Abstract
Metabolism of most diesters of phthalic acid in humans occurs by an initial phase I biotransformation in which phthalate monoesters are formed, followed by a phase II biotransformation in which phthalate monoesters react with glucuronic acid to form their respective glucuronide conjugates. The phase II conjugation increases water solubility and facilitates urinary excretion of phthalate, and reduces the potential biological activity because the putative biologically active species is the monoester metabolite. In this study, we report percentages of glucuronidation of four common phthalate monoesters, monoethyl (mEP), monobutyl (mBP), monobenzyl (mBzP), and mono-2-ethylhexyl phthalate (mEHP) in a subset of urine (mEP n=262, mBP n=283, mBzP n=328, mEHP n=119) and serum (mEP n=93, mBP n=149, mEHP n=141) samples from the general US population. The percentages of free and conjugated monoester excreted in urine differed for the various phthalates. For the more lipophilic monoesters (i.e., mBP, mBzP, and mEHP), the geometric mean of free monoester excretion ranged from 6 to 16%. The contrary was true for the most hydrophilic monoester, mEP, for which about 71% was excreted in urine as its free monoester. Furthermore, percentages of free and conjugated monoesters were similar for mEP, mBP and mEHP among serum and urine samples. Serum mBzP was largely below the method limit of detection. Interestingly, the serum mEP and mBP levels were less than 3% and 47%, respectively, of their urinary levels, whereas the level of mEHP was similar both in urine and serum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Public concern has increased in recent years about the potential health risks associated with exposure to phthalic acid diesters, commonly known as phthalates. Phthalates are a group of ubiquitous industrial chemicals used in a variety of consumer products such as solvents, additives and plasticizers (Nassberger et al. 1987; ATSDR 1995, 1997, 1999, 2000). Some phthalates and their monoester metabolites are known to cause carcinogenic, toxic or endocrine-modulating effects in animals (Autian 1973, 1982; Douglas et al. 1986; Doull et al. 1999; Banerjee et al. 2002). Phthalate exposure of humans can occur through oral, dermal or inhalation routes. Dermal exposure is considered the major route of exposure to diethyl phthalate (DEP) that is found in hygiene products such as soap, shampoo, and conditioners. In contrast, for phthalates that are used mainly as plasticizers, such as di-2-ethylhexyl phthalate (DEHP), oral or inhalation exposures predominate. In addition, for dialysis and blood transfusion patients, direct exposure (Mettang et al. 1996) to DEHP can occur through intravenous injection.
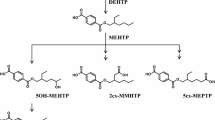
Understanding the metabolism of the various phthalates is important from a risk-assessment standpoint. In general, biotransformation or metabolism of xenobiotics most frequently results in detoxification of the chemical and facilitates excretion from the body. However, this may not be the case for phthalates. For example, dibutyl phthalate (DBP) and DEHP are known to be metabolized to the bioactive monoesters monobutyl phthalate (mBP) and mono-2-ethylhexyl phthalate (mEHP), upon phase 1 biotransformation, and are excreted in urine (Heindel and Powell 1992; Fig. 1). The phthalate monoesters, formed during the phase I biotransformation, can be excreted unchanged or they can undergo phase II biotransformation, which is catalyzed by the enzyme uridine 5′-diphosphoglucuronyl transferase (UGT) to produce glucuronide-conjugated monoesters. Alternatively, the phthalate monoesters may be further metabolized to produce more hydrophilic oxidative products (Albro et al. 1973; Albro and Thomas 1973; Albro and Moore 1974; ATSDR 1997, 2000; Barr et al. 2003; Silva et al. 2003) and their glucuronide conjugates (Fig. 2). The oxidative products and their glucuronide conjugates were not measured in this study.
Phthalate monoesters are valuable biomarkers for exposure to phthalate diesters, and have been measured in previous exposure assessment studies (Blount et al. 2000b; Brock et al. 2002; CDC 2001, 2003; Duty et al. 2002). In most of these studies, total phthalate monoester concentrations (i.e., free plus glucuronide-bound) were measured. However, from a toxicological standpoint, it may be important to note the relative degree of the bioactive free monoester metabolite to its respective conjugated, less bioactive, metabolite.
In this study, we report the relative degree of free monoester metabolite (phase 1) with respect to its glucuronide conjugated metabolite (phase 2) for four phthalate diesters, DEP, benzylbutylphthalate (BzBP), DBP, and DEHP in a subset of human urine and serum samples from the general US population.
Materials and methods
Subjects
The monoester metabolites of phthalates have been routinely measured since 1999 as a part of the ongoing National Health and Nutrition Examination Survey (NHANES-1999) conducted by the US National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC). NHANES was designed to provide information from the civilian, non-institutionalized population that is representative of the general US population. Total phthalate monoesters, which include both free and glucuronidated forms, were measured in a subset of samples collected during 1999 and 2000. The distribution of the results from 1999 were reported in CDC’s National Report on Human Exposure to Environmental Chemicals (CDC 2001, 2003), and the composite 1999–2000 data will be reported elsewhere. From approximately 2300 samples analyzed during 1999 and 2000, 328 samples were randomly selected for analysis of the free monoester only. Although NHANES was designed to provide representative information, our random sampling for free monoester analysis was a convenience sampling and the urine and serum samples were not necessarily matched for the same person. However, the participants from whom samples were obtained and analyzed represented a diverse population comprising persons from both sexes and all races, ethnicities, age groups (age 6 years and above) and geographic regions in the United States.
Analytical methods
The analytical methods used to quantify both urinary and serum phthalate metabolites have been reported (Blount et al. 2000a; Kato et al. 2003; Silva et al. 2003). Briefly, the urine and serum samples were spiked with 13C4-labeled monoester internal standards, 4-methylumbelliferone (quantified as a surrogate to evaluate the completion of the deglucuronidation) and its labeled internal standard, were subjected to an enzyme hydrolysis with β-glucuronidase enzyme (Escherichia coli K12; Roche Biomedical, Mannheim, Germany) and extracted by solid-phase extraction. The extracts were concentrated and resuspended in water. The extractions were repeated, eliminating the deglucuronidation step for analysis of the free monoester. The urinary phthalate monoesters were resolved (Silva et al. 2003) by a high-performance liquid chromatography (HPLC; Waters 2690, Milford, MA, USA) system equipped with a Betasil phenyl column (Keystone Scientific, Bellefonte, PA, USA) using a nonlinear water/acetonitrile solvent gradient and analyzed by atmospheric pressure chemical ionization-tandem mass spectrometry (APCI-MS/MS; ThermoFinnigan TSQ 7000; San Jose, CA, USA). The serum phthalate monoesters (Kato et al. 2003) were chromatographically resolved by an Agilent 1100 HPLC (Wimington, DE, USA), using the same water/acetonitrile solvent gradient, and were analyzed by electrospray ionization (ESI) tandem mass spectrometry (PE Sciex API 3000; Applied Biosystems, Foster City, CA, USA). Concentrations were calculated using linear regression lines from isotope dilution calibration plots. Bound concentrations were determined by subtracting the free monoester concentrations from the total monoester concentrations for a given sample. Data points for total analyte concentrations below detection limits were eliminated from the study, and urinary data were not creatinine-corrected. Reagent blanks, quality control samples and standards were analyzed along with the unknown human samples. The serum samples were acid-treated upon receipt before extractions to avoid hydrolysis of diester phthalates to monoesters by serum enzymes. We assumed the contribution of mEHP from blood products contaminated with plastics containing DEHP was minimal.
Results and discussion
In the samples tested, we observed significant interindividual variations in the ratios of free monoester to their respective bound monoester (Table 1), which were not consistent among analytes (Fig. 3). Our data strongly suggest phase II biotransformation by glucuronidation as the major route of detoxification for DBP, BzBP, and DEHP, but not for DEP. In the population studied the urinary mEP concentrations ranged over three orders of magnitude (Table 1). We observed urinary mEP predominantly in its free form (Fig. 4A) with a geometric mean of 71% of total monoester, whereas urinary mBP, mBzP, and mEHP were present predominantly in their glucuronide-bound form (Fig. 4B–D) with geometric means of 6, 7 and 16%, respectively (Table 1). In more than half of the samples analyzed, less than 5% of total urinary mBP, mBzP and mEHP were present as the free monoesters (Fig. 4). Furthermore, more than three-quarters of persons excreted 10% or less of mBP and mBzP as the free monoester (Fig. 4B,C). A statistical correlation analysis of the study population for free mBP and mBzP levels indicated similar distributions (P<0.001). This suggests relatively similar metabolic pathways for DBP and BzBP, or may reflect metabolism of BzBP to produce both mBP and mBzP.
Percentage of the free form of urinary phthalate monoesters (mEP monoethyl phthalate, mBP monobutyl phthalate, mBzP monobenzyl phthalate, mEHP mono-2-ethylhexyl phthalate) in a subset of a human population from National Health and Nutrition Examination Survey-1999. (solid points indicate 5th and 95th percentiles, horizontal bars indicate the medians, boxes enclose the 25th and 75th percentiles, and error bars are standard deviations)
Frequency of detection of free urinary and serum monoester phthalates. A Urinary mEP with inset A′ presenting serum mEP. B Urinary mBP with inset B′ presenting serum mBP. C Urinary mBzP; serum levels of mBzP were not detectable. D Urinary mEHP with inset D′ presenting serum mEHP (which may show falsely elevated levels due to DEHP hydrolysis by serum enzymes)
The high propensity of mBP, mBzP, and mEHP toward glucuronidation reduces the effective concentration of the free bioactive species for target organs, thus potentially minimizing the adverse effects of exposure to these chemicals. The differences in glucuronidation patterns of mEP, when compared with those of mEHP, mBP, and mBzP, demonstrated obvious differences in phase II metabolism of these phthalates. Perhaps these differences can be attributed to the relative solubility of these monoesters in urine. Because glucuronidation tends to make xenobiotic chemicals and their reaction products more soluble in urine, and thus more easily eliminated, the more nonpolar or lipophilic phthalates such as mBP, mBzP, and mEHP may require more polar moieties added to them to make them appreciably soluble in urine. Conversely, the more hydrophilic compounds, such as mEP, may be more easily or rapidly eliminated in the urine, making them less available for phase II metabolism.
In serum, we found only three phthalate metabolites, mEP, mBP and mEHP, at concentrations high enough for quantification (Table 2). Interestingly, glucuronide distribution patterns of these metabolites in serum closely resembled those of urine (Fig. 4). Similar to the pattern in urine, mBP and mEHP were found predominantly glucuronidated in serum, whereas mEP was found mostly free. We observed markedly lower serum mEP levels compared with the urinary levels (Fig. 5), likely indicating that mEP is more readily excreted in urine. The pattern remained unchanged irrespective of whether levels were compared with matched urine and serum samples or unmatched samples (Fig. 5A,B). The much higher levels of mEP in urine than in serum suggest that DEP is absorbed unchanged into the bloodstream, and that the kidneys may be the primary organ for phase I biotransformation of DEP to produce the monoester mEP. If formed in the kidneys, mEP may pass directly into the urine without reaching the liver for the phase II biotransformation, thus explaining the significantly low urinary and serum levels of glucuronidated mEP. This mechanism of DEP metabolism increases the effective concentration of DEP for systemic circulation. Our preliminary data suggest presence of high levels of DEP in serum, supporting our theory. However, further research is needed to confirm this proposed mechanism. Furthermore, mEP is smaller and more hydrophilic than the other three monoester phthalates; therefore, glucuronidation may not be needed to facilitate excretion. The comparable levels of mBP and mEHP in urine and serum suggest that for DBP and DEHP both hydrolysis and glucuronidation occur, probably in the liver, so producing mostly the glucuronidated form of the monoester metabolites.
Geometric mean concentrations (in nanograms per milliliter, ppb) of total monoethyl phthalate (mEP), monobutyl phthalate (mBP), mono-2-ethylhexyl phthalate (mEHP) and monobenzyl phthalate (mBzP) in urine and serum. A Urine and serum samples not necessarily from the same individuals. B Urine and serum samples from the same individuals
In plots of the percentage of free monoester versus the log total monoester concentration, we observed no concentration-dependent changes in the relative free and glucuronide-bound concentrations among the analytes (Fig. 6), thus indicating no apparent saturation of glucuronyl transferase enzyme at exposed concentrations. The percentage of free mEP, mBP, mBzP and mEHP found in urine remained scattered with geometric means, 71, 6, 7 and 16%, respectively, and showed no apparent decrease with increased concentration of total phthalate as should occur if enzyme saturation occurred. We plotted the concentrations of the free phthalate monoesters in the urine against the total phthalate concentration for each monoester (Fig. 7). The free urinary phthalate concentrations of mEP, mBP and mBzP increased (P<0.0001) with increasing concentrations of total combined free and glucuronidated forms. The correlation was not significant for mEHP. Because the concentrations of mEHP found in urine were low with a significant number of samples having concentrations of free mEHP below or close to the limit of detection, the analytical procedure may have introduced some errors in the detection making it difficult to interpret correlation. The high correlation of free mEP with total combined mEP may also indicate less interindividual variability in metabolism of DEP than in the other phthalates. Differences in metabolism have been documented with age, sex, race, ethnicity, diseases, pregnancy, and other factors. Because of a limited sample size, we were not able to assess differences in glucuronidation efficiencies among different demographic groups.
Our data show higher systemic circulation of glucuronides of mBP and mEHP than of their free metabolites, potentially reducing the possible toxic effects associated with the free monoesters upon exposure to their parent compounds DBP, and DEHP. For DEP, the free monoester is the predominant excretion product, despite significantly low serum mEP concentration. In our previous studies, the total urinary phthalate concentration, consisting of free and conjugated forms, was used for exposure assessment. Although glucuronide may serve as the transport form of the free phthalates, because the free form of the phthalate metabolite may be more bioactive than its parent compound (e.g., mBP, mEHP) the amount of the free form provides valuable information regarding the biologically effective dose of the active toxicant and the risks associated with the exposure.
References
Albro PW, Moore B (1974) Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr 94:209–218
Albro PW, Thomas RO (1973) Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochim Biophys Acta Biochim 306:380–390
Albro PW, Thomas R, Fishbein L (1973) Metabolism of diethylhexyl phthalate by rats. Isolation and characterization of the urinary metabolites. J Chromatogr A, J 76:321–330
ATSDR (US Agency for Toxic Substances and Disease Registry) (1995) Toxicological profile for diethyl phthalate (DEP). US Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles
ATSDR (US Agency for Toxic Substances and Disease Registry) (1997) Toxicological profile for di-n-octyl phthalate (DNOP). U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles/tp95.html
ATSDR (US Agency for Toxic Substances and Disease Registry) (1999) Toxicological profile for di-n-butyl phthalate (DBP)... U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles
ATSDR (US Agency for Toxic Substances and Disease Registry) (2000) Toxicological profile for di(2-ethylhexyl)phthalate (DEHP).. U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles/
Autian J (1973) Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect 4:3–26
Autian J (1982) Antifertility effects and dominant lethal assays for mutagenic effects of DEHP. Environ Health Perspect. 45:115–118
Banerjee S, Thuillier R, Culty M, Papadopoulos V, Brown TR, Banerjee PP (2002) In utero exposure to di(2-ethylhexyl) phthalate alters growth, tissue organization, and the expression of androgen receptor protein of rat prostate. Biol Reprod 66:254
Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM (2003) New directions in the quantitation of human exposure to phthalates. Environ Health Perspect Online, 24 February 2003, DOI 10.1289/ehp.6074
Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW (2000a) Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem 72:4127–4134
Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW (2000b) Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect 108:979–982
Brock JW, Caudill SP, Silva MJ, Needham LL, Hilborn ED (2002) Phthalate monoesters levels in the urine of young children. Bull Environ Contamin Toxicol 68:309–314
CDC (Centers for Disease Control and Prevention) (2001) National report on human exposure to environmental chemicals 2001 (NCEH Pub. No. 01-0164). CDC National Center for Environmental Health, Division of Laboratory Sciences, Atlanta GA
CDC (Centers for Disease Control and Prevention) (2003) National report on human exposure to environmental chemicals 2003 (NCEH Pub. No. 01-0164). CDC, National Center for Environmental Health, Division of Laboratory Sciences, Atlanta GA
Douglas GR, Hugenholtz AP, Blakey DH (1986) Genetic toxicology of phthalate-esters—mutagenic and other genotoxic effects. Environ Health Perspect 65:255–262
Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M (1999) A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Toxicol Appl Pharmacol 29:327–357
Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani D, Hauser R (2002) Urinary phthalate monoesters at general population exposure levels are associated with altered semen quality. Epidemiology 13:657
Heindel JJ, Powell CJ (1992) Phthalate ester effects on rat Sertoli-cell function-in vitro—effects of phthalate side-chain and age of animal. Toxicol Appl Pharmacol 115:116–123
Kato K, Silva MJ, Brock JW, Reidy JA, Malek NA, Hodge CC, Nakazawa H, Needham LL, Barr DB (2003) Quantitative detection of nine phthalate metabolites in human serum using reversed phase high performance liquid chromatography/electrospray ionization–tandem mass spectrometry. J Anal Toxicol (in press)
Mettang T, Thomas S, Kiefer T, Fischer FP, Kuhlmann U, Wodarz R, Rettenmeier AW (1996) Uraemic pruritus and exposure to di(2-ethylhexyl)phthalate (DEHP) in haemodialysis patients. Nephrol DialTransplant 11:2439–2443
Nassberger L, Arbin A, Ostelius J (1987) Exposure of patients to phthalates from polyvinyl-chloride tubes and bags during dialysis. Nephron 45:286–290
Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW (2003) Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization–tandem mass spectrometry. J Chromatogr B 789/2:393–404
Acknowledgements
The authors acknowledge the National Center for Health Statistics for conducting NHANES. Use of trade names is for identification only and does not constitute endorsement by the US Department of Health and Human Services or the Centers for Disease Control and Prevention. The experiments comply with the current laws of the country in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00204-005-0653-9
Rights and permissions
About this article
Cite this article
Silva, M.J., Barr, D.B., Reidy, J.A. et al. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol 77, 561–567 (2003). https://doi.org/10.1007/s00204-003-0486-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0486-3