Abstract
Amphetamines are a class of psychotropic drugs with high abuse potential, as a result of their stimulant, euphoric, emphathogenic, entactogenic, and hallucinogenic properties. Although most amphetamines are synthetic drugs, of which methamphetamine, amphetamine, and 3,4-methylenedioxymethamphetamine (“ecstasy”) represent well-recognized examples, the use of natural related compounds, namely cathinone and ephedrine, has been part of the history of humankind for thousands of years. Resulting from their amphiphilic nature, these drugs can easily cross the blood–brain barrier and elicit their well-known psychotropic effects. In the field of amphetamines’ research, there is a general consensus that mitochondrial-dependent pathways can provide a major understanding concerning pathological processes underlying the neurotoxicity of these drugs. These events include alterations on tricarboxylic acid cycle’s enzymes functioning, inhibition of mitochondrial electron transport chain’s complexes, perturbations of mitochondrial clearance mechanisms, interference with mitochondrial dynamics, as well as oxidative modifications in mitochondrial macromolecules. Additionally, other studies indicate that amphetamines-induced neuronal toxicity is closely regulated by B cell lymphoma 2 superfamily of proteins with consequent activation of caspase-mediated downstream cell death pathway. Understanding the molecular mechanisms at mitochondrial level involved in amphetamines’ neurotoxicity can help in defining target pathways or molecules mediating these effects, as well as in developing putative therapeutic approaches to prevent or treat the acute- or long-lasting neuropsychiatric complications seen in human abusers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increased interest concerning the involvement of mitochondria in drug-evoked neuronal injury and, ultimately, neurotoxicity, based on the fact that mitochondria are central players of adenosine 5′-triphosphate (ATP) synthesis and calcium buffering in neurons. As such, these organelles play a critical role in regulating the adequate neuronal function and survival, and are postulated to constitute an important target in many pathological conditions. Once thought to be solitary and rigidly structured, it is now acknowledged that these organelles are highly dynamic and communal in neurons. The dynamic processes involved in regulating neuronal mitochondrial function enable mitochondrial recruitment to critical subcellular compartments, content interchange among mitochondria, control of mitochondrial shape, communication of mitochondria with cytosol and mitochondrial quality control. As such, mitochondria can readily adapt to changes in cellular requirements resulting from either physiological perturbations or toxicological insults. However, when mitochondrial function is disrupted, cellular dysfunction ensues, which ultimately might lead to brain injury.
Of particular concern is the long-lasting neuronal dysfunction associated with exposure to amphetamines, (a class of widely abused psychostimulant drugs derived from β-phenylethylamine) (Carvalho et al. 2012; Cuyas et al. 2013; Gouzoulis-Mayfrank and Daumann 2009), with an methyl group in the α-carbon that renders them resistant to monoaminoxidase (MAO) metabolism (Kuhar et al. 1999) (Fig. 1). Resulting from their amphiphilic nature, these drugs can easily cross the blood–brain barrier and elicit its well-known psychotropic effects (Young and Glennon 1986). Amphetamines are substrates for transporters associated with the uptake of the biogenic amines, such as dopamine (DA), noradrenaline (NA), and serotonin (5-HT) (Carvalho et al. 2012). They either diffuse into or are taken up by nerve terminals via these transporters and subsequently cause the release of monoamines into the synaptic cleft. Amphetamines also disrupt vesicular storage of monoamines and prevent neurotransmitters’ uptake into vesicles, thus increasing the cytoplasmic concentrations of the neurotransmitters and, consequently, making them more readily available for reverse transport into the synaptic cleft. In addition, amphetaminic compounds also inhibit the metabolism of monoamine transmitters by partially inhibiting MAO enzymes (Matsumoto et al. 2014; Ramsay and Hunter 2002), and increase the synaptic levels of monoamines by inhibiting their reuptake (Kuczenski et al. 1995; Rothman et al. 2000; Sulzer et al. 1995, 2005). The acute effects of these drugs include euphoria, alertness, decreased appetite, increased locomotor activity, and hyperthermia (Yamamoto et al. 2010). On the other hand, studies in rodents and primates have demonstrated the ability of amphetamines to cause long-lasting deficits in dopaminergic and 5-hydroxytriptaminergic brain areas, as confirmed by decreased function of monoamine transporters, tyrosine hydroxylase (TH), and tryptophan hydroxylase (Frey et al. 1997; Hotchkiss et al. 1979; Villemagne et al. 1998; Wagner et al. 1980), as well as nerve terminal degeneration (Axt and Molliver 1991; Ricaurte et al. 1982, 1984). Notably, in humans, a persistent reduction in most dopaminergic markers, including DA content (Wilson et al. 1996), TH (Wilson et al. 1996), and dopamine transporter (DAT) levels (Volkow et al. 2001a, b; Wilson et al. 1996), decreased vesicular monoamine transporter 2 binding (Johanson et al. 2006), and in serotonin transporter (5-HTT) functions (Kish et al. 2009; Sekine et al. 2006) were reported in chronic methamphetamine (METH) users. Similarly, in 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) abusers, decreases in 5-HTT functions have been observed in multiple brain regions (McCann et al. 2005). AMPH was also reported to reduce brain activity in several regions during cognitive tasks, in which alterations in dopaminergic activation caused by the drug was suggested as a major trigger factor (Willson et al. 2004). Neuronal symptoms, such as psychiatric illness, including psychotic states and anxiety-like disorders, aggressiveness, and impulsiveness are frequently associated with repeated exposure to these drugs over time (Akiyama 2006; Grelotti et al. 2010; Sato 1992).
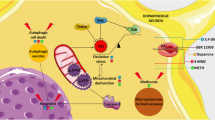
Although several factors have been suggested to be involved in the neuronal injury associated with long-lasting abuse of amphetamines, current and emerging studies have focused on the involvement of mitochondria-dependent pathways. Abnormal mitochondrial function is closely associated with deregulation on bioenergetic metabolism, calcium signaling, mitochondrial deoxyribonucleic acid (mtDNA) integrity, mitochondrial dynamics, including organelles’ biogenesis, fusion/fission, transport and selective degradation by mitophagy, and increased production of reactive oxygen species (ROS), in turn impacting on a wide range of cellular processes, ultimately leading to neuronal toxicity, or even death, and, consequently, brain injury.
The aim of this review is to highlight the evidence supporting the involvement of mitochondrial dysfunction in amphetamines’ neuronal effects, to a better understanding of the mechanisms underlying their well-documented neurotoxic potential.
Studies linking inhibition of tricarboxylic acid cycle function to amphetamines-induced neurotoxicity
The tricarboxylic acid (TCA) cycle is a central part of the energetic metabolism that contributes to the generation of ATP by the chemical breakdown of carbohydrates, fats, and proteins. It consists in a series of chemical reactions, catalyzed by enzymes occurring inside mitochondria, called the mitochondrial matrix (Raimundo et al. 2011). A schematic representation of the mitochondrial TCA cycle is illustrated in Fig. 2. Generically, The TCA cycle can be divided into two stages: oxidative, in which citrate (six carbon atoms) is converted to succinyl-CoA (four carbons) releasing two CO2 molecules; and reductive, the successive oxidations of succinate to fumarate, fumarate to malate, and malate to oxaloacetate. The first reaction of the cycle is the condensation of acetyl-coenzyme A with oxaloacetate to form citrate, catalyzed by citrate synthase. Citrate is subsequently converted to isocitrate by aconitase, an enzyme that contains a non-heme 4Fe–4S cluster. The conversion of isocitrate in α-ketoglutarate is the first oxidative decarboxylation of the cycle, which is catalyzed by isocitrate dehydrogenase and occurs in three forms: nicotinamide adenine dinucleotide oxidized form (NAD+)-dependent and localized at mitochondria (1), as well as nicotinamide adenine dinucleotide phosphate oxidized form-dependent and localized at either mitochondria (2) or the cytoplasm (3). The α-ketoglutarate dehydrogenase complex catalyzes the conversion of α-ketoglutarate to succinyl coenzyme A (succinyl-CoA) and CO2. Succinyl-CoA is the precursor for heme synthesis in animals. Posteriorly, succinyl-CoA generates succinate and guanosine 5′-triphosphate (GTP) or ATP, by a reaction catalyzed by succinate-CoA ligase (SUCL), which is a dimer of an α subunit (SUCLG1) and one of the β subunits, either ATP-forming (SUCLA2) or GTP-forming (SUCLG2). Succinate is oxidized to fumarate by succinate dehydrogenase (SDH). The SDH reaction is part of both the citrate cycle and the electron transport chain (ETC), where it is referred as complex II. All other oxidative steps of the cycle generate nicotinamide adenine dinucleotide reduced form (NADH) to feed complex I of the ETC, whereas the electrons removed from succinate are channeled through flavin adenine dinucleotide reduced form to ubiquinone. Fumarate hydratase catalyzes the hydration of the double bond in fumarate, generating malate. The last reaction of the cycle recycles oxaloacetate from malate. This reaction is catalyzed by malate dehydrogenase and couples the oxidation of malate with the reduction of NAD+.
Modulation of mitochondrial tricarboxylic acid (TCA) cycle and electron transport chain (ETC) functioning by amphetamines. TCA cycle: mediators of TCA cycle and enzymes described to be targeted by amphetamines. The TCA cycle consists in a series of chemical reactions catalyzed by enzymes occurring inside the mitochondria, particularly in the mitochondrial matrix. Amphetamines have been described to modulate the activity of key enzymes of the TCA cycle, including citrate synthase [1], aconitase [2], isocitrate dehydrogenase [3], succinate dehydrogenase [4], and malate dehydrogenase [5]. ETC: Mediators of the mitochondrial ETC and proteins described to be targeted by amphetamines. The ETC, also called mitochondrial respiratory chain, comprises five multi-subunit protein complexes located in the inner mitochondrial membrane (IMM). The adenosine nucleotide translocator (ANT), an IMM’s protein, is responsible for the electrogenic 1:1 exchange of adenosine 5′-diphosphate (ADP) for adenosine 5′-thriphosphate (ATP). In vivo studies have reported the amphetamines’ ability to inhibit ETC’s complexes I [6], II [7], III [8], IV [9], and V [10], which have been supported by additional in vitro settings. Inhibition of ETC functioning may trigger mitochondrial membrane potential (ΔΨm) dissipation, ATP depletion, and increased formation of reactive oxygen species (ROS)
Increased evidences suggest that amphetaminic compounds may target critical enzymes of the TCA cycle. In vivo studies have demonstrated a significant inhibition of the citrate synthase and SDH activities in several areas of rat’s brain, including prefrontal cortex, hippocampus, striatum, and amygdala, around 2 h after multiple administrations of METH (0.5, 1 or 2 mg/kg, i.p., singly or 0.25 mg/kg, i.p., once daily, 15 days) (Feier et al. 2012, 2013). Furthermore, METH-induced decreased activity of malate dehydrogenase was also found in rat’s hippocampus, striatum, and amygdala around 2 h after the last dose, following repeated administration of METH (0.25 mg/kg, i.p., once daily, 15 days) (Feier et al. 2013). Studies addressing the influence of METH (4 × 10 mg/kg, i.p., once every hour) on mitochondrial metabolic networks found decreased urinary levels of TCA cycle’s intermediates, such as aconitase, α-ketoglutarate, malate, fumarate, succinate, oxaloacetate/pyruvate, and isocitrate/citrate, in rat urine collected between 0 and 24 h after the last METH injection (Shima et al. 2011). In the urine samples collected between the 72- and 96-h period, no differences were found in these markers, as compared to control rats (Shima et al. 2011). These findings suggest that the inhibitory effect of METH on TCA cycle functioning could be reversible and, therefore, time-limited. Other studies investigating protein expression profiles, using a 2-DE-based proteomic approach, have also reported a decreased expression of isocitrate dehydrogenase and aconitase in rat’s amygdala 4 h after METH administration (1 mg/kg, i.p.) (Iwazaki et al. 2008).
In rats, around 2 h after acute administration of d-AMPH (2 mg/kg, i.p., singly), an inhibition of the citrate synthase activity in amygdala and striatum was also revealed (Feier et al. 2012). In turn, reduced SDH activity was also reported in rat’s striatum around 2 h after d-AMPH administration (2 mg/kg, i.p., singly) (Feier et al. 2012). Similarly, long-term exposure to d-AMPH (i.p., once daily, for 20 days—initial dose of 5 mg/kg/day and subsequently increased by 1 mg/kg every 5 days, up to a total of 8 mg/kg/day on days 16–20), also resulted in an inhibition of citrate synthase in rat’s brain 24 h after the last d-AMPH injection (Valenzuela et al. 1987). Using another administration scheme (2 mg/kg, i.p., once daily, for 7 days, followed by another single injection of 2 mg/kg at the 15th day), similar reductions in citrate synthase, SDH, and malate dehydrogenase activities were documented in prefrontal cortex, striatum, and hippocampus of rats, 2 h after the last d-AMPH injection (Valvassori et al. 2013).
Increased levels of TCA cycle’s intermediates, including fumarate and malate, were also reported in brain tissue of rats 60 min after AMPH administration (5 mg/kg, i.p.). Likewise, increased levels of α-ketoglutarate were even reported after 5 min (2.5 mg/kg, i.p.) or 30 min (5 or 15 mg/kg, i.p.) of exposure to AMPH (Berntman et al. 1978). Thus, since METH was shown to reduce urinary levels of TCA cycle’s intermediates between the 0 and 24-h period after administration (Shima et al. 2011), this suggests the existence of a biphasic effect of these drugs on the TCA cycle functioning: a stimulation at early stages, probably as a result of their acute effects, followed by a phase of reduced mitochondrial activity, which may result from a direct or indirect inhibition of the TCA cycle’s enzymes.
Since neurons are critically dependent on mitochondrial energy metabolism for maintaining their integrity and functionality, it is likely to consider that impaired regulation of the TCA cycle may play a role on the neurotoxic effects mediated by AMPH-like drugs. Taking into account the above-referred findings, the TCA cycle’s enzymes described to be targeted by amphetamines are indicated in Fig. 2.
Studies linking inhibition of electron transport chain’s complexes function to amphetamines-induced neurotoxicity
Mitochondria provide cellular energy by converting oxygen and nutrients into ATP, via oxidative phosphorylation (Ho et al. 2012), which occur on the ETC, localized in the inner mitochondrial membrane. A schematic representation of the mitochondrial ETC is illustrated in Fig. 2. Although glycolytic metabolism of glucose is highly relevant in many organs, in the brain, ATP is mainly produced via oxidative phosphorylation. Oxidation of glucose in the TCA cycle supplies high-energy electrons in the form of NADH or flavin adenine dinucleotide reduced form to undergo oxidative phosphorylation, which involves the flow of these high-energy electrons along the ETC, from complex I (NADH dehydrogenase) and complex II (succinate dehydrogenase) to complex IV (cytochrome c oxidase), and finally to molecular oxygen.
Along the flow of electrons through the ETC, there is a concomitant pumping of protons in complex I, III (ubiquinol cytochrome c oxidoreductase) and IV, from the mitochondrial matrix to the mitochondrial inter-membrane space, creating a electrochemical gradient, also known as proton-motive force, across the inner membrane (Mitcheel 1961). Complex V, also called ATP synthase, utilizes this electrochemical gradient to drive adenosine 5′-diphosphate (ADP) phosphorylation to generate ATP, by channeling the protons back to the matrix (Hatefi et al. 1975; Mitcheel 1961). The proton-motive force has two components: the mitochondrial membrane potential (ΔΨm), which arises from the net movement of positive charge across the inner mitochondrial membrane, and the pH gradient. At any given time, the ΔΨm, typically between −150 and −180 mV, reflects the balance between processes that contribute to the generation of the proton gradient and those that consume it (Ho et al. 2012; Vafai and Mootha 2012). The proton-motive force is best known for driving ATP synthesis through oxidative phosphorylation, but is also linked to many other processes.
The nicotinamide nucleotide transhydrogenase, which plays an important role in ROS homeostasis, relies on the proton-motive force to regenerate mitochondrial nicotinamide adenine dinucleotide phosphate reduced form. Furthermore, it is coupled to solute and ion transport across the inner membrane, by which its collapse can halt essential biosynthetic reactions, such as Fe–S clusters’ biogenesis and proteins’ import. The importance of the proton-motive force, namely the ΔΨm, is exemplified by the fact that glycolytic ATP can be consumed by complex V that is run in reverse to defend ΔΨm during states of ETC inhibition (Vafai and Mootha 2012).
Increased evidences suggest that a fully functional mitochondrial ETC is important to prevent the neurotoxic effects of amphetamines. This theory received particular attention after the observation that inhibitors of the ETC could enhance MDMA- (Nixdorf et al. 2001) and METH-induced (Albers et al. 1996) neurotoxicity, both in mice (Albers et al. 1996) and rats (Nixdorf et al. 2001). Whereas the direct infusion of MDMA [100 µM, at 1.8 (Darvesh and Gudelsky 2005) or 2 µL/min (Nixdorf et al. 2001), for 8 h] into the rat’s striatum did not affect DA or 5-HT tissue content 5 (Darvesh and Gudelsky 2005) or 7 (Nixdorf et al. 2001) days later, co-administration of MDMA with malonate (inhibitor of mitochondrial complex II) produced long-term depletion of both DA and 5-HT content (Darvesh and Gudelsky 2005; Nixdorf et al. 2001). Similarly, infusion of malonate (2–3 µmol) in caudate nucleus, followed by systemic administration of METH (5, 2.5 mg/kg, i.p., 2-h interval), resulted in greater damage to dopaminergic neurons (DA depletion and TH inhibition), 5–7 days later, than that observed for METH or malonate alone (Albers et al. 1996).
Other studies revealed that substrates of energy metabolism attenuated MDMA- (Darvesh and Gudelsky 2005), METH- (Stephans et al. 1998), and d-AMPH-induced (Wan et al. 1999) neurotoxicity. Perfusion of nicotinamide (precursor for the electron carrier molecule NADH, 1 mM) or ubiquinone (an electron-carrying coenzyme of the ETC, 100 µM) in rat’s striatum, beginning 2 h prior to the first MDMA injection (10 mg/kg, i.p., every 2 h, four times) and ending 6 h after the last injection of MDMA, significantly attenuated MDMA-induced 5-HT depletion 5 days later (Darvesh and Gudelsky 2005). Striatal infusion of decylubiquinone (ubiquinone analogue) or nicotinamide for 6 h, beginning immediately after the last METH injection (3 × 10 mg/kg, i.p., every 2 h, followed by a last i.p., injection of 5 mg/kg, 2 h later), significantly attenuated the METH-induced striatal DA depletions, measured 1 week later (Stephans et al. 1998). In the same way, pretreatment with nicotinamide (500 mg/kg, i.p.) 3 h before d-AMPH administration (10 mg/kg, i.p.) significantly attenuated d-AMPH-induced acute striatal reduction in the ATP/ADP ratio (3 h after d-AMPH administration) and long-term striatal DA depletion (7 days later) (Wan et al. 1999). Despite this, the first direct evidence that AMPH-like compounds may interfere with the ETC function was provided by Burrows and co-workers (Burrows et al. 2000). In that work, it was revealed that both METH (4 × 10 mg/kg, i.p., every 2 h) and MDMA (4 × 15 mg/kg, i.p., every 2 h) induced a significant reduction in the complex IV activity in substantia nigra, nucleus accumbens, and striatum of rats, 2 h after the last injection of drugs (Burrows et al. 2000).
Many other studies have reported an inhibition of mitochondrial ETC complexes’ activity by amphetamines, namely complex I (Feier et al. 2012, 2013), complex II–III (Brown et al. 2005; Feier et al. 2012, 2013), and complex IV (Feier et al. 2012, 2013; Prince et al. 1997), in striatum and other DA-containing brain areas of rat. Consistently, METH (5 mg/kg, i.p., four times, 2-h interval) was also found to decrease profoundly the cytochrome c oxidase activity in the mitochondrial fraction of rat’s frontal cortex 12 h after the last injection (Bachmann et al. 2009). Furthermore, a significant decrease in complex I activity in mitochondrial P2 homogenate from mice’s brain was also associated with METH, both in vivo (10 or 20 mg/kg, i.p., twice, 2 h apart), 5 days after the last METH injection, and in vitro (1–10 µM), in a concentration-dependent manner, after a 60-min exposure period (Thrash et al. 2010). In turn, decreased expression of complex I, as revealed 24 h after METH administration (30 mg/kg, i.p., one daily, for 7 days), was also associated with drug-induced neurotoxicity in mice (Klongpanichapak et al. 2006). Likewise, in SH-SY5Y cells, METH (1.68 mM, 24 or 48 h) was also shown to cause a time-dependent decrease in complexes IV (subunits I, II and IV) and V (β subunit) expression, though no alterations were found on the expression profile of complexes I, II, and III (Wu et al. 2007). Furthermore, an up-regulation of cytochrome c oxidase subunit I (COXI) gene and a down-regulation of the genes codifying for nicotinamide adenine dinucleotide dehydrogenase subunit II (NDII) in substantia nigra (Barrett et al. 2001) and ventral midbrain (Xie et al. 2002), 12 h after METH administration [single dose of 45 mg/kg, subcutaneous (s.c.)], as revealed by a microarray hybridization approach, were linked to drug-induced dopaminergic neuronal injury in mice. Of note, the COXI gene expression alterations revealed by microarray hybridization in the ventral midbrain, which were observed even after 24 h of METH exposure (single dose of 45 mg/kg, s.c.), were consistently correlated with changes in messenger ribonucleic acid (mRNA) levels (Xie et al. 2002). Notably, by preventing METH-induced hyperthermia (one cage containing METH-treated animals was placed on ice during the entire dosing regimen), the inhibition of mitochondrial complexes II–III observed in striatal mitochondria of rats administered with METH (10 mg/kg, s.c., four times, 2-h interval), was not rescued 1 h after the last injection, thus suggesting that METH’s effects on mitochondrial function occur independently of its hyperthermic effects (Brown et al. 2005). Furthermore, the co-administration of the N-methyl-D-aspartate receptor antagonist MK-801 or the peroxynitrite scavenger 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato Iron (III) attenuated the METH-induced inhibition of the mitochondrial complex II (Brown et al. 2005), thus providing strong evidences for a correlation among METH-induced effects on glutamatergic system (excitotoxicity), oxidative stress, and mitochondrial function inhibition (Brown et al. 2005).
d-AMPH (2 mg/kg, i.p., one daily, for 14 days) was shown to induce a marked inhibition of complexes I, II, III and IV of the ETC in rat’s hippocampus, striatum, prefrontal frontal cortex, and amygdala, as measured around 2 h after the last injection (Moretti et al. 2011; Valvassori et al. 2010). A reduction in the activity of complex I, in the amygdala, and complexes III and IV in hippocampus, striatum, prefrontal frontal cortex, and amygdala was also found in rats about 2 h after intraperitoneal administration of a single dose of 2 mg/kg of d-AMPH (Feier et al. 2012). Despite this, previous studies in NT2 rho0 cells [cells with trace-to-no complex I, II/III, and IV activity, but with normal ΔΨm, as compared to NT2 rho+ cells (Cardoso et al. 2001)] revealed more pronounced toxic effects, as compared to the effects observed in NT2 rho+ cells, following AMPH exposure (1 or 2 mM, for 24 h), indicating that the absence of fully functional ETC renders cells more sensitive to AMPH’s toxic effects (Cunha-Oliveira et al. 2006).
MDMA (10 mg/kg, i.p., four times, 2-h interval) was also shown to cause an inhibition of complex I and II activity in rat’s striatum, 12 h after the last injection (Quinton and Yamamoto 2006). In mouse’s striatum, high doses of MDMA (10, 20, 30 mg/kg, i.p., every 2 h) were also shown to decrease the activity of mitochondrial complex I, 1, 3, 6, 12, or 24 h after the last MDMA injection (Puerta et al. 2010). Furthermore, deletions in the genes coding for nicotinamide adenine dinucleotide dehydrogenase subunit I (NDI) and NDII of the mitochondrial complex I and for COXI of the mitochondrial complex IV were found in isolated mitochondria from several brain areas of rat, including prefrontal cortex, hippocampus, striatum, raphe nuclei, amygdala, substantia nigra, and ventral tegmental area, 2 weeks after MDMA administration (10 mg/kg, i.p., four times, 2-h interval) (Alves et al. 2007, 2009a). Notably, the effects of MDMA on mtDNA were almost completely rescued by co-administration of the monoamine oxidase (MAO)-B inhibitor selegiline (Alves et al. 2007) or acetyl-L-carnitine (Alves et al. 2009a). In these studies, apart from the deletions on mtDNA, MDMA also decreased the expression of the subunits NDII and COXI of the mitochondrial complexes I and IV, respectively (Alves et al. 2007, 2009a, b), which were also almost completely prevented by co-administration of the MAO-B inhibitor selegiline (Alves et al. 2007) or acetyl-L-carnitine (Alves et al. 2009a), but not by inhibiting MAO-A with clorgyline (Alves et al. 2009b) (for better understanding about the involvement of MAO in the neurotoxic effects of MDMA and other amphetamines see Sect. “Involvement of monoamine oxidase in the neurotoxicity of amphetamines”).
From these studies, it is likely to assume that an interference with the ETC functioning may be an important feature in mediating the amphetamines-induced neurotoxicity. Taking into account the above-referred findings, the ETC’s complexes described to be targeted by amphetamines are presented in Fig. 2.
Changes in oxygen consumption and ATP levels as an index of amphetamine-induced impairment of mitochondrial electron transport chain functioning
One of the most expected consequences resulting from a perturbation of mitochondrial ETC functioning is an impairment of ATP generation. Indeed, many studies have associated ATP depletion with amphetamines-induced neurotoxic effects.
An initial study, evaluating the relationship between energy impairment and METH’s effects in dopaminergic neurons, showed striatal ATP depletion 1.5 h after METH administration to mice (10 mg/kg, i.p., four times, 2-h interval). These effects of METH on ATP levels revealed to be selective for the striatum, as ATP concentrations were not affected in cerebellar cortex and hippocampus (Chan et al. 1994). This study raised the possibility that perturbations of mitochondrial energy metabolism play a role in METH-induced dopaminergic neurotoxicity.
Another in vivo study reported a quick decrease in ATP brain levels (30 and 45 min) after METH administration (5 mg/kg, i.p.) to rats (Shiba et al. 2011). Simultaneously, ADP and adenosine 5′-monophosphate were increased, resulting in decreased energy charge rate. This effect was finely correlated with increased levels of lactate (metabolite of the glycolytic system) (Shiba et al. 2011), which indicates a major role for glycolytic pathways’ impairment in that effect. Despite this, oxygen partial pressure in rat’s cortex and striatum temporarily increased after METH administration (5 mg/kg), and then reduced to a steady state in both brain areas (Shiba et al. 2011), thereby suggesting that METH-induced alterations in ETC function may, at least in part, be involved in lowering ATP brain levels.
Other in vitro studies have consistently reported the amphetamines’ ability, including METH (Ajjimaporn et al. 2005), AMPH (Klongpanichapak et al. 2007), or MDMA’s metabolite (Capela et al. 2007b) in decreasing neuronal ATP levels. Using human dopaminergic SK-N-SH cells as experimental model, Ajjimaporn et al. (2005) correlated decreased cell viability with ATP depletion following 24 h of METH exposure (0.1–1 mM). A further study, using the same experimental model, also associated AMPH (0.25–1 mM, for 24 h)-induced cell death with ATP depletion, an effect partially attenuated by the antioxidant melatonin (Klongpanichapak et al. 2007). In primary cultures of cortical neurons, the toxic effects observed after exposure to the MDMA’s metabolite 5-(glutathion-S-yl)-N-methyl-α-methyldopamine [5-(GSH)-N-Me-α-MeDA, 400 µM], for 6 h, also resulted in decreased neuronal ATP, which was efficiently attenuated by the antioxidant N-acetylcysteine (Capela et al. 2007b). Despite this, a role for mitochondrial ETC impairment in this effect has not been consistently provided, thus opening the possibility that an impairment of glycolytic pathways may also contribute to this effect. Nevertheless, since most brain ATP is generated by mitochondria (mitochondria are thought to produce more than 90 % of neuronal ATP) (Ho et al. 2012; Van Laar and Berman 2013), this raises the possibility that changes in ETC function may be crucial determinants contributing to the ATP depletion induced by amphetamines.
Studies in freshly isolated mitochondria from M213 cells, an immortalized rat striatal cell line, also sustained the METH’s ability (2 mM, after 2 and 4 h) to decrease succinate-supported mitochondrial respiration (Deng et al. 2002). Nevertheless, considering the limited amount of data concerning the role of ATP depletion on amphetamines’ neurotoxic effects, more studies are needed in this field, to appraise its real impact in producing neuronal degeneration and toxicity.
Changes in mitochondrial membrane potential as an index of amphetamines-induced impairment of mitochondrial electron transport chain functioning
The maintenance of a correct ETC functioning is critical in supporting an adequate mitochondrial function. As such, it is likely to consider that modifications in the activity of the ETC’s complexes, including direct or indirect inhibition or modified expression, may result in ΔΨm dissipation, thus affecting the rate of ATP generation through the complex V.
Increased evidences have attributed a role for changes on mitochondrial polarization status in amphetamines-induced neurotoxicity. Cell culture studies with mice’s cortical, striatal, or mesencephalic astrocytes showed a disruption of the ΔΨm following METH exposure (4 mM), with cortical cells showing lower responsiveness to METH’s effects (in both striatal and mesencephalic astrocytes, a dissipation of the ΔΨm occurred as early as 8 h after METH exposure, although just observed after 12 h in cortical cells) (Lau et al. 2000). Through flow cytometry methodologies, another study revealed decreased ΔΨm as an early event (from 1 h) associated with METH (1.68 mM)-induced cytotoxicity in SH-SY5Y cells (Wu et al. 2007). In mesencephalic dopaminergic neuronal cells (N27 cell line), METH exposure (1 or 2 mM), for 3, 6, or 12 h, also produced a dramatic reduction in the intensity of orange red spots and predominance of green staining of JC-1 dye, consistent with loss of the ΔΨm (Lin et al. 2012).
In cultured cortical neurons from rat, AMPH (0.5 mM, 24 h) was shown to reduce the rhodamine 123 (RHO 123) accumulation, suggesting, therefore, disruption of the ΔΨm (Cunha-Oliveira et al. 2006).
Studies in synaptosomes from whole mouse’s brain revealed that the toxic effects triggered by MDMA’s metabolites N-methyl-α-methyldopamine (N-Me-α-MeDA, 200 µM) and 5-(glutathion-S-yl)-α-methyldopamine [5-(GSH)-α-MeDA, 200 µM] did not rely in modifications on mitochondrial polarization status, suggesting that mitochondrial-dependent pathways did not appear to play a major role on MDMA’s toxic effects (Barbosa et al. 2012).
Although, to date, studies reporting the amphetamines’ ability to target ΔΨm are limited, studies using immortalized human HepG2 cells (model of human hepatocytes) have reported the ability of other AMPH-like drugs, including MDMA, 4-methylthioamphetamine and d-AMPH, to disrupt ΔΨm (Silva et al. 2013a, b), agreeing, therefore, with the results evidenced in brain cells.
Reactive oxygen species production as an index of mitochondrial electron transport chain functioning impairment caused by amphetamines
The main site of superoxide \(\text{O}^{\cdot-}_{2}\) production is considered to be the ETC in the mitochondria, thus leading to consider that mitochondrial deficits play a crucial role in the pathogenesis of brain injury, by initiating and promoting oxidative stress (Adam-Vizi 2005). Studies in isolated mitochondria have identified complexes I and III as major sites of ROS production in cells. Under physiological conditions, \(\text{O}^{\cdot-}_{2}\) produced in the mitochondria has a very short half-life, since it is efficiently dismutated by manganese superoxide dismutase (SOD) in the mitochondrial matrix (Forman and Azzi 1997) or by copper/zinc SOD in the intermembrane space and cytosol (Fridovich 1995) giving rise to the formation of \(\text{O}^{\cdot-}_{2}\) and hydrogen peroxide (H2O2) (Vafai and Mootha 2012). Notably, METH (4 × 3 mg/kg, i.p., 2-h apart)-induced long-term striatal toxicity, as measured 1 week later, was attenuated in transgenic mice over-expressing the human mitochondrial SOD, as compared with the correspondent non-transgenic littermates (Maragos et al. 2000), which indicates that a perturbation of mitochondrial function, with consequent increased ROS formation, may have contributed to the observed neurotoxic effects. Indeed, inhibition of respiratory complexes of in situ mitochondria was associated with enhanced ROS generation, though different complexes had to be inhibited to different degrees in order to induce excessive ROS production (Sipos et al. 2003).
Amphetamines have been demonstrated to inhibit complexes within the ETC (see Sect. “Studies linking inhibition of electron transport chain’s complexes function to amphetamines-induced neurotoxicity”). Therefore, this inhibition is likely to increase ROS and contribute to amphetamine-induced neurotoxicity. In accordance, there are several reports indicating that various inhibitors of the ETC increase ROS formation. For example, the metabolite of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, 1-methyl-4-phenylpyridinium (MPP+), inhibits the complex I of the ETC (Schapira 2010) and increases ROS formation in mice’s striatum (Castro-Caldas et al. 2012). 3-Nitropropionic acid, a complex II inhibitor, also increases the rate of ROS formation (Liot et al. 2009). These data suggest that inhibition of complexes within the ETC alters the balance between ROS formation and antioxidant systems, such that there is a net increase in ROS accumulation, which may damage neurons. Thus, considering that amphetamines-induced neurotoxicity has been linked to inhibition of the mitochondrial complexes of the ETC, it is likely to consider that ROS may play a crucial role in amphetamines’ neurotoxic effects. Indeed, many studies have implicated increased ROS formation on the neurotoxic effects triggered by AMPH-like compounds.
Studies in striatal and mesencephalic astrocytes revealed METH (4 mM, for 8–48 h)-induced ROS formation together with ΔΨm dissipation (Lau et al. 2000), thus suggesting a main involvement of mitochondria on these effects. From this study, subsequent studies also demonstrated the potential of METH to induce ROS formation in striatal synaptosomes (Escubedo et al. 2005; Pubill et al. 2005), cerebellar granule neurons (Jiménez et al. 2004), SH-SY5Y cells (Langsdorf and Chang 2011; Wu et al. 2007), and immortalized human brain microvascular endothelial cells (Zhang et al. 2009). In mouse’s brain, METH (10 or 20 mg/kg, i.p., twice, 2-h apart)-induced inhibition of mitochondrial complex I was also associated with increased generation of ROS, as ascertained 5 days after the last METH administration (Thrash et al. 2010). In nuclear factor E2-related factor 2-deficient knockout mice, unlike their wild-type littermates, METH (10 mg/kg, i.p., on gestational days 16 and 17—total of two doses) did not increase the mRNA levels of ROS-protective hemeoxygenase-1, nicotinamide adenine dinucleotide phosphate quinoneoxidoreductase, and oxoguanineglycosylase1 in fetal brain tissue, as measure 6 and 24 h after the METH regime (Ramkissoon and Wells 2013). These results indicate a crucial role for this transcription factor in regulating physiological response to METH.
Repeated administration of low doses of d-AMPH (2 or 4 mg/kg, i.p., one daily, for 7 days) increased the formation of \(\text{O}^{\cdot-}_{2}\) in submitochondrial particles from rat’s hippocampus and prefrontal cortex, approximately 2 h after the last d-AMPH injection (Frey et al. 2006). In SK-N-SH cells, d-AMPH (1 mM)-induced neurotoxicity relied on ROS production and ATP depletion (Klongpanichapak et al. 2007), thus suggesting a role for mitochondria-dependent pathways.
As reported by earlier studies, MDMA administration to rats also resulted in increased ROS formation (Colado et al. 1997, 1999; Shankaran et al. 1999). Similar observations were reported in cerebellar granule neurons (Jiménez et al. 2004) and mouse striatal synaptosomes (Chipana et al. 2006, 2008) exposed to MDMA. Nevertheless, other studies showed that not only the amphetaminic compounds MDMA and its N-demetylated analogue 3,4-methylenedioxyamphetamine, but also MDMA’s metabolites 5-(GSH)-α-MeDA and 2,5-bis(glutathion-S-yl)-α-methyldopamine increased ROS formation in both human 5-HTT- and DAT-transfected SK-N-MC cells, in a concentration- and time-dependent manner (Jones et al. 2004). In accordance, subsequent studies established that the neurotoxic effects mediated by 5-(GSH)-α-MeDA and other MDMA’s metabolites, namely α-methyldopamine (α-MeDA), N-Me-α-MeDA, 5-(GSH)-N-Me-α-MeDA, 5-(N-acetylcysteine-S-yl)-α-methyldopamine [5-(NAC)-α-MeDA], and 5-(N-acetylcysteine-S-yl)-N-methyl-α-methyldopamine [5-(NAC)-N-Me-α-MeDA], in cultured cortical neurons from rat (Capela et al. 2007b) or mouse brain synaptosomes (Barbosa et al. 2012) relied on ROS formation. Notably, increased ROS formation was also demonstrated in SH-SY5Y differentiated cells exposed to the mixture of MDMA and six of its metabolites [α-MeDA, N-Me-α-MeDA, 5-(GSH)-α-MeDA, 5-(GSH)-N-Me-α-MeDA, 5-(NAC)-α-MeDA and 5-(NAC)-N-Me-α-MeDA] at in vivo relevant concentrations (each compound at equimolar concentrations of 10 or 20 µM), during 6 h (Barbosa et al. 2014b), thereby indicating that MDMA’s effects in vivo may result from a combined effect among parent compound and metabolites. Considering that mitochondrial ETC is the main site of ROS production into the cell (Adam-Vizi 2005), these results suggest a role for mitochondria in these effects. Nevertheless, MDMA’s metabolites-induced ROS formation in mouse brain synaptosomes was independent of the mitochondrial polarization status (Barbosa et al. 2012), thus indicating that this effect also relies on mitochondrial-independent pathways. Indeed, the mechanisms underlying the toxicity of MDMA’s metabolites are thought to involve in the inherent reactivity of their catechol moiety (Carvalho et al. 2004). MDMA’s metabolites, remaining redox actives, are prone to oxidation, originating from the corresponding ortho-quinones (Macedo et al. 2007; Spencer et al. 1998), which may further undergo redox cycling to the corresponding semi-quinone radicals with consequent ROS formation (Erives et al. 2008). Additionally, other pathways have been described to contribute to amphetamine-induced ROS formation, including MAO metabolism of monoamine neurotransmitters, DA auto-oxidation, hyperthermia, or glutamate (Brown and Yamamoto 2003; Capela et al. 2009). Therefore, it is likely to consider that other pathways, independent on mitochondria, may also be key determinants contributing to the ROS generation triggered by AMPH-like compounds.
Involvement of monoamine oxidase in the neurotoxicity of amphetamines
MAO is a family of flavin adenine dinucleotide-containing enzymes located in the mitochondrial outer membrane (Fišar 2012). There are three functionally distinct domains in MAO molecule: substrate domain, flavin-binding domain, and a region that attaches the protein to the mitochondrial membrane (Edmondson et al. 2009).
Monoamine metabolism of endogenous or exogenous monoamines by MAO involves oxidative deamination (using O2 as the electron acceptor) to the corresponding aldehyde and ammonia (from primary amines) or substituted amine (in the case of secondary amines), with generation of H2O2 (Vilar et al. 2012). Indeed, in the brain, metabolism of monoamines by MAO constitutes the main source of H2O2. Under physiological conditions, H2O2 is then inactivated by glutathione peroxidase. However, in the presence of transition metals, it can be converted, through the Fenton reaction, to the highly reactive hydroxyl radical, which presents widespread deleterious effects (Fišar 2012).
Two isoforms of MAO enzymes, MAO-A and MAO-B, are present in most mammalian tissues, which are distinguished by their sensitivities to the acetylenic inhibitors clorgyline and L-deprenyl (selegiline), and by their substrate specificities. Whereas MAO-A is inhibited by low concentrations of clorgyline and is more active in catalyzing the oxidation of 5-HT and NA, MAO-B is selectively inhibited by low concentrations of L-deprenyl and is more active toward benzylamine and 2-phenylethylamine. DA, adrenaline, tryptamine, and tyramine might be oxidized by both forms (Alves et al. 2007; Youdim et al. 2006).
The administration of amphetamines results in a phase of abrupt increase in the extravesicular levels of monoamine neurotransmitters inside nerve endings, mainly 5-HT, DA, and NA, which are essentially metabolized by MAO (Alves et al. 2007; Barbosa et al. 2012; Capela et al. 2009; Carvalho et al. 2012). Furthermore, amphetamines have been also shown to partially inhibit MAO activity, both in vitro and in vivo (Ask et al. 1985; Green and El Hait 1980; Scorza et al. 1997).
Studies with human recombinant MAO sustained METH and p-methoxymethamphetamine ability to inhibit MAO enzymes, although with lower effect on MAO-B (METH, IC50 = 41 µM for MAO-A and >200 µM for MAO-B; p-methoxymethamphetamine, IC50 = 1.7 µM for MAO-A and 58 µM for MAO-B) (Matsumoto et al. 2014). d-AMPH was reported to inhibit MAO-A by competing with the flavin to its active site in the enzyme (Ramsay and Hunter 2002). In the same way, the AMPH metabolite p-hydroxyamphetamine [p-OHA, 80 µg/mouse, intracerebroventricular] appreciably inhibited MAO-A activity, without affecting MAO-B activity, in homogenates of the mouse’s striatum, hypothalamus, and the rest of the forebrain, as measured 20, 40, and 60 min after metabolite administration. In contrast, in the same study, another AMPH metabolite, p-hydroxynoradrenaline (80 µg/mouse, intracerebroventricular), did not inhibit any type of MAO (Arai et al. 1990). Nevertheless, when assessing in vitro the effect of these metabolites, p-OHA (1 µM, 5 min) and p-hydroxynoradrenaline (10 µM, 5 min), on intra- and extrasynaptosomal MAO-A activity, using mouse’s forebrain homogenates, it was found an inhibition of the intrasynaptosomal deamination of 5-HT by MAO-A for both metabolites, with p-OHA being more potent (Arai et al. 1990). Taken together, these results clearly suggested that p-OHA might accumulate inside 5-HT nerve terminals through the uptake system and, concomitantly, inhibit MAO-A activity.
MDMA was shown to competitively inhibit 5-HT metabolism by rat’s brain MAO-A (IC50 value of 44 µM) and MAO-B (IC50 value of 370 µM) activities, showing, therefore, a selective inhibition of MAO-A (Leonardi and Azmitia 1994). Consistently, studies with human recombinant MAO reported an highly selective inhibition of MAO-A (IC50 = 34 µM), with lower inhibition of MAO-B (IC50 = 110 µM) (Matsumoto et al. 2014). Studies in brain mitochondria reported an inhibition of MAO-B not only by MDMA, but also by its oxidative metabolite α-MeDA, with IC50 values of 1.00 ± 0.23 mM and 70.10 ± 13.20 µM, respectively (Escubedo et al. 2011). p-Methoxyamphetamine was also shown to be an highly selective inhibitor of MAO-A, possessing only weak activity against the MAO-B (Green and El Hait 1980; Matsumoto et al. 2014). o-Methoxyamphetamine and m-methoxyamphetamine were also documented to inhibit MAO both in vitro and in vivo with potencies comparable with or less than that of d-AMPH (Green and El Hait 1980). Additionally, inhibition of MAO enzymes has been also reported for other amphetaminic derivates, namely 4-methylthioamphetamine (IC50 = 0.25 µM for MAO-A and 65 µM for MAO-B) and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI, IC50 = 37 µM for MAO-A and >200 µM for MAO-B) (Matsumoto et al. 2014). As such, it is of general consensus that amphetamines show selective inhibition to MAO-A, with a lower inhibitor activity against MAO-B.
Probably, the most convincing evidence toward the contribution of MAO activity to the neurotoxicity of amphetamines has been revealed from studies using MAO inhibitors and MAO-B-deficient mice. Mitochondrial toxicity (lipid peroxidation and protein carbonylation) and deletions in the genes of NDI and NDII subunits of the mitochondrial complex I and COXI subunit of the mitochondrial complex IV induced by MDMA (10 mg/kg, i.p., four times, 2-h interval), in an adolescent rat model (postnatal day 45), were almost completely attenuated by MAO-B inhibition with L-deprenyl (2 mg/kg, i.p., 30 min before the first MDMA injection), as ascertained 2 weeks later (Alves et al. 2007). Notably, the particular vulnerability of hippocampus to MDMA-induced mtDNA deletions revealed in this study (Alves et al. 2007) was further supported by a recent work, in which MDMA-induced increased expression of MAO-B in rat’s brain was restricted to the hippocampal region (Cuyas et al. 2013). Furthermore, in rats, MAO-B inhibition with L-deprenyl (2 mg/kg, i.p., 30 min before MDMA injection) (Sprague and Nichols 1995a, b) or by using an antisense oligonucleotide targeted at MAO-B [0.5 µL/h of a 50 µM solution (600 pmol/day), for 7 days before MDMA administration] (Falk et al. 2002) also attenuated the 5-hydroxytryptaminergic neurotoxicity induced by MDMA (40 mg/kg, s.c.) 7 days later, in a similar extension to those that were observed in MAO-B-deficient mice (Fornai et al. 2001).
On the other hand, studies evaluating the role of MAO-A in the neurotoxicity induced by amphetaminic compounds have shown contrary results to those observed for MAO-B. Inhibition of MAO-A by clorgyline administration (1 mg/kg, i.p., 30 min before the first MDMA injection) had no protective effect on the alterations induced by MDMA (10 mg/kg, i.p., four times, 2-h interval) in rat’s brain mitochondria (increased lipid peroxidation, protein carbonylation, and decreased expression of the ETC’s subunit NDII), as measured 2 weeks later (Alves et al. 2009b). Particularly, MAO-A inhibition even intensified MDMA-induced decreased expression of COXI subunit (Alves et al. 2009b). Indeed, it has been suggested that the neurotoxicity induced by MDMA may be explained with the following sequence of events: (1) depletion of 5-HT from 5-hydroxytryptaminergic neurons; (2) increase in DA synthesis and release, in part attributable to the stimulation of serotonin 2A receptors; (3) increase in extracellular DA; (4) transport of DA into 5-HT nerve terminals by the 5-HT transporter; (5) and deamination of DA inside 5-HT nerve terminals by MAO-B, with the consequent formation of H2O2 and related oxidative stress, leading to the selective 5-HT neuronal degeneration (Sprague and Nichols 1995b). Considering that MAO-A is expressed predominantly in catecholaminergic neurons (Shih et al. 1999), it might be hypothesized that its inhibition will increase the availability of DA for an uptake by 5-hydroxytryptaminergic neurons and subsequent MAO-B metabolism. This hypothesis may explain the lack of protective effects, or even potentiation of toxicity, mediated by MAO-A inhibition against MDMA-induced mitochondrial neurotoxicity (Alves et al. 2009b).
Furthermore, microdialysis studies have evaluated the role of MAO-A inhibition in MDMA-induced changes in 5-HT brain levels under normal and elevated ambient temperatures. In experiments conducted at a normal room temperature of 22 °C, the co-administration of MDMA with MAO-A inhibitors [clorgyline (10 mg/k, i.p., 24 h before MDMA) or moclobemide (20 mg/kg, i.p., 60 min before MDMA)] potentiated the increase in extracellular 5-HT levels caused by MDMA (10 mg/kg, i.p.) alone in rat’s brain (Freezer et al. 2005; Hewton et al. 2007). However, under the same conditions, the increased extracellular 5-HT brain levels in rats administrated with p-methoxyamphetamine (10 mg/kg, i.p.) were not significantly modified by co-administration of clorgyline or moclobemide (Freezer et al. 2005; Hewton et al. 2007). In experiments performed at an elevated room temperature of 30 °C, similar results were found, either for MDMA (10 mg/kg, i.p.) or for p-methoxyamphetamine (10 mg/kg, i.p.), by inhibiting MAO-A with moclobemide (20 mg/kg, i.p., 60 min before drug) (Stanley et al. 2007) thereby suggesting that the effect of MAO-A inhibition was independent on the hyperthermic response associated with these drugs.
Some cases of human deaths have been reported to be a consequence of the concomitant ingestion of AMPH-like drugs and MAO-A inhibitors, which have been attributed to the 5-HT syndrome (Pilgrim et al. 2010, 2012; Vuori et al. 2003).
Amphetamines and mitochondrial biogenesis
The mitochondrial biogenesis, the beginning of the mitochondrial “lifecycle,” encompasses the coordinated synthesis of nuclear DNA- and mtDNA-encoding proteins, together with membrane synthesis and the proper targeting and folding of ETC’s subunits. Nevertheless, the continuous mitochondrial renewal is sustained by a physiological equilibrium between organelles’ biogenesis and selective degradation through autophagic processes (mitophagy) (Herrmann et al. 2012; Zhu et al. 2013). An increased mitochondrial biogenesis reflect a long-term adaptive response, though it is not always required to meet transiently increased energetic needs. Thus, transient changes in energy demands can be met by increases in the expression of a mitochondrial subset of genes or of crucial regulators and by the enhancement of mitochondrial function (Hock and Kralli 2009). Some physiological states, such as caloric restriction, endurance exercise, and long-lasting cold exposure, and exogenous agents, including, thiazolidinediones and resveratrol, are described to increase mitochondrial biogenesis (Hock and Kralli 2009; Onyango et al. 2010).
A complex network of hormone- or growth factor-initiated intracellular signaling pathways and the resultant activation of nuclear transcription factors regulate mitochondrial biogenesis (Zhu et al. 2013). Among these, peroxisome proliferator-activated receptor gamma co-activator 1α, nuclear respiratory factors 1 and 2, which control expression of nuclear-encoded mitochondrial structural proteins (Wu et al. 1999), and transcription factor A mitochondrial, a nuclear-encoded transcription factor crucial for replication, transcription, and maintenance of mtDNA (Kang and Hamasaki 2005), are the major players. Additionally, other factors, including hormones, second messengers, such as calcium, endothelial nitric oxide synthase or cyclic adenosine 5′-monophosphate, and kinase pathways, including protein kinase A, mitogen-activated protein kinases, or adenosine 5′-monophosphate-activated protein kinase catalytic subunit α-2, control mitochondrial biogenesis at different levels, by regulating PGC-1α expression, protein localization, or post-translational modifications (Devaux et al. 2010; Zhu et al. 2013). The nascent mitochondrial proteins, synthezised as pre-proteins, need to be imported, processed, and efficiently assembled in mitochondria, through complex processes, tightly regulated by post-transcriptional mechanisms, and the target of rapamycin (TOR) signalling pathway (Devaux et al. 2010).
Accumulated evidences along years suggest that amphetamines might target mitochondrial biogenesis. Using a microarray hybridization approach, Xie and co-workers (Xie et al. 2002) and Barrett and co-workers (Barrett et al. 2001) correlated an up-regulation of COXI gene and a down-regulation of the genes codifying for NDII subunit of the ETC in the ventral midbrain and substantia nigra of mice, respectively, to METH (45 mg/kg, s.c.)-induced dopaminergic neuronal injury, as measured 12 h after METH administration. Notably, the expression patterns revealed by microarray hybridization were consistently correlated with changes in mRNA levels and attenuated by DAT inhibition with WIN35428 (12.5 mg/kg, i.p., immediately before METH) (Xie et al. 2002). In the same way, a study evaluating the molecular mechanisms underlying METH’s neurotoxic effects linked increased mitochondrial mass and decreases in mtDNA copy number and mitochondrial protein content per mitochondrion to the neurotoxicity induced by METH (1.68 mM, 48 h) in human dopaminergic neuroblastoma SH-SY5Y cells (Wu et al. 2007), adding considerable evidences that METH target mitochondrial biogenesis. Other studies indicating an impairment of the protein kinase B (Akt)/mammalian target of TOR pathway in METH-induced neurotoxicity (Gonçalves et al. 2012; Kongsuphol et al. 2009; Li et al. 2012), strongly suggest a role for mitochondrial biogenesis alteration in its neurotoxic effects.
A study with the hallucinogenic amphetaminic compound DOI, a potent serotonin 2A receptor agonist, revealed increased mitochondrial biogenesis in rabbit renal proximal tubular cells following DOI exposure (10 or 20 µM, 24 h), as ascertained by the increased mitochondrial respiratory rate under normal or uncoupled state (Beeson et al. 2010). Nevertheless, another study published in the same year, using the same experimental model, revealed increased activity of the PGC-1α promoter and increased expression of ATP synthase β and nicotinamide adenine dinucleotide dehydrogenase 1 sub-complex 8 following DOI exposure (3 or 10 µM) for 24 h, thereby revealing, unequivocally, DOI’s ability to modify mitochondrial biogenesis (Rasbach et al. 2010). Furthermore, by using the 5-HT receptor antagonist AMI-193 or by genetic silencing of PGC-1α, DOI-induced PGC-1α expression and biogenesis of mitochondrial proteins were blocked, respectively, suggesting that 5-HT receptors may mediate the transcription of this mitochondrial biogenesis regulator (Rasbach et al. 2010). Despite this, although it remains unclear whether similar effects may mediate DOI’s neuronal effects, these evidences strongly suggest that similar mechanisms may be involved at brain level.
ROS have been described as one of the signaling molecules in inducing mitochondrial biogenesis, as a compensatory mechanism in ETC deficits, by inducing the biogenesis regulator PGC-1α (Acin-Perez et al. 2009). ROS generation also results in an increase of the PGC-α-target sirtuin-3, a NAD+-dependent deacetylase, which, in turn, activates mitochondrial SOD by deacetylation (Kim et al. 2011). Additionally, besides \(\text{O}^{\cdot-}_{2}\) and H2O 2 (Cruz et al. 2007), NO has also been described to regulate mitochondrial biogenesis (Wadley et al. 2007). Considering the accumulated evidences correlating increased ROS formation to amphetamine-induced neurotoxicity (Barbosa et al. 2012, 2014b; Capela et al. 2007b; Colado et al. 1997; Frey et al. 2006; Shankaran et al. 1999; Thrash et al. 2010), it is reasonable to consider that ROS-regulated pathways may modify mitochondrial response to biogenesis stimuli and, thus, to contribute to the neurotoxicity of amphetamines.
Can impaired mitochondrial quality control play a role on amphetamine-related brain effects?
Mitochondria have developed several mechanisms that act to generate and maintain organelle homeostasis (Baker et al. 2011; Tatsuta and Langer 2008). The first line of defense is provided by a highly conserved intraorganellar proteolytic system that conducts the surveillance of protein quality control within mitochondria. Molecular chaperones and energy-dependent proteases monitor the folding and assembly of mitochondrial proteins and selectively remove excess and damaged proteins from the organelle (Koppen and Langer 2007; Tatsuta and Langer 2008). The second level of mitochondrial quality control arises from the dynamic nature of the organelle itself. Mitochondria are constantly fusing and dividing, mediated by dynamin-like guanosine 5′-triphosphatases (GTPases) in the inner and outer mitochondrial membranes, assigning an important role for components regulating mitochondrial dynamics (Otera and Mihara 2011). However, severe damage of mitochondria impairs fusion and results in fragmentation of mitochondria, which are then selectively removed by an autophagic process, termed mitophagy (Fischer et al. 2012). The selective degradation of defective mitochondria is critical for proper neuronal function, health, and survival, since it protects them from releasing oxidants and apoptosis triggers. At this level, phosphatase and tensin homolog-induced putative kinase 1 (PINK1)/Parkin signaling pathway acts as a key regulator of mitophagy, thus allowing degradation of small and surrounding fusion-deficient mitochondria with sustained depolarization (Twig and Shirihai 2011). Parkin is localized in the cytosol, but it translocates to mitochondria with reduced ΔΨm where it ubiquitinates protein targets (Narendra et al. 2008). The adaptor protein p62 then binds ubiquitinated mitochondrial proteins and LC3 on autophagosomes, thus recruiting autophagic membranes for mitochondrial clearance (Youle and Narendra 2011). PINK1 is a serine/threonine kinase that recruits Parkin to depolarized mitochondria. In mitochondria with intact ΔΨm, PINK1 is imported and degraded by presenilin-associated rhomboid-like protein (Jin et al. 2010). In mitochondria with reduced ΔΨm, PINK1 is no longer degraded and accumulates in the outer mitochondrial membrane where it can recruit Parkin through direct interaction (Kim et al. 2008; Matsuda et al. 2010). Through its E3 ubiquitin ligase activity, Parkin also ubiquitinates mitochondrial proteins, including VDAC1 (Geisler et al. 2010), Miro (Liu et al. 2012; Wang et al. 2011b), and fusion mediators [mitofusins (Mfns)] (Gegg et al. 2010). Additionally, PINK1/Parkin axis also regulates turnover of specific ETC’s components (Vincow et al. 2013), suggesting additional roles for the PINK1/Parkin pathway in regulating mitophagy.
A recent study demonstrated that the cell death induced by METH (2 mM, 12 h)-induced cell death in a dopaminergic neuronal model, the N27 cell line, was closely associated with induction of mitophagy (Lin et al. 2012). Furthermore, over-expression of LC3 partially protected against the apoptotic cell death induced by METH (2 mM, 24 h), as measured by the extent of DNA fragmentation, suggesting a neuroprotective role for autophagy in METH-induced neurotoxicity (Lin et al. 2012). Additionally, striatal tissue isolated from METH-administrated (4 × 20 mg/kg, i.p, 2-h interval) rats also exhibited elevated LC3 1 and 7 days post-METH administration, suggesting that METH-induced autophagy may serve as an adaptive strategy for inhibiting mitochondria-mediated apoptotic cell death (Lin et al. 2012).
Another study, in PC12 cells, also provided robust cellular evidence that upon METH exposure, PINK1 increased the total number of mitochondria, concurrently recruited beclin1 (a protein essential for initiation of the autophagic process), Parkin, and ubiquitin, and enhanced the clearance of damaged mitochondria. Furthermore, in the absence of functional PINK1, and upon METH-induced autophagy stress, a failure of the autophagy system at large was observed, with marked accumulation of dysfunctional mitochondria and dramatic increase in apoptotic cell death (Lenzi et al. 2012). In accordance with these findings, Castino et al. (2008) showed typical apoptotic cell death in PC12 cells exposed to METH (1 µM, 12 h), when the autophagy/mitophagy pathway was impaired or blocked.
Using Parkin knockout mice, Takamatsu and co-workers (Takamatsu et al. 2011) attempted to clarify the role of Parkin in MDMA-induced hyperthermia, one of the causal factors of neuronal damage. Notably, an enhanced hyperthermic response in either heterozygous or homozygous knockout mice for Parkin administered with MDMA (30 mg/kg, i.p.) was found, as compared with respective wild-type littermates (Takamatsu et al. 2011). Taken together, these data suggest the PINK1/Parkin signaling pathway as a promised target to counteract the toxic effects mediated by METH and MDMA. Despite this, whether similar explanations may be extrapolated to other AMPH-like drugs remains unclear.
Effects of amphetamines on mitochondrial fusion/fission equilibrium
Mitochondria are highly dynamic and complex organelles that, undergoing regulated movement throughout the cell, continuously alter their shape, ranging between two opposite processes, fusion and fission, in response to several stimuli and cellular metabolic demands. These opposite processes are crucial in sustaining the robust structure and function of mitochondria (Saxton and Hollenbeck 2012a). Mitochondrial shapes can range from punctuate structures to tubular networks, which are probably physically contiguous structures constructed via fusion of single bean-like mitochondria (Popov et al. 2005). Continuous movements of mitochondria allow a physical contact that facilitates their fusion, thus creating single large mitochondria. Mitochondrial fusion allows the exchange of mtDNA and other matrix components between neighboring mitochondria, including damaged or senescent mitochondria, thus conferring protection against mtDNA mutations and maintaining a healthy oxidative phosphorylation (Chan 2006; Ranieri et al. 2013). Fission events permit mitochondrial separation to daughter cells during mitosis (Taguchi et al. 2007), control organelle size and shape for an efficient mitochondrial distribution in neuronal processes (Ishihara et al. 2004; Kageyama et al. 2012), allow the recovery of damaged mitochondria for degradation by mitophagy (Twig et al. 2008a), and regulate apoptosis through segregation of the most critically injured mitochondria (Youle and van der Bliek 2012).
Mitochondrial fusion is a multi-step process that requires the coordinated fusion of both outer and inner mitochondrial membranes, ultimately resulting in mixing of matrix contents. In mammalian cells, three large GTPases mediate mitochondrial fusion. Mitofusin 1 (Mfn1) and Mitofusin 2 (Mfn2), anchored to the outer membranes of adjacent mitochondria, induce outer membrane fusion, whereas optic atrophy 1 protein (OPA1), a protein residing in the intermembrane space, mediates fusion of the inner membranes (Song et al. 2009). Mfns have their amino- and carboxyl-terminals directed to the cytosol (Rojo et al. 2002), which forming homo- or hetero-protein complexes allow mitochondrial tethering and fusion through GTP hydrolysis (Koshiba et al. 2004). Whereas the Mfn’s amino-terminal GTPase domain is required for fusion activity, carboxyl-terminal domain coordinates the docking of mitochondria to one another through antiparallel binding to the carboxyl-terminal domains of Mfn molecules on adjacent mitochondria. Additionally, Mfn1 appears to mediate tethering of mitochondria more efficiently than Mfn2 (Ishihara et al. 2004). OPA1 acts as a hetero oligomeric complex, formed by a larger size OPA1 (L-OPA1) and the smaller size OPA1 (S-OPA1), in the inner mitochondrial membrane fusion (Cipolat et al. 2004). However, in mediating mitochondrial fusion, OPA1 functionality appears to require Mfn1, but not Mfn2 (Cipolat et al. 2004). Additionally, OPA1 was also described to play a key role in cristae organization and remodeling of the inner mitochondrial membrane (Cipolat et al. 2006; Frezza et al. 2006), by a mechanism independent of the mitochondrial fusion (Frezza et al. 2006).
Since knockout of Mfn1 or Mfn2 resulted in the formation of small fragmented mitochondria (Chen et al. 2007a), and that cells lacking Mfns exhibited several cellular defects, including poor cell growth, heterogeneity of inner mitochondrial membrane potential and decreased respiration (Chen et al. 2003), this indicates that mitochondrial fusion plays a crucial role in maintaining mitochondrial functionality.
Mitochondrial fission is mediated by dynamin-related protein 1 (Drp1), a member of the dynamin family of GTPases. When mitochondrial fission is stimulated, Drp1 translocates from the cytosol to specific sites on the mitochondrial outer membrane and homo-oligomerizes, forming spiral chains around mitochondria, which, using GTP hydrolysis, constrict and twist tubule to initiate mitochondrial fission (Ingerman et al. 2005; Smirnova et al. 2001). Nevertheless, it has been suggested that tubules of the endoplasmic reticulum wrap around and squeeze mitochondria at the early stage of division, prior to the assembly of Drp1 filaments on mitochondria (Friedman et al. 2011). After mitochondrial fission, Drp1 spirals likely disassemble from mitochondria for future rounds of mitochondrial fission (Ingerman et al. 2005; Smirnova et al. 2001).
In yeast, Drp1 attaches to mitochondria by binding to mitochondrial fission protein 1 (Fis1), a protein anchored to the mitochondrial outer membrane, through the adaptor protein Mdv1 (or its paralogue Caf4) (Mozdy et al. 2000). However, in mammalians, though a yeast Fis1 orthologous has been identified (hFis1) (James et al. 2003), orthologous for Mdv1 and Caf4 are not described, and the evidences supporting a role for hFis1 in mitochondrial fission are mixed. Supporting a role in fission events, over-expression of Fis1 in mammalian cells promoted mitochondrial fragmentation, and Fis1 inhibition resulted in mitochondrial elongation (Yoon et al. 2003). Similarly, in Fis1-null cells, mitochondria presented an elongated and interconnected morphology, when compared to their respective wild-type counterparts, thus indicating that Fis1 plays a role in fission events (Losón et al. 2013). Despite this, in another study, it was shown that the conditional knockdown of Fis1 did not disrupt mitochondrial morphology or Drp1 recruitment to mitochondria (Otera et al. 2010). Similarly, a recent study also reported independence on Drp1 in Fis1-regulated mitochondrial fission (Onoue et al. 2013). Thus, in mammalians, other outer mitochondrial membrane proteins have been described as putative mitochondrial receptors for Drp1. One of the molecules is mitochondrial fission factor (mff), whose knockdown resulted in mitochondrial elongation (Gandre-Babbe and van der Bliek 2008), by a mechanism independent on Fis1 (Losón et al. 2013). Subsequent observations indicating that Mff and Drp1 colocalize in puncta on mitochondria and that the exogenous expression of Mff increased the amount of Drp1 recruited to mitochondria and induced extensive mitochondrial fission (Otera et al. 2010), led to consider Mff as a strong candidate for Drp1 receptor in the outer mitochondrial membrane.
Alternatively, mitochondrial elongation factor 1 (Zhao et al. 2011), also identified as MiD49/51 (Palmer et al. 2011), was reported to promote mitochondrial fusion instead of fission, which by blocking GTP binding to the GTPase domain of Drp1 does not allow the conformational change of Drp1 multimers required for membrane fission. However, it remains unclear why MiD49/51 causes mitochondrial elongation instead of fission. According to one model, Fis1 can partially reverse the elongated mitochondrial phenotype by sequestering mitochondrial elongation factor 1 and, consequently, unblocking Drp1 GTP hydrolysis, thus leading to organelle fission (Oettinghaus et al. 2012; Zhao et al. 2011). On the other hand, a recent study demonstrated that MiD49/51 over-expression enhanced mitochondrial recruitment of Drp1, though an increased inhibitory phosphorylation of Drp1 on Serine637 was observed [decreased phosphorylation of Drp1 at Serine637 facilitates its recruitment to mitochondria (Cereghetti et al. 2008)]. However, after carbonyl cyanide m-chlorophenyl hydrazone treatment, this phosphorylation was reduced and mitochondrial fission ensues, suggesting that MiD49/51 may recruit Drp1 to mitochondria, but maintains it in an inactive state until a cellular signal triggers fission (Losón et al. 2013). In addition, the observation that B cell lymphoma-extra-long (Bcl-xL) caused a significant increase in Drp1 GTPase activity in cultured hippocampal neurons also suggests that Bcl-xL may act as a GTPase-activating protein for Drp1 in mediating mitochondrial fission (Li et al. 2008).
Increased evidences suggest that amphetaminic compounds may target fusion/fission processes, resulting in alterations of mitochondrial fusion/fission equilibrium. The cell death caused by METH (300 µM, 24 h) in rat’s hippocampal progenitor neural cells was associated with Drp1 oligomerization and consequent translocation to mitochondrial surface, by a calcium-independent mechanism, resulting in a phenotype of increased mitochondrial fragmentation (Tian et al. 2009). Furthermore, METH (2 mM, 12 h)-induced neurotoxicity in mesencephalic dopaminergic cells was closely associated with more rounded and swollen mitochondria (Lin et al. 2012).
Recently, studies conducted by our research group showed a perturbation of mitochondrial fusion/fission equilibrium associated with MDMA. Using cultured neurons from mice’s hippocampus, MDMA (1.6 mM), following a short incubation period of 90 min, led to an increased fragmentation of axonal mitochondria, a phenotype closely correlated with an impairment of axonal mitochondrial trafficking (Barbosa et al. 2014d). Similar results were further described for the mixture of MDMA and six of its major metabolites [α-MeDA, N-Me-α-MeDA, 5-(GSH)-α-MeDA, 5-(GSH)-N-Me-α-MeDA, 5-(NAC)-α-MeDA and 5-(NAC)-N-Me-α-MeDA], at in vivo relevant concentrations (each compound at a equimolar concentration of 10 µM), following an exposure period of 24 h (Barbosa et al. 2014e).
Additionally, another study associated a disruption of the inner mitochondrial membrane cristae to METH-induced neurotoxicity (Lin et al. 2012). Thus, this suggests that AMPH-like drugs, namely METH, may target key regulators of mitochondrial cristae remodeling, such as Opa1 (Cipolat et al. 2006; Frezza et al. 2006), mitofilin (Yang et al. 2012), or PINK1/Parkin pathway (Poole et al. 2008). Despite this, more studies are needed to clarify whether these proteins and pathways are implicated in the neurotoxicity of amphetamines, and what pathways drive to alterations in these proteins and/or mechanisms.
Many studies have demonstrated that excessive mitochondrial fragmentation plays an active role in apoptosis (Barsoum et al. 2006; Estaquier and Arnoult 2007; Frank et al. 2001; Pereira et al. 2010), autophagic cell death (Barsoum et al. 2006; Twig et al. 2008a, b), and necrosis (Wang et al. 2012; Zhou et al. 2012). Furthermore, impaired mitochondrial fragmentation, resulting from a failure of mitochondrial quality control mechanisms, like fusion/fission events, triggered by some neurotoxicants, such as rotenone or MPP+, has been associated with a collapse of mitochondrial network and neuronal injury or death (Büeler 2009; Qi et al. 2012; Wang et al. 2011a). Accordingly, a reduction in mitochondrial fission results in longer and more interconnected mitochondrial tubules and prevents the cell death (Barsoum et al. 2006; Yuan et al. 2007). Therefore, these data suggest a potential new mechanism underlying the neuronal effects of amphetamines.
Effects of amphetamines on neuronal trafficking of mitochondria
In neurons, essential constituents and mitochondria, which are generally synthesized or generated in the cell body, are delivered through the neuronal processes to their final destination, the synaptic terminals. Owing to their unique metabolic requirements, these areas do not display a uniform mitochondrial distribution. The large size of many neurons (up to 1 m in humans), which precludes rapid diffusion of ATP from one end of the cell to the other implies, therefore, that the energy production must be spatially matched to local energy usage. In neurons, areas with high energy requirements, such as presynaptic and postsynaptic terminals, active growth cones and axonal branches, myelination boundaries, sites of axonal protein synthesis, and nodes of Ranvier are enriched in mitochondria, compared to other cellular domains (Sheng and Cai 2012). Furthermore, though mitochondrial biogenesis may occur locally in the axon, generation of new organelles mainly occurs within the cell body. Additionally, as the degradation of organelles, such as mitochondria, ensues in the cell body, dysfunctional mitochondria need to return to the soma for an efficient degradation (mitophagy) through the autophagy-lysosomal system (Ashrafi and Schwarz 2013). As such, neurons require highly efficient mechanisms for mitochondrial transport regulation from and to the cell body to enable the rapid redistribution of mitochondria to different areas, in order to supply increased metabolic requirements.
The cytoskeleton, constituted by microtubules, actin filaments, and neurofilaments (also called intermediate filaments), is responsible for the maintenance of the highly specialized structure of neurons, also allowing axonal growth and coordinated transport and stable docking events (Sau et al. 2011). Microtubules, formed by polymers of α- and β-tubulin, are polarized structures with the “plus” end toward to the terminal and the “minus” end toward to the cell body. However, dendrites presented a mixed polarity (Sau et al. 2011; Saxton and Hollenbeck 2012b). Cytoskeletal filaments are linked to microtubule-associated proteins, such as Tau protein, which permit their stabilization (Saxton and Hollenbeck 2012b). Kinesin motors, linked to the outer mitochondrial membrane protein Miro1/2, through the adaptor proteins Trak1/2 (also known as OIP106 and OIP98/Grif-1, respectively) mediate “plus” end-directed mitochondrial transport, whereas cytoplasmic dynein mediates mitochondrial transport toward the “minus” ends of microtubules (MacAskill et al. 2010; Schwarz 2013; Sheng and Cai 2012).
Since neuronal mitochondrial trafficking is tightly regulated in response to changes in the local energetic state and metabolic demand, it requires the existence of highly coordinated mechanisms to regulate the movement of these organelles along neuronal processes and their docking to supply specific biological needs. At this level, cytosolic calcium (Chang et al. 2006, 2011; Macaskill et al. 2009; Rintoul et al. 2003; Wang and Schwarz 2009), microtubule-associated proteins (Ebneth et al. 1998; Jiménez-Mateos et al. 2006; Llorens-Martín et al. 2011; Stamer et al. 2002; Stoothoff et al. 2009), and PINK1/Parkin (Weihofen et al. 2009) are perhaps the best characterized regulators of mitochondrial transport, though other regulators like mitochondrial calcium (Chang et al. 2011), Mfn2 (Misko et al. 2010, 2012), or histone deacetylases (Chen et al. 2010; Kim et al. 2010) are also described.
The apparent relevance of impaired neuronal trafficking of intracellular organelles and materials in the context of amphetamines-induced neurotoxicity was firstly provided by Callahan and co-workers (Callahan et al. 2001). In that study, to further appraise the 5-hydroxytryptaminergic neurotoxic potential of MDMA and fenfluramine, the authors used tritiated proline to examine anterograde transport along ascending axonal projections originating in the rostral raphe nuclei of rats. They found a marked reduction in anterograde transport of labeled materials to various forebrain regions known to receive 5-hydroxytryptaminergic innervation, 3 weeks after MDMA exposure (20 mg/kg, s.c., twice daily, for 4 days) or fenfluramine (10 mg/kg, i.p., four times, 2-h interval). Furthermore, these transport perturbations were associated with lasting decrements in 5-HT axonal markers (5-HT and 5-hydroxyindoleacetic acid content), thus suggesting a role for impaired axonal transport in developing neurotoxic effects (Callahan et al. 2001). However, this study did not establish differentiation between neuronal transport of mitochondria or of other intracellular organelles or materials, not allowing, thus, a clear appraisement of the real impact of AMPH-like drugs on mitochondrial trafficking.
One year later, another study, using a microarray hybridization approach, reported a decreased expression of the microtubule-associated protein Tau in the mouse’s ventral midbrain 24 h after METH administration (45 mg/kg, s.c.) (Xie et al. 2002), suggesting a feasible alteration of neuronal mitochondrial trafficking by METH. In support of this observation, MDMA was further reported to increase Tau phosphorylation in mice’s hippocampus (Busceti et al. 2008) and to disrupt microtubular system in 5-hydroxytryptaminergic axons of rat’s frontal cortex (Ádori et al. 2011).
In the last years, two studies reported an alteration of mitochondrial movement in hippocampal neurons by monoamine neurotransmitters 5-HT and DA. Acting through the serotonin 1A receptor subtype, 5-HT increased Akt activity and, consequently, decreased glycogen synthase 3β (GSK3β) activity, thus promoting axonal transport of mitochondria (Chen et al. 2007b). Although the molecular target of the Akt-GSK3β signaling pathway remained unclear, this observation highlights the possibility that 5-HT might act as an extracellular modulator in regulating neuronal ATP distribution by controlling axonal mitochondrial distribution. By contrast, DA and DAD2 receptor agonists inhibited mitochondrial movement via the same Akt-GSK3β signaling cascade (Chen et al. 2008), suggesting that the distribution of neuronal mitochondria may occur through a conserved regulatory mechanism. Additionally, the Akt-GSK3β signaling pathway likely achieves this through the regulation of kinesin–cargo interactions (Morfini et al. 2002; Pigino et al. 2003). Considering that the acute and sustained release of monoamine neurotransmitters, including 5-HT and DA, from nerve endings is by far the most studied acute effect of amphetamines in the brain (Capela et al. 2009; Carvalho et al. 2012), these studies provided strong evidences that amphetamines’ brain effects may rely on neuronal mitochondrial trafficking alterations.
Recent studies conducted by our research group constituted the first direct evidence that AMPH-like compounds, namely MDMA, target the neuronal mitochondrial trafficking (Barbosa et al. 2014d, e). In one of these studies, MDMA (1.6 mM), after a short incubation period of 90 min, was shown to reduce the overall mitochondrial movement along axonal processes of cultured neurons from mice’s hippocampus, in a time-dependent manner. This effect was closely associated with increases in intracellular free calcium levels, both in cell body and neuronal extensions, increased phosphorylation of Tau protein, at Thr181 residue, and excessive fragmentation of axonal mitochondria (Barbosa et al. 2014d). Over-expressing experiments with wild-type Miro1 or its mutant version (Miro1 ΔEF) lacking the EF hand calcium-binding domains (it does not respond to calcium levels alterations) revealed an independence on Miro1 regulatory functions through cytosolic calcium in MDMA-induced mitochondrial trafficking impairment (Barbosa et al. 2014d). Nevertheless, experiments in Tau-null neurons revealed a partial reversion of mitochondrial transport deficits, in a similar fashion to those observed in wild-type neurons over-expressing a GSK3β-kinase death construct (GSK3β Aln9, which lacks kinase activity—in neurons, Tau phosphorylation is largely dependent on GSK3β) (Barbosa et al. 2014d), supporting a main role for Tau protein and GSK3β in MDMA’s mitochondrial trafficking phenotype. Lastly, mitochondrial fusion/fission-regulated events were also shown to play a crucial role in MDMA-induced mitochondrial trafficking arrest. Over-expression of wild-type Mfn2 or Drp1 K38A (mutant Drp1 construct that lacks fission properties) partially recovered the MDMA’s mitochondrial transport phenotype, although over-expression of Mfn2 R94Q (mutant Mfn2 construct that lacks fusion and transport properties) did not (Barbosa et al. 2014d). This indicates that fully functional Mfn2 was required to reverse MDMA’s effects and suggest that MDMA might alter fusion mechanisms.
Since MDMA and its metabolites have been shown to co-exist in the brain following peripheral administration of MDMA (Chu et al. 1996; Erives et al. 2008; Jones et al. 2005), in another study, our research group introduced a new approach, in which parent compound and six of its major in vivo metabolites [α-MeDA, N-Me-α-MeDA, 5-(GSH)-α-MeDA, 5-(GSH)-N-Me-α-MeDA, 5-(NAC)-α-MeDA and 5-(NAC)-N-Me-α-MeDA] were combined as a mixture, at in vivo relevant concentrations (each compound at a equimolar concentration of 10 µM), and its effects on neuronal mitochondrial trafficking were further appraised. Using this experimental design, a reduction in overall mitochondrial motility along axonal processes of cultured hippocampal neurons following exposure to the mixture for 24 h was shown. Additionally, this effect was shown to rely on mitochondrial fusion/fission-dependent mechanisms, since the over-expression of wild-type Mfn2 or Drp1 K38A almost completely rescued the mitochondrial trafficking arrest caused by this mixture (Barbosa et al. 2014e). Taking into account the above-referred findings, a mechanistic pathway for the deregulation of neuronal mitochondrial trafficking caused by MDMA and by the mixture of MDMA and its metabolites was postulated, which is illustrated in Fig. 3. To a more detailed review about the role of mitochondrial trafficking impairment in the context of amphetamines-induced neuronal degeneration and toxicity see Barbosa et al. (2014c).
Postulated mechanisms for the mitochondrial trafficking impairment induced by MDMA and its metabolites in cultured hippocampal neurons. MDMA, as well as the mixture of MDMA and its metabolites, impair axonal transport of mitochondria, in both anterograde and retrograde directions [1]. Intracellular calcium raises, both in the cell body and neuronal extensions [2], are associated with the transport deficits induced by MDMA, though these effects most likely rely on Miro1-independent mechanisms [2]. Nevertheless, MDMA increases Tau phosphorylation at Threonine 181 residue [3], which may contribute partially to its effects on mitochondrial neuronal trafficking, most likely due to microtubules destabilization [4]. MDMA’s effects on mitochondrial transport also depended on GSK3 activity [5], a main kinase for Tau protein. Furthermore, MDMA, as well the mixture of MDMA and its metabolites, increase the fragmentation of axonal mitochondria. Over-expression of fully functional Mitofusin 2 (Mfn2) or dynamin-related protein 1 (Drp1) K38A, a Drp1 construct that down-regulates mitochondrial fission, partially rescue the trafficking deficits triggered either by MDMA or by the mixture of MDMA and its metabolites. Nevertheless, over-expression of Mfn2 R94Q, a Charcot-Marie-Tooth neuropathy 2A (CMT2A) mutant protein with impaired fusion and transport properties, does not recover the MDMA’s and mixture’s mitochondrial phenotype. This indicates that MDMA, as well the mixture of MDMA and its metabolites, might alter mitochondrial fusion/fission mechanisms [6] and, thus, compromise mitochondrial transport
Other studies, analyzing the impact of recognized neurotoxins, such as MPP+, on mitochondrial transport, have reported disrupted mitochondrial motility in dopaminergic neurons, by oxidative stress-related pathways (Kim-Han et al. 2011), and in PC12 cells, by modification of the microtubules’ dynamics (Cartelli et al. 2010). Nevertheless, to date, whether similar mechanisms may mediate the mitochondrial trafficking impairment caused by amphetaminic compounds remains unclear. More studies are needed in this field to appraise the target pathways modified by these drugs and their real impact in amphetamines’ neuronal effects.
Mitochondrial-dependent apoptosis triggered by amphetamines
The apoptotic-signaling cascade may be shared into two major pathways, extrinsic and intrinsic pathways, which are triggered by soluble molecules that bind to plasma membrane receptors or by mitochondrial stimuli, respectively. In the intrinsic pathway, also called mitochondrial pathway, mitochondria are involved and play a crucial role in integrating and circulating death signals triggered inside the cells, such as DNA damage, oxidative stress, hypoxia, or growth factor deprivation, which through regulatory cell death pathways result in outer mitochondrial membrane permeabilization (Sinha et al. 2013).
Permeabilization of the outer mitochondrial membrane allows the release of at least five apoptogenic proteins/mediators from the mitochondrial intermembrane space, cytochrome c, second mitochondrial activator of caspases/direct inhibitor of apoptotic proteins (IAPs)-binding protein with low PI (Smac/DIABLO), high temperature requirement protein A2 (HtrA2/Omi), apoptosis-inducing factor (AIF), and endonuclease G (Ola et al. 2011; Yuan and Akey 2013). Cytochrome c, Smac/DIABLO, and HtrA2/Omi are caspase-dependent factors, whereas AIF and endonuclease G constitute caspase-independent elements. These proteins participate in the apoptotic cascade at different levels. Cytochrome c, the main mediator of apoptosis, was the first mitochondrial protein with apoptotic function identified and established the general importance of mitochondria in apoptosis (Cai et al. 1998). Smac/DIABLO and HtrA2/Omi suppress the ability of IAPs to inhibit caspases. Endonuclease G and AIF are involved in DNA fragmentation. AIF also mediates chromatin condensation (Ola et al. 2011). The B cell lymphoma 2 (Bcl-2) superfamily of proteins, which encompasses two subcategories, anti-apoptotic and pro-apoptotic proteins, regulates the release of these apoptotic mediators.
Accumulating evidences indicate a major role for mitochondrial-dependent pathways in amphetamines-mediated neuronal death. The involvement of the different mitochondrial proteins in this event is detailed in the following sections.
Role of Bcl-2 superfamily of proteins in mediating amphetamines-induced neuronal apoptosis
Anti-apoptotic Bcl-2 family multi-domain proteins include Bcl-2, Bcl-xL, Bcl-2-related protein A1, Bcl-w, and Bcl-2-related ovarian killer, which contain BH-(1–4) domains. The Bcl-xL and Bcl-2 have a carboxy-terminal hydrophobic transmembrane tail domain, which helps in localizing the proteins in outer mitochondrial membrane. However, Bcl-2 also resides in the nuclear and endoplasmic reticulum membrane, being translocated to outer mitochondrial membrane upon an apoptotic signal. Myeloid cell leukemia factor-1 is another anti-apoptotic Bcl-2 protein, which contains, however, three BH domains (BH-1, -2, and -3) (Brunelle and Letai 2009; Chipuk et al. 2010; Moldoveanu et al. 2014; Ola et al. 2011).
Pro-apoptotic Bcl-2 family of proteins are divided into two subgroups according to the number of BH domains [Bcl-2-associated X protein (Bax) and Bcl-2-associated death promoter] or proteins that have only the BH3 domain [e.g., BH3 interacting domain death agonist (Bid)]. Bcl-xS is not included in any of these two groups, since it has only BH3 and BH4 domains. Members of BH3-only protein include hara-kiri, Bid, Bcl-2-interacting mediator of cell death (Bim), Bcl-2 modifying factor, p53-upregulated modulator of apoptosis (Puma), Noxa, Bcl-2 antagonist of cell death (Bad), and Bcl-2 interacting killer. Bid, Bad, and Bim have cytosolic location with respect to mitochondria. Following death signal, BH3 domain-only proteins can neutralize or depress anti-apoptotic Bcl-2 proteins or directly activate pro-apoptotic proteins, thus inducing apoptosis (Brunelle and Letai 2009; Chipuk et al. 2010; Moldoveanu et al. 2014; Ola et al. 2011).
Many factors, including mitochondrial release of certain apoptogenic factors, such as cytochrome c (Kluck et al. 1997) or AIF (Susin et al. 1996), changes in the mitochondrial membrane permeability transition pore (Marchetti et al. 1996; Marzo et al. 1998), or the recruitment of downstream apoptotic cell death executors, such as caspases (Ola et al. 2011; Ulukaya et al. 2011) and DNases (Shimizu et al. 1996), regulate Bcl-2 superfamily of proteins’ effects. Thus, the ultimate cellular responses to pro- and anti-apoptotic signals may be dependent either on transcriptional and translational changes or on interactions between Bcl-2 homologs and structurally unrelated proteins, as well as on their proteolytic cleavage. Consequently, cellular death is a result of adverse ratios of death promoters to death inhibitors (Brunelle and Letai 2009; Chipuk et al. 2010).
Initial studies in cultured neurons showed that the neuronal death induced by METH (500 µM), d-AMPH (500 µM), MDMA (500 µM), or 3,4-methylenedioxyamphetamine (500 µM) was closely associated with a differential regulation of the Bcl-X splice variants, with inhibition of the anti-apoptotic Bcl-xL-related proteins, but induction of the pro-apoptotic Bcl-xS variant, as ascertained after 1, 24, or 96 h of exposure to the drugs (Stumm et al. 1999). This study strongly corroborates a previous finding showing a significant protection against METH-induced apoptosis in immortalized CSM14.1 neuronal cells over-expressing Bcl-2 protein (Cadet et al. 1997). Following these initial reports, increased observations have documented a crucial role for Bcl-2 superfamily members in amphetamines-mediated apoptotic cascade.
A study using complementary DNA array analyses revealed an up-regulation of the pro-apoptotic proteins Bax and Bid in mouse’s brain 16 h after METH administration (40 mg/kg, i.p.) (Cadet et al. 2001). Another study, using a more detailed time course and RT-PCR and Western blotting approaches, further confirmed the METH’s ability (40 mg/kg, i.p.) to cause significant up-regulation of the pro-death Bcl-2 family genes Bax, Bcl-2-associated death promoter, Bad, and Bid in mouse’s brain. The up-regulation of Bcl-2 pro-death genes preceded apoptotic cell death, which appeared at approximately 16 h post-drug administration and was maximal at around 24 h after METH injection (40 mg/kg, i.p.) (Jayanthi et al. 2001). In contrast to the pro-death genes, cluster analysis of anti-apoptotic Bcl-2 family genes Bcl-2, Bcl-xL, and Bcl-w showed a downward profile, in a time-dependent fashion (Jayanthi et al. 2001). Additionally, based on the pattern of protein expression obtained from the Western blotting analysis, this study provided information about the relative ratios between pro- and anti-apoptotic proteins. Substantial increases in the pro-death/anti-death ratios for Bad/Bcl-xL, Bad/Bcl-2, and Bax/Bcl-2 were shown (Jayanthi et al. 2001), being this last further supported by another in vivo setting, as shown in rat’s frontal cortex 12 h after METH exposure (4 × 10 mg/kg, i.p., 2-h interval) (Bachmann et al. 2009).
Other in vivo studies also revealed increased expression of pro-apoptotic proteins Bax and Bad and reduced levels of the Bcl-2 in striatum 30 min to 24 h after METH administration (four intra-peritoneal injections of saline given at 2-h intervals, each followed by a dose of 10 mg/kg of METH 30 min later), in a DA D1 and D2 receptors-sensitive manner (Beauvais et al. 2011). Additionally, by combining Bax knockout mice and a RT-PCR-based approach, Ryu and co-workers (Ryu et al. 2007) revealed a substantial reduction in Bax mRNA levels in mice’s striatum, hippocampus, midbrain, and cerebellum 1 day after METH exposure (0.5 mg/kg, i.p., once daily, for 4 consecutive days), as compared to wild-type littermates. These results further support a major role for Bax in mediating METH’s brain effects.
Mechanistically, in vitro settings, using M213 rat striatal and SH-SY5Y human neuroblastoma cells, based on RT-PCR and Western blotting approaches, have also sustained the METH’s ability (2 mM) to modify the pattern of expression of pro- and anti-apoptotic proteins: increased expression of the pro-apoptotic protein Bax and reduced levels of the anti-apoptotic regulator Bcl-2 (Deng et al. 2002; Wisessmith et al. 2009). When taken together, these data indicate that METH administration might cause a shift in the intrinsic ratio of death promoters to death repressors that may trigger neuronal death.
Animal studies in the mice model have also associated an up-regulation of the pro-apoptotic protein Bax and decreased Bcl-2 mRNA levels with AMPH-induced striatal apoptosis (4 × 10 mg/kg, i.p., 2-h apart), which were consistently traduced in changes in protein expression levels. Additionally, AMPH-induced TUNEL-positive cells were substantially reduced in Bax knockout mice, suggesting a major role for the pro-apoptotic protein Bax in mediating AMPH’s striatal toxicity (Krasnova et al. 2005).
Increased evidences have also suggested a major role for Bcl-2 superfamily of proteins in mediating MDMA’s neurotoxic effects. Studies in rats administrated with MDMA (5, 10, or 20 mg/kg, i.p., twice daily, for 7 days) revealed a dose-dependent up-regulation of Bax and down-regulation of Bcl-2 in the hippocampus, as ascertained 7 days after the last MDMA injection. Notably, these effects were evident by evaluating both mRNA and protein expression levels (Soleimani Asl et al. 2012). In another study using a MDMA regime of 10 mg/kg, once daily, for 7 days, it was also found a marked up-regulation of Bax and down-regulation of Bcl-2 in the hippocampus 3 days after the last MDMA administration, as revealed by both mRNA and protein expression levels (Mehdizadeh et al. 2012). This suggests that MDMA’s effects on Bax and Bcl-2 expression patterns endure in time. Furthermore, NAC protected against the differential effects of MDMA on Bax and Bcl-2 mRNA and protein expression patters (Soleimani Asl et al. 2013, 2015), indicating a major role for oxidative stress-related pathways in these effects. A subsequent study also revealed an up-regulation of Bax and down-regulation of Bcl-2 genes, consistently traduced in modified expression levels of these proteins, in rat’s striatum 2 weeks after MDMA administration (10 mg/kg, i.p., once daily, for 7 days) (Soleimani et al. 2012). This indicates that MDMA’s effects on Bcl-2 superfamily of proteins are not restricted to the hippocampus region. Additionally, adenosine 2A receptors seemed to have a role in the apoptotic effects of MDMA, via Bax and Bcl-2 pathways, in rat’s striatum, since an agonist of this receptor decreased the toxicity of MDMA, whereas the antagonist increased it (Soleimani et al. 2012).
Despite this, a recent study in immortalized human HepG2 cells reported the ability of several AMPH-like drugs, namely METH (3 mM), MDMA (1.3 mM), 4-methylthioamphetamine (0.5 mM), and d-AMPH (1.7 mM), as well as their mixture (1.6 mM of mixture, constituted by 790 μM METH, 334 μM MDMA, 113 μM 4-methylthioamphetamine, and 363 μM d-AMPH) to up-regulate bim and puma genes’ expression and protein levels, as assessed after 8 or 12 h of exposure, respectively. This constitutes the first report indicating an involvement of these proteins in amphetamines’ toxic effects (Silva et al. 2013a). Nevertheless, to date, it remains unclear whether similar effects are found at neuronal level. Therefore, more studies are needed to better understand the involvement of these regulators in the neuronal apoptotic death profile triggered by this class of compounds.
Involvement of cytochrome c and caspase activation in amphetamines-induced neuronal death
Cytochrome c is a 12.3 kDa nuclear DNA-encoded heme-containing protein located in the mitochondrial intermembrane space. The cytochrome c precursor, apocytochrome c, is synthesized in the cytoplasm and can spontaneously insert into the mitochondrial outer membrane by a non-receptor mediated process. Following heme incorporation by the cytochrome c heme lyase, protein refolds and is further released into the mitochondrial intermembrane space (Cai et al. 1998).
As a mitochondrial response to apoptosis trigger stimuli, cytochrome c, along with other proteins, is released from mitochondria into the cytosol, and consequently forms a complex with apoptotic protease activating factor-1 (Apaf-1) and ATP/2′-deoxyadenosine 5′-triphosphate, the apoptosome, a multi-protein platform comprising a seven-spoked wheel-shaped complex crucial in the recruitment and subsequent activation of caspase-9. Once activated, caspase-9 processes executioner caspases, such as caspase-3, caspase-6, and caspase-7, thereby promoting the execution of apoptosis (Bratton and Salvesen 2010; Yuan and Akey 2013). Nevertheless, IAPs may bind to active caspase-9 and caspase-3 blocking the caspase cascade, thus inhibiting apoptosis (Bratton et al. 2001).
Several studies have reported the amphetamines’ ability to trigger mitochondrial cytochrome c release. Immunocytochemistry analysis revealed a marked increase in the cytochrome c release from mitochondria in cerebellar granule cells after METH exposure (4 mM) for 48 h, which was tightly correlated with caspase-9, caspase-6, and caspase-3 activation (effects seen at 24-h time-point) (Jiménez et al. 2004). Further, other in vitro studies in M213 rat striatal (Deng et al. 2002) or N27 rat mesencephalic dopaminergic neuronal cell lines (Lin et al. 2012) have also revealed mitochondrial cytochrome c release (Deng et al. 2002; Lin et al. 2012), caspase-9 cleavage (Deng et al. 2002), and activation of the caspase-3-mediated downstream cell death executioner pathway following METH exposure (Lin et al. 2012). In vivo studies in mice (40 mg/kg dosis, i.p.) (Jayanthi et al. 2004) or rat [4 × 5 mg/kg, i.p., 2-h interval (Bachmann et al. 2009) or four i.p., injections of saline given at 2-h intervals, each followed by a dose of 10 mg/kg of METH 30 min later (Beauvais et al. 2011)] also correlated METH-induced neuronal apoptosis to decreased mitochondrial cytochrome c content (Bachmann et al. 2009; Jayanthi et al. 2004) and correspondent increase in its cytosolic fraction (Beauvais et al. 2011; Jayanthi et al. 2004). This effect was associated with Apaf-1 formation and cleavage of caspase-9, caspase-6, and caspase-3 to their active forms, but not of caspase-7, indicating a mitochondrial-dependent apoptotic death profile (Jayanthi et al. 2004). Additionally, METH-induced mitochondrial cytochrome c release in rat’s striatum has been described to rely on DA D1 receptors’ activation (Beauvais et al. 2011), thus suggesting a role for these receptors in mediating apoptotic response. When taken together with in vitro findings, these in vivo data implicate a formal role for mitochondrial cytochrome c in METH-induced neuronal death.
Other studies have also reported an involvement of cytochrome c activation-dependent pathways in AMPH-induced neuronal apoptotic death. In PC12 cells, apoptotic features, characterized by chromatin condensation (1 mM, 48 h) (Oliveira et al. 2002), decreased mitochondrial cytochrome c content, and caspase-3 activation (300 µM, 5 h) (Oliveira et al. 2003), were closely associated with AMPH-induced cell death. Further reports in cultured neurons linked AMPH (0.5 mM, 24 h)-induced neuronal death to increased caspase-9 and caspase-3 proteolytic processing and activity, but not of caspase-8, and decreased overall mitochondrial cytochrome c staining, thereby suggesting a mitochondrial-dependent apoptosis trigger stimuli (Cunha-Oliveira et al. 2006).
Studies with MDMA have also reported a major role for cytochrome c in MDMA-induced neuronal death. Findings from in vitro studies have demonstrated an apoptotic death profile in cerebellar granule cells, characterized by chromatin condensation, caspase-6, caspase-9, and caspase-3 activation and mitochondrial cytochrome c release following MDMA exposure (4 mM, 24–48 h) (Jiménez et al. 2004). Nevertheless, studies in hippocampal cultured neurons (400 µM, 24 h) found no changes in both cytosolic and mitochondrial cytochrome c content, but increased caspase-8- and caspase-3-like activities after 48 h of exposure (Capela et al. 2013), also suggesting a role for the extrinsic pathway in apoptosis activation. Other reports in cultured cortical neurons from rat or SH-SH5Y-differentiated cells have also revealed increased caspase-3 activity following exposure to MDMA (200–800 µM, for 48 h) (Capela et al. 2007a) or its metabolites N-Me-α-MeDA (100 µM, 24 h) (Barbosa et al. 2014a), 5-(NAC)-α-MeDA (200 µM, 24 h) (Capela et al. 2007b), or 5-(NAC)-N-Me-α-MeDA (200 µM, 24 h) (Capela et al. 2007b) or to the mixture of MDMA and six of its major in vivo metabolites, each compound at a equimolar concentration of 10 µM, for 24 h (Barbosa et al. 2014b). Nevertheless, a direct involvement of mitochondrial-dependent pathways in this effect was not established. Additionally, evidences indicating only a partial rescue of MDMA-induced neuronal death by caspase-3 inhibition (Capela et al. 2013) further support the existence of a combined effect of mitochondrial-dependent and mitochondrial-independent pathways in MDMA-mediated apoptotic neuronal response.
In vivo studies have also reported increased caspase-3 immunoreactivity in neurons of the cortex of rats exposed to MDMA (40 mg/kg, i.p.) for 24 h (Warren et al. 2007). In the rostral forebrain and hippocampus of neonatal rats, MDMA administration (2 × 10 mg/kg, s.c.) resulted in a twofold increase in the number of cleaved caspase-3-immunoreactive cells, as ascertained 4 days later (Meyer et al. 2004). In mice, MDMA (3 × 5 mg/kg, i.p.) also produced a marked induction of caspase-3 activity in the amygdala and hippocampus, as measured 7 days later (Tamburini et al. 2006).
Studies with the hallucinogenic amphetaminic compound DOI have also established a role for cytochrome c-regulated pathways in mediating its neurotoxic effects. Using cultured hippocampal neurons, Capela and co-workers (Capela et al. 2013) reported increased cytosolic cytochrome c content (50 µM, 24 h) and increased caspase-8- and caspase-3-like activities (50 µM, 48 h) as early events associated with DOI-induced neuronal death. These data, combined with the observation indicating only a partial recovery of DOI-induced neuronal death by caspase-3 inhibition (Capela et al. 2013), further suggest a tight regulation of mitochondrial-dependent and mitochondrial-independent apoptotic pathways in mediating DOI’s neurotoxic effects.
Overall, these data suggest a major role for cytochrome c-mediated trails and activation of the caspases-mediated downstream cell death executioner pathway in amphetamines’ neurotoxic effects.
Smac/DIABLO involvement in amphetamines-induced neuronal apoptosis
Smac/DIABLO is a protein, codified by a nuclear gene, released from mitochondria to cytosol after an apoptosis trigger stimuli. It contains an amino-terminal that acts as a mitochondrial targeting signal, being its mature form originated by the cleavage of this signal (Du et al. 2000; Martinez-Ruiz et al. 2008). The Smac/DIABLO pro-apoptotic effect is mediated by its interaction with IAPs, releasing caspases from them, instead of direct activation on Apaf-1, cytochrome c, or procaspase-9. Thus, it enhances an efficient activation of caspases and apoptosis execution (Du et al. 2000; Srinivasula et al. 2001). A particular Smac/DIABLO’s amino-terminal motif, consisting of four amino acids, Ala–Val–Pro–Ile, mediates its interaction with IAPs (Chai et al. 2000; Wu et al. 2000). Other structural and biochemical data had established that Smac/DIABLO requires the formation of homodimers to interact with IAPs (Chai et al. 2000).
Although limited in scope, some studies have established a role for Smac/DIABLO in the neurotoxic effects mediated by AMPH-like drugs. In vivo studies analyzing the cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades in METH-induced neuronal apoptosis have revealed the METH’s ability to trigger mitochondrial Smac/DIABLO release, after a 40 mg/kg, i.p., dosis (Jayanthi et al. 2004), and an increase in its cytosolic amount after a single i.p. METH dosis of 40 mg/kg (Jayanthi et al. 2004), or 4 i.p. METH dosis of 10 mg/kg, at 2-h intervals (Imam et al. 2005), in mouse’s striatum. Additionally, the increase in cytosolic Smac/DIABLO amount was more pronounced in the striatum of mice heterozygous for Nurr1 (Imam et al. 2005), a transcription factor of the orphan nuclear receptor of the steroid/thyroid hormone receptor superfamily that plays an important role in the proper development of dopaminergic neurons (Perlmann and Wallén-Mackenzie 2004). This reveals an increased susceptibility of dopaminergic neurons to METH’s effects.
Recently, another study associated Smac/DIABLO activation with MDMA (16 h after an i.p., dosis of 20 mg/kg)-induced apoptosis in rat’s liver (Cerretani et al. 2011). Nevertheless, to date, it remains unclear whether similar effects are found at neuronal level. Therefore, more studies are needed to better understand the involvement of this regulator in the neuronal apoptotic death profile triggered by this class of compounds.
AIF involvement in amphetamines-induced neuronal apoptosis
AIF, a mitochondrial flavin adenine dinucleotide-containing flavoprotein oxidoreductase, is a caspase-independent element of the apoptosis cascade located in the mitochondrial inter-membrane space with its amino-terminal anchored in the inner mitochondrial membrane (Nikoletopoulou et al. 2013). Upon an apoptosis trigger stimuli, AIF undergoes proteolysis by calpains or cathepsins into a soluble form (tAIF), and then translocates to the nuclei, via its carboxyl-terminal domain nuclear localization sequence, leading to chromatin condensation and large-scale DNA degradation, in a caspase-independent manner (Krantic et al. 2007; Nikoletopoulou et al. 2013; Sevrioukova 2011). Other AIF’s properties include the maintenance of the ETC’s complex I functionality and peroxide scavenging activity (Delettre et al. 2006). Additionally, since it displays NADH oxidase activity, also contributes to the generation of \(\text{O}^{\cdot-}_{2}\) (Miramar et al. 2001). Consequently, the reduction in assembled complex I associated with AIF deficiency is anticipated to have a profound effect on neuronal mitochondrial function (Nicholls 2009; Vahsen et al. 2004).
Increased evidences have indicated an involvement of the mitochondrial protein AIF in mediating neuronal apoptosis caused by AMPH-like drugs. In vivo studies revealed the METH’s ability (40 mg/kg, i.p.) to cause mitochondrial AIF release with consequent nuclear translocation, via transit through the cytosol, in mice’s striatum (Jayanthi et al. 2004). Studies in cultured hippocampal neurons further associated DOI (50 µM, 24 h)-induced neuronal apoptosis with an increase in both mitochondrial and cytosolic AIF 67 kDa subunit. However, mitochondrial AIF-processed 57 kDa subunit remained unchanged, and cytosolic AIF-processed 57 kDa subunit was undetectable. Furthermore, following DOI exposure, nuclear AIF levels also remained undetectable (Capela et al. 2013). In the same way, MDMA-induced hippocampal neuronal death (400 µM, 24 h) was also linked to increased mitochondrial AIF subunit 67 kDa content, though no differences were found in cytosolic compartment, as well as in the AIF-processed subunit 57 kDa in mitochondria. Similarly to DOI, cytosolic AIF processed 57 kDa subunit and nuclear AIF remained undetectable following MDMA exposure (Capela et al. 2013). An additional in vitro study in cultured cortical neurons reported a nuclear apoptotic morphology following AMPH exposure (0.5 mM) for 24 h, though no changes in mitochondrial AIF amount were found, thereby suggesting a role for other mediators, like endonuclease G, in mediating DNA fragmentation and neuronal death (Cunha-Oliveira et al. 2006). From these studies, it is likely to assume that AIF may play a role in amphetamines-induced neuronal apoptosis, though other regulators might also be determinants in mediating this effect.
Taking into account the above-referred findings, the mitochondrial proteins involved in mitochondrial-dependent pathways of neuronal death described to be targeted by amphetamines are presented in Fig. 4.
Postulated mechanisms of amphetamine-induced mitochondrial-dependent apoptotic neuronal death. Both in vivo and in vitro studies have reported the amphetamines’ ability to up-regulate the pro-death genes BH3 interacting domain death agonist (bid) [1], B cell lymphoma 2 (Bcl-2)-associated X protein (bax) [2], Bcl-2-associated death promoter (bak) [3], and Bcl-2 antagonist of cell death (bad) [4], which have been consistently correlated with increased protein expression. Studies in an in vitro liver model have also reported the ability of several AMPH-like drugs to up-regulate Bcl-2 interacting mediator of cell death (bim) [5] and p53-upregulated modulator of apoptosis (puma) [6] genes’ expression and protein levels, although their role in amphetamine-induced neuronal death remains uncharacterized. In turn, amphetamines also cause a downward profile of the antiapoptotic Bcl-extra-long (Bcl-xL) [7] and Bcl-2 [8] genes. These changes result in mitochondrial membrane permeabilization (MMP), with consequent release of apoptosis-inducing factor (AIF) [9], cytochrome c [10], and second mitochondrial activator of caspases/direct inhibitor of apoptotic proteins (IAPs)-binding protein with low PI (Smac/DIABLO) [11]. The release of these apoptogenic factors has been reported by several studies to trigger caspases’ activation [12, 14–16], leading to mitochondrial-dependent apoptotic cell death. Some studies have also reported the amphetamines’ ability to trigger activation of caspase 8 [13], thus suggesting a tight regulation of mitochondrial-dependent and mitochondrial-independent apoptotic pathways in mediating amphetamines’ neuronal toxicity and death
Concluding remarks
One of the most feared and debated issue concerning the abuse of amphetamines is related to their neurotoxic potential. Considering that in Europe, it is estimated that 11.4 million of people have taken AMPH-like stimulants at some point in their lives (EMCDDA 2014), this constitutes an important health issue. As such, studies analyzing the pathways and mechanisms mediating amphetamines’ neurotoxic effects are of striking importance. In this field, earlier studies had suggested that amphetamines’ neurotoxicity is essentially characterized by damage to monoaminergic terminals, and accumulating evidence have clearly implicated mitochondria as a direct target of amphetamines’ neurotoxicity. These events include alterations on TCA cycle’s enzymes functioning, inhibition of mitochondrial ETC’s complexes, perturbations of mitochondrial clearance mechanisms, and interference with mitochondrial dynamics, including mitochondrial biogenesis, fusion/fission, trafficking, and mitophagy, as well as oxidative modifications in mitochondrial macromolecules. Additionally, other studies indicate that the neuronal toxicity induced by amphetamines rely on the activation of several mitochondrial pathways, whose roles have been well predictable in animal models and in in vitro settings. In this field, pathways involving the release of mitochondrial proteins with consequent activation of the caspases-mediated downstream cell death executioner pathway, closely regulated by Bcl-2 superfamily of proteins, have been greatly implicated in amphetamines’ neurotoxic effects.
The elucidation of such mechanism can help in developing putative therapeutic approaches to prevent or treat the acute- and long-lasting neuropsychiatric complications associated with the abuse of amphetamines.
Future perspectives
Neurons, perhaps more than other cell types, depend on mitochondria for their function and survival. Therefore, it is reasonable to consider that pathological alterations in normal mitochondrial functioning and pathways might be key players in developing neuronal degeneration and toxicity associated with amphetamines. Despite this, the real involvement of mitochondrial-dependent mechanisms in mediating amphetamines’ neurotoxic effects remains poorly understood, and, therefore, more studies are needed in this research field. For example, it is of high interest to ascertain whether the increase in ROS formation triggered by amphetamines is the cause or a consequence of amphetamines-induced mitochondrial dysfunction. Since ROS might, directly or indirectly, activate some cell signaling pathways, including protein kinases, it is important to appraise how mitochondria may mediate neuronal toxicity and death: Are mitochondria key players in this process or constitute targets of other cell death executioner pathways?
On the other hand, mitochondria are known to interact with other intracellular organelles, including endoplasmic reticulum. As such, it is important to ascertain how these functional interactions impact mitochondrial response and toxicity of amphetamines.
Accumulating evidences have also attributed to amphetamines’ metabolites a major role in mediating their neurotoxic effects. Thus, it is attracting to analyze the exact role of metabolism in amphetamines-induced mitochondrial dysfunction and toxicity: Can metabolites target directly mitochondrial components and, thus, impair mitochondrial functioning? Can amphetamines and their metabolites activate dissimilar intracellular pathways? Can these pathways act in a combined manner in causing mitochondrial dysfunction?
Additionally, more information is needed on the specific role of hyperthermia and oxidative mechanisms, as well as their interactions, in amphetamines-induced mitochondrial dysfunction and toxicity.
References
Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G (2009) Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med 1:392–406
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7:1140–1149
Ádori C, Lőw P, Andó RD et al (2011) Ultrastructural characterization of tryptophan hydroxylase 2-specific cortical serotonergic fibers and dorsal raphe neuronal cell bodies after MDMA treatment in rat. Psychopharmacology 213:377–391
Ajjimaporn A, Swinscoe J, Shavali S, Govitrapong P, Ebadi M (2005) Metallothionein provides zinc-mediated protective effects against methamphetamine toxicity in SK-N-SH cells. Brain Res Bull 67:466–475
Akiyama K (2006) Longitudinal clinical course following pharmacological treatment of methamphetamine psychosis which persists after long-term abstinence. Ann N Y Acad Sci 1074:125–134
Albers DS, Zeevalk GD, Sonsalla PK (1996) Damage to dopaminergic nerve terminals in mice by combined treatment of intrastriatal malonate with systemic methamphetamine or MPTP. Brain Res 718:217–220
Alves E, Summavielle T, Alves CJ et al (2007) Monoamine oxidase-B mediates ecstasy-induced neurotoxic effects to adolescent rat brain mitochondria. J Neurosci 27:10203–10210
Alves E, Binienda Z, Carvalho F et al (2009a) Acetyl-l-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience 158:514–523
Alves E, Summavielle T, Alves CJ et al (2009b) Ecstasy-induced oxidative stress to adolescent rat brain mitochondria in vivo: influence of monoamine oxidase type A. Addict Biol 14:185–193
Arai Y, Se KK, Kinemuchi H et al (1990) Selective inhibition of MAO-A in serotonergic synaptosomes by two amphetamine metabolites, p-hydroxyamphetamine and p-hydroxynorephedrine. Neurochem Int 17:587–592
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20:31–42
Ask AL, Fagervall I, Florvall L, Ross SB, Ytterborn S (1985) Inhibition of monoamine oxidase in 5-hydroxytryptaminergic neurones by substituted p-aminophenylalkylamines. Br J Pharmacol 85:683–690
Axt KJ, Molliver ME (1991) Immunocytochemical evidence for methamphetamine-induced serotonergic axon loss in the rat brain. Synapse 9:302–313
Bachmann RF, Wang Y, Yuan P et al (2009) Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage. Int J Neuropsychopharmacol 12:805–822
Baker MJ, Tatsuta T, Langer T (2011) Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol 3(7)
Barbosa DJ, Capela JP, Oliveira JMA et al (2012) Pro-oxidant effects of Ecstasy and its metabolites in mouse brain synaptosomes. Br J Pharmacol 165:1017–1033
Barbosa DJ, Capela JP, Silva R et al (2014a) “Ecstasy”-induced toxicity in SH-SY5Y differentiated cells: role of hyperthermia and metabolites. Arch Toxicol 88:515–531
Barbosa DJ, Capela JP, Silva R et al (2014b) The mixture of “ecstasy” and its metabolites is toxic to human SH-SY5Y differentiated cells at in vivo relevant concentrations. Arch Toxicol 88:455–473
Barbosa DJ, Serrat R, Mirra S et al (2014c) MDMA impairs mitochondrial neuronal trafficking in a Tau- and Mitofusin2/Drp1-dependent manner. Arch Toxicol 88:1561–1572
Barbosa DJ, Serrat R, Ferreira LM et al (2014d) Neuronal mitochondrial trafficking impairment: the cause or a consequence of neuronal dysfunction caused by amphetamine-like drugs. J Drug Alcohol Res 3:235868
Barbosa DJ, Serrat R, Mirra S et al (2014e) The mixture of “ecstasy” and its metabolites impairs mitochondrial fusion/fission equilibrium and trafficking in hippocampal neurons, at in vivo relevant concentrations. Toxicol Sci 139:407–420
Barrett T, Xie T, Piao Y et al (2001) A murine dopamine neuron-specific cDNA library and microarray: increased COXI expression during methamphetamine neurotoxicity. Neurobiol Dis 8:822–833
Barsoum MJ, Yuan H, Gerencser AA et al (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25:3900–3911
Beauvais G, Atwell K, Jayanthi S, Ladenheim B, Cadet JL (2011) Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS ONE 6:e28946
Beeson CC, Beeson GC, Schnellmann RG (2010) A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem 404:75–81
Berntman L, Carlsson C, Hägerdal M, Siesjö BK (1978) Circulatory and metabolic effects in the brain induced by amphetamine sulphate. Acta Physiol Scand 102:310–323
Bratton SB, Salvesen GS (2010) Regulation of the Apaf-1–caspase-9 apoptosome. J Cell Sci 123:3209–3214
Bratton SB, Walker G, Srinivasula SM et al (2001) Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J 20:998–1009
Brown JM, Yamamoto BK (2003) Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther 99:45–53
Brown JM, Quinton MS, Yamamoto BK (2005) Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. J Neurochem 95:429–436
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122:437–441
Büeler H (2009) Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp Neurol 218:235–246
Burrows KB, Gudelsky G, Yamamoto BK (2000) Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur J Pharmacol 398:11–18
Busceti CL, Biagioni F, Riozzi B et al (2008) Enhanced tau phosphorylation in the hippocampus of mice treated with 3,4-methylenedioxymethamphetamine (“ecstasy”). J Neurosci 28:3234–3245
Cadet JL, Ordonez SV, Ordonez JV (1997) Methamphetamine induces apoptosis in immortalized neural cells: protection by the proto-oncogene, bcl-2. Synapse 25:176–184
Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B (2001) Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: evidence from cDNA array. Synapse 41:40–48
Cai J, Yang J, Jones DP (1998) Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1366:139–149
Callahan BT, Cord BJ, Ricaurte GA (2001) Long-term impairment of anterograde axonal transport along fiber projections originating in the rostral raphe nuclei after treatment with fenfluramine or methylenedioxymethamphetamine. Synapse 40:113–121
Capela JP, Fernandes E, Remiao F, Bastos ML, Meisel A, Carvalho F (2007a) Ecstasy induces apoptosis via 5-HT2A-receptor stimulation in cortical neurons. Neurotoxicology 28:868–875
Capela JP, Macedo C, Branco PS et al (2007b) Neurotoxicity mechanisms of thioether ecstasy metabolites. Neuroscience 146:1743–1757
Capela JP, Carmo H, Remião F, Bastos ML, Meisel A, Carvalho F (2009) Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol 39:210–271
Capela JP, da Costa Araújo S, Costa VM et al (2013) The neurotoxicity of hallucinogenic amphetamines in primary cultures of hippocampal neurons. Neurotoxicology 34:254–263
Cardoso SM, Santos S, Swerdlow RH, Oliveira CR (2001) Functional mitochondria are required for amyloid b-mediated neurotoxicity. FASEB J 15:1439–1441
Cartelli D, Ronchi C, Maggioni MG, Rodighiero S, Giavini E, Cappelletti G (2010) Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+-induced neurodegeneration. J Neurochem 115:247–258
Carvalho M, Remiao F, Milhazes N et al (2004) The toxicity of N-methyl-alpha-methyldopamine to freshly isolated rat hepatocytes is prevented by ascorbic acid and N-acetylcysteine. Toxicology 200:193–203
Carvalho M, Carmo H, Costa VM et al (2012) Toxicity of amphetamines: an update. Arch Toxicol 86:1167–1231
Castino R, Lazzeri G, Lenzi P et al (2008) Suppression of autophagy precipitates neuronal cell death following low doses of methamphetamine. J Neurochem 106:1426–1439
Castro-Caldas M, Carvalho AN, Rodrigues E et al (2012) Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol Neurobiol 46:475–486
Cereghetti GM, Stangherlin A, de Brito OM et al (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105:15803–15808
Cerretani D, Bello S, Cantatore S et al (2011) Acute administration of 3,4-methylenedioxymethamphetamine (MDMA) induces oxidative stress, lipoperoxidation and TNFα-mediated apoptosis in rat liver. Pharmacol Res 64:517–527
Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y (2000) Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406:855–862
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125:1241–1252
Chan P, Di Monte DA, Luo JJ, DeLanney LE, Irwin I, Langston JW (1994) Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. J Neurochem 62:2484–2487
Chang DTW, Honick AS, Reynolds IJ (2006) Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci 26:7035–7045
Chang KT, Niescier RF, Min KT (2011) Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc Natl Acad Sci USA 108:15456–15461
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200
Chen H, McCaffery JM, Chan DC (2007a) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130:548–562
Chen S, Owens GC, Crossin KL, Edelman DB (2007b) Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol Cell Neurosci 36:472–483
Chen S, Owens GC, Edelman DB (2008) Dopamine inhibits mitochondrial motility in hippocampal neurons. PLoS ONE 3:e2804
Chen S, Owens GC, Makarenkova H, Edelman DB (2010) HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS ONE 5:e10848
Chipana C, Camarasa J, Pubill D, Escubedo E (2006) Protection against MDMA-induced dopaminergic neurotoxicity in mice by methyllycaconitine: involvement of nicotinic receptors. Neuropharmacology 51:885–895
Chipana C, Camarasa J, Pubill D, Escubedo E (2008) Memantine prevents MDMA-induced neurotoxicity. Neurotoxicology 29:179–183
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR (2010) The BCL-2 family reunion. Mol Cell 37:299–310
Chu T, Kumagai Y, DiStefano EW, Cho AK (1996) Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51:789–796
Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101:15927–15932
Cipolat S, Rudka T, Hartmann D et al (2006) Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126:163–175
Colado MI, O’Shea E, Granados R, Murray TK, Green AR (1997) In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (“ecstasy”) and p-chloroamphetamine but not the degeneration following fenfluramine. Br J Pharmacol 121:889–900
Colado MI, O’Shea E, Esteban B, Granados R, Green AR (1999) In vivo evidence against clomethiazole being neuroprotective against MDMA (‘ecstasy’)-induced degeneration of rat brain 5-HT nerve terminals by a free radical scavenging mechanism. Neuropharmacology 38:307–314
Cruz CM, Rinna A, Forman HJ, Ventura ALM, Persechini PM, Ojcius DM (2007) ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282:2871–2879
Cunha-Oliveira T, Rego AC, Cardoso SM et al (2006) Mitochondrial dysfunction and caspase activation in rat cortical neurons treated with cocaine or amphetamine. Brain Res 1089:44–54
Cuyas E, Robledo P, Pizarro N et al (2013) 3,4-Methylenedioxymethamphetamine induces gene expression changes in rats related to serotonergic and dopaminergic systems, but not to neurotoxicity. Neurotox Res 25:161–169
Darvesh AS, Gudelsky GA (2005) Evidence for a role of energy dysregulation in the MDMA-induced depletion of brain 5-HT. Brain Res 1056:168–175
Delettre C, Yuste VJ, Moubarak RS et al (2006) AIFsh, a novel apoptosis-inducing factor (AIF) pro-apoptotic isoform with potential pathological relevance in human cancer. J Biol Chem 281:6413–6427
Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL (2002) Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology 42:837–845
Devaux F, Lelandais G, Garcia M, Goussard S, Jacq C (2010) Posttranscriptional control of mitochondrial biogenesis: spatio-temporal regulation of the protein import process. FEBS Lett 584:4273–4279
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33–42
Ebneth A, Godemann R, Stamer K et al (1998) Overexpression of Tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol 143:777–794
Edmondson DE, Binda C, Wang J, Upadhyay AK, Mattevi A (2009) Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry 48:4220–4230
EMCDDA (2014) European drug report 2014: trends and developments. European Monitoring Centre for Drugs and Drug Addiction, Lisbon
Erives GV, Lau SS, Monks TJ (2008) Accumulation of neurotoxic thioether metabolites of 3,4-(±)-methylenedioxymethamphetamine in rat brain. J Pharmacol Exp Ther 324:284–291
Escubedo E, Chipana C, Pérez-Sánchez M, Camarasa J, Pubill D (2005) Methyllycaconitine prevents methamphetamine-induced effects in mouse striatum: involvement of alpha7 nicotinic receptors. J Pharmacol Exp Ther 315:658–667
Escubedo E, Abad S, Torres I, Camarasa J, Pubill D (2011) Comparative neurochemical profile of 3,4-methylenedioxymethamphetamine and its metabolite alpha-methyldopamine on key targets of MDMA neurotoxicity. Neurochem Int 58:92–101
Estaquier J, Arnoult D (2007) Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ 14:1086–1094
Falk EM, Cook VJ, Nichols DE, Sprague JE (2002) An antisense oligonucleotide targeted at MAO-B attenuates rat striatal serotonergic neurotoxicity induced by MDMA. Pharmacol Biochem Behav 72:617–622
Feier G, Valvassori SS, Lopes-Borges J et al (2012) Behavioral changes and brain energy metabolism dysfunction in rats treated with methamphetamine or dextroamphetamine. Neurosci Lett 530:75–79
Feier G, Valvassori SS, Varela RB et al (2013) Lithium and valproate modulate energy metabolism in an animal model of mania induced by methamphetamine. Pharmacol Biochem Behav 103:589–596
Fišar Z (2012) Cannabinoids and monoamine neurotransmission with focus on monoamine oxidase. Prog Neuropsychopharmacol Biol Psychiatry 38:68–77
Fischer F, Hamann A, Osiewacz HD (2012) Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci 37:284–292
Forman HJ, Azzi A (1997) On the virtual existence of superoxide anions in mitochondria: thoughts regarding its role in pathophysiology. FASEB J 11:374–375
Fornai F, Giorgi FS, Gesi M, Chen K, Alessrì MG, Shih JC (2001) Biochemical effects of the monoamine neurotoxins DSP-4 and MDMA in specific brain regions of MAO-B-deficient mice. Synapse 39:213–221
Frank S, Gaume B, Bergmann-Leitner ES et al (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1:515–525
Freezer A, Salem A, Irvine RJ (2005) Effects of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and para-methoxyamphetamine on striatal 5-HT when co-administered with moclobemide. Brain Res 1041:48–55
Frey K, Kilbourn M, Robinson T (1997) Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol 334:273–279
Frey BN, Valvassori SS, Gomes KM et al (2006) Increased oxidative stress in submitochondrial particles after chronic amphetamine exposure. Brain Res 1097:224–229
Frezza C, Cipolat S, Martins de Brito O et al (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126:177–189
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Friedman JR, Lackner LL, West M, Di Benedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334:358–362
Gandre-Babbe S, van der Bliek AM (2008) The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19:2402–2412
Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AHV, Taanman JW (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19:4861–4870
Geisler S, Holmström KM, Skujat D et al (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12:119–131
Gonçalves J, Baptista S, Olesen MV et al (2012) Methamphetamine-induced changes in the mice hippocampal neuropeptide Y system: implications for memory impairment. J Neurochem 123:1041–1053
Gouzoulis-Mayfrank E, Daumann J (2009) Neurotoxicity of drugs of abuse—the case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines. Dialogues Clin Neurosci 11:305–317
Green AL, El Hait MA (1980) p-Methoxyamphetamine, a potent reversible inhibitor of type-A monoamine oxidase in vitro and In vivo. J Pharm Pharmacol 32:262–266
Grelotti DJ, Kanayama G, Pope HG (2010) Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: presentation of a case and review of the literature. Am J Psychiatry 167:17–23
Hatefi Y, Hanstein WG, Galante Y, Stiggall DL (1975) Mitochondrial ATP-Pi exchange complex and the site of uncoupling of oxidative phosphorylation. Fed Proc 34:1699–1706
Herrmann JM, Longen S, Weckbecker D, Depuydt M (2012) Biogenesis of mitochondrial proteins. Adv Exp Med Biol 748:41–64
Hewton R, Salem A, Irvine RJ (2007) Potentiation of 3,4-methylenedioxymethamphetamine-induced 5-HT release in the rat substantia nigra by clorgyline, a monoamine oxidase A inhibitor. Clin Exp Pharmacol Physiol 34:1051–1057
Ho P, Ho J, Liu H et al (2012) Mitochondrial neuronal uncoupling proteins: a target for potential disease-modification in Parkinson’s disease. Transl Neurodegener 1:3
Hock MB, Kralli A (2009) Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203
Hotchkiss AJ, Morgan ME, Gibb JW (1979) The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci 25:373–378
Imam SZ, Jankovic J, Ali SF et al (2005) Nitric oxide mediates increased susceptibility to dopaminergic damage in Nurr1 heterozygous mice. FASEB J 19:1441–1450
Ingerman E, Perkins EM, Marino M et al (2005) Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol 170:1021–1027
Ishihara N, Eura Y, Mihara K (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117:6535–6546
Iwazaki T, McGregor IS, Matsumoto I (2008) Protein expression profile in the amygdala of rats with methamphetamine-induced behavioral sensitization. Neurosci Lett 435:113–119
James DI, Parone PA, Mattenberger Y, Martinou JC (2003) hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278:36373–36379
Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL (2001) Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J 15:1745–1752
Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL (2004) Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J 18:238–251
Jiménez A, Jordà EG, Verdaguer E et al (2004) Neurotoxicity of amphetamine derivatives is mediated by caspase pathway activation in rat cerebellar granule cells. Toxicol Appl Pharmacol 196:223–234
Jiménez-Mateos EM, González-Billault C, Dawson HN, Vitek MP, Avila J (2006) Role of MAP1B in axonal retrograde transport of mitochondria. Biochem J 397:53–59
Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191:933–942
Johanson CE, Frey KA, Lundahl LH et al (2006) Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185:327–338
Jones DC, Lau SS, Monks TJ (2004) Thioether metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine inhibit human serotonin transporter (hSERT) function and simultaneously stimulate dopamine uptake into hSERT-expressing SK-N-MC cells. J Pharmacol Exp Ther 311:298–306
Jones DC, Duvauchelle C, Ikegami A et al (2005) Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther 313:422–431
Kageyama Y, Zhang Z, Roda R et al (2012) Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol 197:535–551
Kang D, Hamasaki N (2005) Mitochondrial transcription factor A in the maintenance of mitochondrial DNA. Ann N Y Acad Sci 1042:101–108
Kim Y, Park J, Kim S et al (2008) PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun 377:975–980
Kim JY, Shen S, Dietz K et al (2010) HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci 13:180–189
Kim SH, Lu HF, Alano CC (2011) Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS ONE 6:e14731
Kim-Han JS, Antenor-Dorsey JA, O’Malley KL (2011) The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci 31:7212–7221
Kish SJ, Fitzmaurice PS, Boileau I et al (2009) Brain serotonin transporter in human methamphetamine users. Psychopharmacology 202:649–661
Klongpanichapak S, Govitrapong P, Sharma S, Ebadi M (2006) Attenuation of cocaine and methamphetamine neurotoxicity by coenzyme Q10. Neurochem Res 31:303–311
Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P (2007) Melatonin protects SK-N-SH neuroblastoma cells from amphetamine-induced neurotoxicity. J Pineal Res 43:65–73
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Kongsuphol P, Mukda S, Nopparat C, Villarroel A, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. J Pineal Res 46:199–206
Koppen M, Langer T (2007) Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol 42:221–242
Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305:858–862
Krantic S, Mechawar N, Reix S, Quirion R (2007) Apoptosis-inducing factor: a matter of neuron life and death. Prog Neurobiol 81:179–196
Krasnova IN, Ladenheim B, Cadet JL (2005) Amphetamine induces apoptosis of medium spiny striatal projection neurons via the mitochondria-dependent pathway. FASEB J 19:851–853
Kuczenski R, Segal DS, Cho AK, Melega W (1995) Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci 15:1308–1317
Kuhar MJ, Couceyro PR, Lambert PD (1999) Storage and Release of Catecholamines. In: Siegel GJ, Agranoff BW (eds) Basic neurochemistry: molecular, cellular and medical aspects, vol 6. Lippincott Williams & Wilkins, Philadelphia
Langsdorf EF, Chang SL (2011) Methamphetamine-mediated modulation of MOR expression in the SH-SY5Y neuroblastoma cell line. Synapse 65:858–865
Lau JWS, Senok S, Stadlin A (2000) Methamphetamine-induced oxidative stress in cultured mouse astrocytes. Ann N Y Acad Sci 914:146–156
Lenzi P, Marongiu R, Falleni A et al (2012) A subcellular analysis of genetic modulation of PINK1 on mitochondrial alterations, autophagy and cell death. Arch Ital Biol 150:194–217
Leonardi ET, Azmitia EC (1994) MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac). Neuropsychopharmacology 10:231–238
Li H, Chen Y, Jones AF et al (2008) Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci USA 105:2169–2174
Li Y, Hu Z, Chen B et al (2012) Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol Lett 215:1–7
Lin M, Chandramani-Shivalingappa P, Jin H et al (2012) Methamphetamine-induced neurotoxicity linked to ubiquitin-proteasome system dysfunction and autophagy-related changes that can be modulated by protein kinase C delta in dopaminergic neuronal cells. Neuroscience 210:308–332
Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E (2009) Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ 16:899–909
Liu S, Sawada T, Lee S et al (2012) Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet 8:e1002537
Llorens-Martín M, López-Doménech G, Soriano E, Avila J (2011) GSK3β is involved in the relief of mitochondria pausing in a tau-dependent manner. PLoS ONE 6:e27686
Losón OC, Song Z, Chen H, Chan DC (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24:659–667
Macaskill AF, Rinholm JE, Twelvetrees AE et al (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61:541–555
MacAskill AF, Atkin TA, Kittler JT (2010) Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci 32:231–240
Macedo C, Branco PS, Ferreira LM et al (2007) Synthesis and cyclic voltammetry studies of 3,4-methylenedioxymethamphetamine (MDMA) human metabolites. J Health Sci 53:31–42
Maragos WF, Jakel R, Chesnut D et al (2000) Methamphetamine toxicity is attenuated in mice that overexpress human manganese superoxide dismutase. Brain Res 878:218–222
Marchetti P, Castedo M, Susin SA et al (1996) Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med 184:1155–1160
Martinez-Ruiz G, Maldonado V, Ceballos-Cancino G, Grajeda J, Melendez-Zajgla J (2008) Role of Smac/DIABLO in cancer progression. J Exp Clin Cancer Res 27:48
Marzo I, Brenner C, Zamzami N et al (1998) The permeability transition pore complex: a target for apoptosis regulation by caspases and Bcl-2–related proteins. J Exp Med 187:1261–1271
Matsuda N, Sato S, Shiba K et al (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189:211–221
Matsumoto T, Maeno Y, Kato H et al (2014) 5-hydroxytryptamine- and dopamine-releasing effects of ring-substituted amphetamines on rat brain: a comparative study using in vivo microdialysis. Eur Neuropsychopharmacol 24:1362–1370
McCann UD, Szabo Z, Seckin E et al (2005) Quantitative PET studies of the serotonin transporter in MDMA users and controls using [lsqb]11C[rsqb]McN5652 and [lsqb]11C[rsqb]DASB. Neuropsychopharmacology 30:1741–1750
Mehdizadeh M, Dabaghian F, Nejhadi A et al (2012) Zingiber officinale alters 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rat brain. Cell J 14:177–184
Meyer JS, Grande M, Johnson K, Ali SF (2004) Neurotoxic effects of MDMA (“ecstasy”) administration to neonatal rats. Int J Dev Neurosci 22:261–271
Miramar MD, Costantini P, Ravagnan L et al (2001) NADH oxidase activity of mitochondrial apoptosis-inducing factor. J Biol Chem 276:16391–16398
Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30:4232–4240
Misko AL, Sasaki Y, Tuck E, Milbrandt J, Baloh RH (2012) Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J Neurosci 32:4145–4155
Mitcheel P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148
Moldoveanu T, Follis AV, Kriwacki RW, Green DR (2014) Many players in BCL-2 family affairs. Trends Biochem Sci 3:101–111
Moretti M, Valvassori SS, Steckert AV et al (2011) Tamoxifen effects on respiratory chain complexes and creatine kinase activities in an animal model of mania. Pharmacol Biochem Behav 98:304–310
Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST (2002) Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J 21:281–293
Mozdy AD, McCaffery JM, Shaw JM (2000) Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol 151:367–380
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803
Nicholls DG (2009) Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans 37:1385–1388
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459
Nixdorf WL, Burrows KB, Gudelsky GA, Yamamoto BK (2001) Enhancement of 3,4-methylenedioxymethamphetamine neurotoxicity by the energy inhibitor malonate. J Neurochem 77:647–654
Oettinghaus B, Licci M, Scorrano L, Frank S (2012) Less than perfect divorces: dysregulated mitochondrial fission and neurodegeneration. Acta Neuropathol 123:189–203
Ola MS, Nawaz M, Ahsan H (2011) Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 351:41–58
Oliveira MT, Rego AC, Morgadinho MT, Macedo TRA, Oliveira CR (2002) Toxic affects of opioid and stimulant drugs on undifferentiated PC12 cells. Ann N Y Acad Sci 965:487–496
Oliveira MT, Rego AC, Macedo TA, Oliveira CR (2003) Drugs of abuse induce apoptotic features in PC12 cells. Ann N Y Acad Sci 1010:667–670
Onoue K, Jofuku A, Ban-Ishihara R et al (2013) Fis1 acts as a mitochondrial recruitment factor for TBC1D15 that is involved in regulation of mitochondrial morphology. J Cell Sci 126:176–185
Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH (2010) Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta 1802:228–234
Otera H, Mihara K (2011) Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem 149:241–251
Otera H, Wang C, Cleland MM et al (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191:1141–1158
Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT (2011) MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 12:565–573
Pereira C, Chaves S, Alves S et al (2010) Mitochondrial degradation in acetic acid-induced yeast apoptosis: the role of Pep4 and the ADP/ATP carrier. Mol Microbiol 76:1398–1410
Perlmann T, Wallén-Mackenzie Å (2004) Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res 318:45–52
Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J (2003) Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci 23:4499–4508
Pilgrim JL, Gerostamoulos D, Drummer OH (2010) Deaths involving serotonergic drugs. Forensic Sci Int 198:110–117
Pilgrim JL, Gerostamoulos D, Woodford N, Drummer OH (2012) Serotonin toxicity involving MDMA (ecstasy) and moclobemide. Forensic Sci Int 215:184–188
Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 105:1638–1643
Popov V, Medvedev NI, Davies HA, Stewart MG (2005) Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: a three-dimensional ultrastructural study. J Comp Neurol 492:50–65
Prince JA, Yassin MS, Oreland L (1997) Normalization of cytochrome-c oxidase activity in the rat brain by neuroleptics after chronic treatment with PCP or methamphetamine. Neuropharmacology 36:1665–1678
Pubill D, Chipana C, Camins A, Pallàs M, Camarasa J, Escubedo E (2005) Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol 204:57–68
Puerta E, Hervias I, Goñi-Allo B et al (2010) Methylenedioxymethamphetamine inhibits mitochondrial complex I activity in mice: a possible mechanism underlying neurotoxicity. Br J Pharmacol 160:233–245
Qi X, Qvit N, Su Y, Mochly-Rosen D (2012) Novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126:789–802
Quinton MS, Yamamoto BK (2006) Causes and consequences of methamphetamine and MDMA toxicity. AAPS J 8:337–347
Raimundo N, Baysal BE, Shadel GS (2011) Revisiting the TCA cycle: signaling to tumor formation. Trends Mol Med 17:641–649
Ramkissoon A, Wells PG (2013) Developmental role of nuclear factor E2-related factor 2 in mitigating methamphetamine fetal toxicity and postnatal neurodevelopmental deficits. Free Radic Biol Med 65:620–631
Ramsay RR, Hunter DJB (2002) Inhibitors alter the spectrum and redox properties of monoamine oxidase A. Biochim Biophys Acta 1601:178–184
Ranieri M, Brajkovic S, Riboldi G et al (2013) Mitochondrial fusion proteins and human diseases. Neurol Res Int 2013:293893
Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG (2010) 5-Hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J Pharmacol Exp Ther 332:632–639
Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY (1982) Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res 235:93–103
Ricaurte GA, Seiden LS, Schuster CR (1984) Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res 303:359–364
Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ (2003) Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci 23:7881–7888
Rojo M, Legros F, Chateau D, Lombès A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115:1663–1674
Rothman RB, Partilla JS, Baumann MH, Dersch CM, Carroll FI, Rice KC (2000) Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: a pharmacological strategy for treating stimulant abuse. Synapse 35:222–227
Ryu NK, Yang MH, Jung MS, Jeon JO, Kim KW, Park JH (2007) Gene expression profiling of rewarding effect in methamphetamine treated Bax-deficient mouse. J Biochem Mol Biol 40:475–485
Sato M (1992) A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci 654:160–170
Sau D, Rusmini P, Crippa V et al (2011) Dysregulation of axonal transport and motorneuron diseases. Biol Cell 103:87–107
Saxton WM, Hollenbeck PJ (2012a) The axonal transport of mitochondria. J Cell Sci 125:2095–2104
Saxton WM, Hollenbeck PJ (2012b) The axonal transport of mitochondria. J Cell Sci 125:2095–2104
Schapira AHV (2010) Complex I: inhibitors, inhibition and neurodegeneration. Exp Neurol 224:331–335
Schwarz TL (2013) Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol 5:a011304
Scorza MC, Carrau C, Silveira R, Zapata-Torres G, Cassels BK, Reyes-Parada M (1997) Monoamine oxidase inhibitory properties of some methoxylated and alkylthio amphetamine derivatives: structure–activity relationships. Biochem Pharmacol 54:1361–1369
Sekine Y, Ouchi Y, Takei N et al (2006) Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 63:90–100
Sevrioukova IF (2011) Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal 14:2545–2579
Shankaran M, Yamamoto BK, Gudelsky GA (1999) Involvement of the serotonin transporter in the formation of hydroxyl radicals induced by 3,4-methylenedioxymethamphetamine. Eur J Pharmacol 385:103–110
Sheng Z, Cai Q (2012) Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci 13:77–93
Shiba T, Yamato M, Kudo W, Watanabe T, Utsumi H, Yamada KI (2011) In vivo imaging of mitochondrial function in methamphetamine-treated rats. Neuroimage 57:866–872
Shih JC, Grimsby J, Chen K (1999) Molecular biology of monoamine oxidase A and B: their role in the degradation of serotonin. In: Gothert HB (ed) Serotoninergic neurons and 5-HT receptors in the SNC. Springer, Berlin, pp 655–670
Shima N, Miyawaki I, Bando K et al (2011) Influences of methamphetamine-induced acute intoxication on urinary and plasma metabolic profiles in the rat. Toxicology 287:29–37
Shimizu S, Eguchi Y, Kamiike W et al (1996) Involvement of ICE family proteases in apoptosis induced by reoxygenation of hypoxic hepatocytes. Am J Physiol 271:G949–G958
Silva DD, Carmo H, Lynch A, Silva E (2013a) An insight into the hepatocellular death induced by amphetamines, individually and in combination: the involvement of necrosis and apoptosis. Arch Toxicol 87:2165–2185
Silva DD, Silva E, Carmo H (2013b) Combination effects of amphetamines under hyperthermia—the role played by oxidative stress. J Appl Toxicol 34:637–650
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157–1180
Sipos I, Tretter L, Adam-Vizi V (2003) Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem 84:112–118
Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12:2245–2256
Soleimani M, Katebi M, Alizadeh A, Mohammadzadeh F, Mehdizadeh M (2012) The role of the A2A receptor in cell apoptosis caused by MDMA. Cell J 14:231–236
Soleimani Asl S, Farhadi MH, Moosavizadeh K et al (2012) Evaluation of Bcl-2 family gene expression in hippocampus of 3,4-methylenedioxymethamphetamine treated rats. Cell J 13:275–280
Soleimani Asl S, Mousavizedeh K, Pourheydar B, Soleimani M, Rahbar E, Mehdizadeh M (2013) Protective effects of N-acetylcysteine on 3,4-methylenedioxymethamphetamine-induced neurotoxicity in male Sprague–Dawley rats. Metab Brain Dis 28:677–686
Soleimani Asl S, Saifi B, Sakhaie A, Zargooshnia S, Mehdizadeh M (2015) Attenuation of ecstasy-induced neurotoxicity by N-acetylcysteine. Metab Brain Dis 30:171–181
Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC (2009) Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20:3525–3532
Spencer JPE, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B (1998) Conjugates of catecholamines with cysteine and GSH in Parkinson’s disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem 71:2112–2122
Sprague JE, Nichols DE (1995a) Inhibition of MAO-B protects against MDMA-induced neurotoxicity in the striatum. Psychopharmacology 118:357–359
Sprague JE, Nichols DE (1995b) The monoamine oxidase-B inhibitor L-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J Pharmacol Exp Ther 273:667–673
Srinivasula SM, Hegde R, Saleh A et al (2001) A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410:112–116
Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow E-M (2002) Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol 156:1051–1063
Stanley N, Salem A, Irvine RJ (2007) The effects of co-administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) or para-methoxyamphetamine and moclobemide at elevated ambient temperatures on striatal 5-HT, body temperature and behavior in rats. Neuroscience 146:321–329
Stephans SE, Whittingham TS, Douglas AJ, Lust WD, Yamamoto BK (1998) Substrates of energy metabolism attenuate methamphetamine-induced neurotoxicity in striatum. J Neurochem 71:613–621
Stoothoff W, Jones PB, Spires-Jones TL et al (2009) Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem 111:417–427
Stumm G, Schlegel J, Schäfer T et al (1999) Amphetamines induce apoptosis and regulation of bcl-x splice variants in neocortical neurons. FASEB J 13:1065–1072
Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci 15:4102–4108
Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433
Susin SA, Zamzami N, Castedo M et al (1996) Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 184:1331–1341
Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282:11521–11529
Takamatsu Y, Shiotsuki H, Kasai S et al (2011) Enhanced hyperthermia induced by MDMA in parkin knockout mice. Curr Neuropharmacol 9:96–99
Tamburini I, Blandini F, Gesi M et al (2006) MDMA induces caspase-3 activation in the limbic system but not in striatum. Ann N Y Acad Sci 1074:377–381
Tatsuta T, Langer T (2008) Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27:306–314
Thrash B, Karuppagounder SS, Uthayathas S, Suppiramaniam V, Dhanasekaran M (2010) Neurotoxic effects of methamphetamine. Neurochem Res 35:171–179
Tian C, Murrin LC, Zheng JC (2009) Mitochondrial fragmentation is involved in methamphetaminei-induced cell death in rat hippocampal neural progenitor cells. PLoS ONE 4:e5546
Twig G, Shirihai OS (2011) The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14:1939–1951
Twig G, Elorza A, Molina AJ et al (2008a) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446
Twig G, Hyde B, Shirihai OS (2008b) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777:1092–1097
Ulukaya E, Acilan C, Yilmaz Y (2011) Apoptosis: Why and how does it occur in biology? Cell Biochem Funct 29:468–480
Vafai SB, Mootha VK (2012) Mitochondrial disorders as windows into an ancient organelle. Nature 491:374–383
Vahsen N, Candé C, Brière JJ et al (2004) AIF deficiency compromises oxidative phosphorylation. EMBO J 23:4679–4689
Valenzuela A, Pla A, Villanueva E (1987) Effects of chronic administration of dextroamphetamine on enzymes of energy metabolism in regions of the rat brain. Neuropharmacology 26:627–631
Valvassori SS, Rezin GT, Ferreira CL et al (2010) Effects of mood stabilizers on mitochondrial respiratory chain activity in brain of rats treated with d-amphetamine. J Psychiatr Res 44:903–909
Valvassori SS, Calixto KV, Budni J et al (2013) Sodium butyrate reverses the inhibition of Krebs cycle enzymes induced by amphetamine in the rat brain. J Neural Transm 120:1737–1742
Van Laar VS, Berman SB (2013) The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson’s disease. Neurobiol Dis 51:43–55
Vilar S, Ferino G, Quezada E, Santana L, Friedman C (2012) Predicting monoamine oxidase inhibitory activity through ligand-based models. Curr Top Med Chem 12:2258–2274
Villemagne V, Yuan J, Wong DF et al (1998) Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 18:419–427
Vincow ES, Merrihew G, Thomas RE et al (2013) The PINK1–Parkin pathway promotes both mitophagy and selective respiratory chain turnover In vivo. Proc Natl Acad Sci USA 110:6400–6405
Volkow ND, Chang L, Wang G et al (2001a) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418
Volkow ND, Chang L, Wang GJ et al (2001b) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382
Vuori E, Henry JA, Ojanperä I et al (2003) Death following ingestion of MDMA (ecstasy) and moclobemide. Addiction 98:365–368
Wadley GD, Choate J, McConell GK (2007) NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol 585:253–262
Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J (1980) Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res 181:151–160
Wan FJ, Lin HC, Kang BH, Tseng CJ, Tung CS (1999) d-Amphetamine-induced depletion of energy and dopamine in the rat striatum is attenuated by nicotinamide pretreatment. Brain Res Bull 50:167–171
Wang X, Schwarz TL (2009) The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136:163–174
Wang X, Su B, Liu W et al (2011a) DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson’s disease. Aging Cell 10:807–823
Wang X, Winter D, Ashrafi G et al (2011b) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147:893–906
Wang Z, Jiang H, Chen S, Du F, Wang X (2012) The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148:228–243
Warren MW, Larner SF, Kobeissy FH et al (2007) Calpain and caspase proteolytic markers co-localize with rat cortical neurons after exposure to methamphetamine and MDMA. Acta Neuropathol 114:277–286
Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ (2009) Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry 48:2045–2052
Willson MC, Wilman AH, Bell EC, Asghar SJ, Silverstone PH (2004) Dextroamphetamine causes a change in regional brain activity in vivo during cognitive tasks: a functional magnetic resonance imaging study of blood oxygen level-dependent response. Biol Psychiatry 56:284–291
Wilson JM, Kalasinsky KS, Levey AI et al (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Wisessmith W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B (2009) Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells. J Pineal Res 46:433–440
Wu Z, Puigserver P, Andersson U et al (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Wu G, Chai J, Suber TL et al (2000) Structural basis of IAP recognition by Smac/DIABLO. Nature 408:1008–1012
Wu CW, Ping YH, Yen JC et al (2007) Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol 220:243–251
Xie T, Tong L, Barrett T et al (2002) Changes in gene expression linked to methamphetamine-induced dopaminergic neurotoxicity. J Neurosci 22:274–283
Yamamoto BK, Moszczynska A, Gudelsky GA (2010) Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci 1187:101–121
Yang RF, Zhao GW, Liang ST et al (2012) Mitofilin regulates cytochrome c release during apoptosis by controlling mitochondrial cristae remodeling. Biochem Biophys Res Commun 428:93–98
Yoon Y, Krueger EW, Oswald BJ, McNiven MA (2003) The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23:5409–5420
Youdim MBH, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337:1062–1065
Young R, Glennon RA (1986) Discriminative stimulus properties of amphetamine and structurally related phenalkylamines. Med Res Rev 6:99–130
Yuan S, Akey CW (2013) Apoptosome structure, assembly, and procaspase activation. Structure 21:501–515
Yuan H, Gerencser AA, Liot G et al (2007) Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ 14:462–471
Zhang X, Banerjee A, Banks WA, Ercal N (2009) N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res 1275:87–95
Zhao J, Liu T, Jin S et al (2011) Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J 30:2762–2778
Zhou Z, Han V, Han J (2012) New components of the necroptotic pathway. Protein Cell 3:811–817
Zhu J, Wang KZ, Chu CT (2013) After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 9:1663–1676
Acknowledgments
D.J.B. was supported by a fellowship (SFRH/BD/64939/2009) from “Fundação para a Ciência e a Tecnologia”, Portugal.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Barbosa, D.J., Capela, J.P., Feio-Azevedo, R. et al. Mitochondria: key players in the neurotoxic effects of amphetamines. Arch Toxicol 89, 1695–1725 (2015). https://doi.org/10.1007/s00204-015-1478-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1478-9