roman RM, Fitz JG (1999) Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology 116:964–979
Roman RM, Lomri N, Braunstein G, Feranchak AP, Simeoni LA, Davison AK, Mechetner E, Schwiebert EM, Fitz JG (2001) Evidence for multidrug resistance-1 P-glycoprotein-dependent regulation of cellular ATP permeability. J Membr Biol 183:165–173
Sauer H, Stanelle R, Hescheler J, Wartenberg M (2002) The DC electrical-field-induced Ca2+ response and growth stimulation of multicellular tumor spheroids are mediated by ATP release and purinergic receptor stimulation. J Cell Sci 115:3265–3273
Schlosser SF, Burgstahler AD, Nathanson MH (1996) Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA 93:9948–9953
Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ (2001) Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol 281:C1158–C1164
Stout CE, Constantin JL, Naus CCG, Charles AC (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488
Tamesue S, Sato C, Katsuragi T (1998) ATP release caused by bradykinin, substance P and histamine from intact and cultured smooth muscles of guinea pig vas deferens. Naunyn-Schmiedeb Arch Pharmacol 357:240–244
Walsh DE, Harvey BJ, Urbach V (2000) CFTR regulation of intracellular calcium in normal and cystic fibrosis human airway epithelia. J Membr Biol 177:209–219
Watt WC, Lazarowski ER, Boucher RC (1998) Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulations of P2Y2 receptors in airway epithelia. J Biol Chem 273:14053–14058
Zhao Y, Migita K, Sato C, Usune S, Iwamoto T, Katsuragi T (2007) Endoplasmic reticulum is a key organelle in bradykinin-triggered ATP release from cultured smooth muscle cells. J Pharm Sci 105:57–65
Acknowledgments
This work was supported by Grant-in Aid for Scientific Research (17590234) From the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Uehara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental data 1
Fluorescence scopic (a, stained with α-actin) and phase contrast scopic b pictures were taken from the same cultured cells. (PPT 327 kb)
Rights and permissions
About this article
Cite this article
Zhao, Y., Migita, K., Sun, J. et al. MRP transporters as membrane machinery in the bradykinin-inducible export of ATP. Naunyn-Schmied Arch Pharmacol 381, 315–320 (2010). https://doi.org/10.1007/s00210-009-0490-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-009-0490-0