Abstract
CD44 is a family of single-span transmembrane glycoproteins. Members of this family differ in the extracellular domain where ten variant exons are either excluded or included in various combinations. CD44 isoforms participate in many physiological processes including hematopoiesis, regeneration, lymphocyte homing and inflammation. Most importantly, they are involved in pathological processes and in particular in cancer. In several types of tumors, CD44 together with other antigens specifies for cancer stem cell populations. Mechanistically, CD44 proteins act as receptors for hyaluronan, co-receptor for receptor tyrosine kinases (RTKs) or G-protein-coupled receptors or provide a platform for metalloproteinases. For all these reasons, targeting CD44 may be a successful approach in cancer therapy. In this review, we discuss the various possibilities of targeting CD44. Among these are the production of CD44 ectodomains, antibodies, peptides or aptamers. Also inhibition of CD44 expression has been proposed. Finally, the function of CD44 as a hyaluronan receptor was also taken advantage of. We are convinced that the success of these therapies will depend on an increased understanding of the molecular functions of specific CD44 isoforms in particular in cancer stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
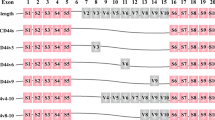
CD44 designates a family of single-span trans-membrane proteins that are encoded by a single gene of about 50 kb of length located on chromosome 11 in humans and on chromosome 2 in mice (reviewed in Orian-Rousseau 2010). The CD44 gene is composed of 20 exons (Fig. 1). Ten of these exons (also known as “constant” exons) are expressed in all isoforms. They also account for the N-terminal extracellular part, the trans-membrane region and the intracellular domain of all members. The ten central exons known as “variant” exons are excised or included in various combinations by alternative splicing in the membrane-proximal stem region. They account for the heterogeneity of this protein family. The last two exons encoding the CD44 cytoplasmic domain are also subjected to alternative splicing. The smallest isoform (CD44s) lacking all variant exons in the extracellular domain is ubiquitously expressed, whereas the expression of variant isoforms is confined to only few tissues and takes place only under specific developmental conditions. Most strikingly, CD44 variant isoforms are expressed in a variety of different cancers, particularly in advanced stages (reviewed in Naor et al. 2002; Orian-Rousseau 2010]. The complexity of the CD44 protein family is further enhanced by post-translational modifications such as N- and O-glycosylations, chondroitin sulfations or heparan sulfate additions (for a more detailed description see Orian-Rousseau and Sleeman 2014; Ponta et al. 2003).
The CD44 proteins are encoded by one single gene. The CD44 gene comprises 20 exons. Ten of these exons (v1–v10) are alternatively spliced. The CD44s isoform does not contain any variant exon. Variant exons (v6 is shown) are included in the stem region of the protein. In humans, exon v1 contains a stop codon and is not found in any isoform. The last two exons encoding the CD44 cytoplasmic domain are also subjected to alternative splicing (reviewed in Ponta et al. 2003)
CD44 came into focus for the first time in cancer research when it was identified as a homing receptor for migrating thymus progenitor cells (O’Neill 1989) and human lymphocytes (Jalkanen et al. 1987; Pals et al. 1989). Furthermore, CD44 appeared to mediate the binding of lymphocytes or lymphoma cells to endothelial cells most likely only upon activation of the lymphocytes (Lesley and Hyman 1992; Oppenheimer-Marks et al. 1990). These functions are not only instrumental for the fate of lymphocytes but are also required for the hematogenic spreading of tumor cells.
Molecular functions of CD44
CD44 is the main receptor for hyaluronan
The migration of lymphocytes as well as the spreading of tumor cells are controlled by CD44 and require interactions with constituents of the extracellular matrix (ECM). A hallmark in the CD44 research was the identification of CD44 as the principal receptor for hyaluronan (HA) (Aruffo et al. 1990). HA is a linear non-sulfated polysaccharide composed of disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine with a MW of 106–107 kDa. HA is particularly abundant in connective tissue and in the lymph and lymph node matrix. HA does not only provide a cellular support and hydrophilic matrix but also regulates cell–cell adhesion, cell migration as well as growth and differentiation (Laurent and Fraser 1992). Consequently, HA is involved in many physiological processes such as wound healing, inflammation, morphogenesis and in pathological processes such as cancer. Furthermore, upon interaction with the cell surface HA forms a “coat” that can act as a cellular barrier (Gately et al. 1984; McBride and Bard 1979) and eventually can protect tumor cells from an immune attack. Interestingly, several tumor cells produce increased amounts of HA or induce the production of HA by surrounding fibroblasts thereby leading to enhanced metastatic spreading (Knudson et al. 1984; Turley and Tretiak 1985; Zhang et al. 1995).
The binding domain of CD44 for HA is located in the N-terminal extracellular part of the molecule. This domain is called the “link region” for its homology with the HA-binding domain of the cartilage link protein that allows network formation between HA and glycosaminoglycans in the ECM (Laurent and Fraser 1992). It is a globular domain with three conserved cysteine bridges and with two BX7B sequences where two basic amino acids (B) are separated by seven non-acidic amino acids (Goetinck et al. 1987; Goldstein et al. 1989; Peach et al. 1993; Yang et al. 1994). This binding domain exists in all CD44 isoforms. However, in some cells, the insertion of variant exons in the stem region results in a loss of HA binding, whereas in other cells, the inclusion of variant exons even enhances HA binding (reviewed in Naor et al. 1997; Orian-Rousseau and Sleeman 2014). Several other factors influence the binding of CD44 to HA. Among these are CD44 aggregation, interaction of CD44 with other cell surface proteins and post-translational modifications of the CD44 protein (reviewed in Naor et al. 1997; Orian-Rousseau and Sleeman 2014). Although not all parameters regulating HA binding to CD44 have been unraveled CD44 turned out to be the most important cellular receptor for HA and seems to be involved in the majority of HA-dependent cellular responses. Additionally, several other HA-binding cellular receptors have been identified (e.g., RHAMM, Lyve1, TLR4, ICAM1), which have functions in rather restricted tissues (reviewed in Naor et al. 1997).
The minimal size of HA fragments binding to CD44 corresponds to six disulfide units. This is important since high molecular weight HA (hHA) in the ECM is degraded by hyaluronidases into smaller fragments (sHA) that still can bind to CD44. Interestingly, hHA and sHA often exert opposite effects on several physiological and pathological processes (for a detailed discussion see Orian-Rousseau and Sleeman 2014).
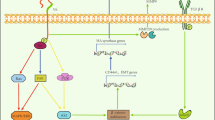
Although HA and CD44s are expressed in nearly all tissues, only few physiological functions requiring their collaboration have been identified. One of which is the already mentioned homing of lymphocytes, where antibodies against CD44 that block the binding to HA, inhibit the binding of lymphocytes to high endothelial venules, a key step in homing (Jalkanen et al. 1987). In addition, the homing of mesenchymal stem cells to the kidney during acute renal failure is instrumental for the healing process and is dependent on CD44–HA interaction (Herrera et al. 2007). Another one is the rolling of lymphocytes in the blood stream, which is one of the first steps in the extravasation of lymphocytes (and metastasizing tumor cells). This step requires the interaction of leukocytes to the endothelial cells of blood vessels. For some T cells, this interaction can be blocked by CD44-specific antibodies and by treatment with soluble HA (DeGrendele et al. 1996, 1997). Furthermore, CD44 antibodies, which prevent the binding of CD44 to HA inhibit the migration of hematopoietic stem cells to the bone marrow (Avigdor et al. 2004). In angiogenesis, the formation of new blood vessels from existing ones, a phenomenon important in wound healing and tumor growth, the interaction of CD44 and HA also appears to be instrumental (Fuchs et al. 2013) (Fig. 2a).
Most common blocking reagents against CD44 isoforms: a CD44 ectodomain [e.g., (Yu et al. 1997), antibodies blocking CD44–HA interaction (Ghatak et al. 2002) or small fragments of HA inhibiting the binding of hHA (Ghatak et al. 2002; Fuchs et al. 2013). b Antibodies against CD44v6 (Heider et al. 1996; Schrijvers et al. 1993) or peptides (Matzke et al. 2005)
CD44 isoforms act as co-receptors
A milestone in the research on CD44 was the identification of the CD44v6 isoform as one of the first metastatic determinants in cancer (Gunthert et al. 1991; Hofmann et al. 1991; Rudy et al. 1993). CD44v6-specific antibodies were able to block the metastatic spreading of rat pancreatic tumor cells. Moreover, the transfection of CD44v4–v7 (the v6 exon is contained in the stem region) cDNA but not of CD44s cDNA into non-metastatic tumor cells conferred metastatic propensity to these cells. These findings prompted a huge number of studies aiming at unraveling the relevance of CD44 isoforms and particular CD44v6 isoforms in all different types and stages of human tumors, and indeed, there is ample of evidence for a correlation between the expression of CD44 isoforms and advanced stages of carcinomas (reviewed in Naor et al. 2002; Orian-Rousseau 2010).
A breakthrough in the understanding of molecular functions of CD44 isoforms in physiological and pathological conditions was the observation that heparan sulfate-modified CD44v3 isoforms are able to bind several heparan sulfate binding growth factors such as FGFs or HB-EGF (Bennett et al. 1995; Jackson et al. 1995). It turned out that such a function is not confined to heparan sulfate-modified CD44 isoforms but can also be provided by other isoforms. Of particular interest for cancer research was the identification of CD44v6 isoforms as co-receptors for the receptor tyrosine kinases (RTKs), Met and VEGFR-2 (Orian-Rousseau et al. 2002; Tremmel et al. 2009). Met and VEGFR-2 activation and subsequent signaling are both dependent on CD44v6 (Orian-Rousseau et al. 2002, 2007). Consequently, the formation of new blood vessels in a human pancreatic xenograft was blocked upon inhibition of CD44v6 (Tremmel et al. 2009). These data point toward a requirement of the co-receptor functions of CD44v6 for these RTKs for tumor progression. In agreement with this assumption is the finding that metastasis of colorectal cancer spheres injected into the murine cecum is dependent on both, CD44v6 and Met (Todaro et al. 2014).
The binding ability of CD44 to ECM components, particularly to HA, and the co-receptor function of CD44 isoforms are features that might explain the contribution of CD44 to tumorigenesis. Furthermore, the functions of CD44 isoforms as co-receptors for several RTKs might also explain why so many different CD44 isoforms exist. Indeed, different isoforms can address different receptors and are specialized for different ligands (reviewed in Orian-Rousseau and Sleeman 2014).
It is worth noting that CD44 proteins collaborate not only with RTKs but are also involved in the CXCL12-CXCR4 axis (Fuchs et al. 2013) and the Wnt signaling pathway (Schmitt et al. 2014). Furthermore, CD44 proteins can contribute to signaling pathways by binding metalloproteinases and thereby facilitating the activation of growth factor pro-forms to the active protein (Yu and Stamenkovic 2000; Yu et al. 2002).
Particularly important in the context of carcinogenesis is the contribution of CD44 to the inhibition of apoptosis (Yu et al. 1997). Several mechanisms have been proposed that account for this function. Among these are the HA-dependent activation of TGFβ1 (Yu and Stamenkovic 2004), the activation of HB-EGF, the activation of osteopontin by CD44v6 containing isoforms and even the inhibition of Fas signaling by CD44 (reviewed in Ponta et al. 2003; Mielgo et al. 2005). A completely different function of CD44 in apoptosis was recently suggested. CD44v6 isoforms account for the formation of a pre-metastatic niche that promotes survival of tumor cells and induces chemo-resistance (Jung et al. 2009, 2011).
The involvement of CD44 in the establishment and progression of several cancers makes it a suitable target for cancer therapy. In this review, we present various ways of targeting CD44 isoforms and discuss the future prospects of these therapies.
Anti-CD44 strategies
Hyaluronan-dependent anti-cancer strategies
There is ample evidence that the CD44–HA interaction is involved in tumor progression (reviewed in Misra et al. 2011; Orian-Rousseau and Sleeman 2014). Therefore, the interference with the binding of CD44 expressed on tumor cells to HA using either the soluble CD44 ectodomain as a competitor or antibodies that specifically block the binding of HA to CD44, impaired tumor growth and metastasis. Indeed, the local administration of the ectodomain of CD44 in mice transfected with human melanoma cells inhibited tumor growth, whereas injection of a mutant, non-HA-binding CD44 ectodomain had no effect (Bartolazzi et al. 1994). Similarly, human melanoma cells transfected with an expression construct for the CD44 ectodomain showed retarded tumor growth when compared to cells transfected with a HA-binding mutant (Ahrens et al. 2001). Most strikingly, the expression of a peptide of 42 amino acid of length that contained three BX7B HA-binding motifs (found in CD44 and other HA-binding proteins) induced apoptosis and inhibited tumor growth of melanoma cells in vivo (Xu et al. 2003).
The expression of the CD44 ectodomain in metastatic murine mammary carcinoma cells also inhibited tumor growth upon the induction of apoptosis and repressed the invasion of tumor cells into the surrounding tissues (Yu et al. 1997) (Fig. 2a). A mutant ectodomain in the HA-binding sequence, however, did not interfere with tumor growth (Peterson et al. 2000). In these mammary carcinoma cells, CD44 recruits the metalloproteinase MMP9 most likely in a HA-dependent manner since MMP9, CD44 and HA are found in clusters (Yu and Stamenkovic 1999). The binding of MMP9 to CD44 allowed the activation of TGFβ1, which resulted in cell survival and metastasis (Yu and Stamenkovic 2004).
Monoclonal CD44-specific antibodies, which interfere with the binding of HA to CD44, have similar effects as the CD44 ectodomain. They led to the inhibition of anchorage-independent growth of murine mammary carcinoma cells and human colon carcinoma cells and induced apoptosis (Ghatak et al. 2002) (Fig. 2a). Furthermore, HA oligosaccharides had similar effects and inhibited tumor growth in vivo most likely by interfering with the binding of hHA to CD44 (Ghatak et al. 2002) (Fig. 2a). This is an example of apparent opposing effects of hHA and sHA.
The binding of HA to CD44 can lead to the internalization of HA (Culty et al. 1992, 1994). This feature and the unique properties of HA, namely its bio-degradability, its bio-compatibility and non-immunogenicity makes HA a good candidate for drug delivery applications. Several labs have shown that HA can be covalently coupled with drugs can efficiently target CD44-expressing cells (Akima et al. 1996; Luo et al. 2000; Pouyani and Prestwich 1994; Yadav et al. 2008). HA contains multiple functional residues (hydroxyl and carboxylic acid) on the HA backbone, which can be used to form HA-drug conjugates. Upon internalization, the drug is released mainly by enzymatic hydrolysis. Several preclinical studies have shown that the anti-cancer properties are efficiently improved by the covalently coupling of drugs to HA. For example, the coupling of the anti-mitotic chemotherapeutic agent paclitaxel to HA increased its solubility and selectively targeted the CD44-dependent human ovarian, colon and breast cancer cells (Luo and Prestwich 1999).
HA can also be covalently or non-covalently coupled with nanoparticles (NPs). An in-depth description of the potential of HA-based nanocarriers can be found in the reviews by (Choi et al. 2012; Ghosh et al. 2012; Misra et al. 2011). Here, we describe only a few examples. Several versions of HA-coupled nanocarriers loaded with anti-cancer drugs were examined and have demonstrated advantages for cancer treatments in animal models. Their non-modified counterparts showed no such advantages. For example, coupling of high molecular weight HA to lipid-based NPs enhanced their circulation time and improved the specificity of tumor targeting (Mizrahy et al. 2014). Interestingly, coupling of sHA did not show this effect. Several anti-cancer drugs such as epirubicin, doxorubicin, paclitaxel or mitomycin c were incorporated in the inner hydrophobic part of HA-nanocarriers and were tested for their therapeutic efficacy (Eliaz et al. 2004; Eliaz and Szoka 2001; Peer and Margalit 2004). In all cases, the encapsulation of the drugs led to a significant improvement of their efficacy.
Examples of more recently developed NPs with extremely high efficient tumor targeting, optimal release of encapsulated drugs due to fine-tuning of the pH conditions and extremely low cytotoxicity are found in the following papers: Qiu et al. (2014); Song et al. (2014a, b). Some of these combinations have made it to clinical trials. One example is a combination of HA and paclitaxel, a highly hydrophobic anti-cancer drug, referred to as ONCOFID™-P undergoing phase II clinical study in Europe for treatment of refractory bladder cancer (reviewed in Choi et al. 2012).
Antibody-based strategies against CD44
Monoclonal antibodies against CD44v6 in head and neck cancer
Expression studies for several CD44 isoforms revealed that they are particular abundant in advanced stages of carcinoma. This is particularly true for CD44v6 (reviewed in Naor et al. 2002; Orian-Rousseau 2010). Since the expression of CD44v6 is particularly high and homogenous in human head and neck carcinoma (HNSCC), HNSCC was considered promising for treatment with the CD44v6 antibodies (Heider et al. 1996; Schrijvers et al. 1993) (Fig. 2b). Two monoclonal antibodies were used. The first one, designated BIWA, was derived from mice injected with the human CD44v6 part (Heider et al. 1996). The second one, named U36, was obtained from a screen for specific epitopes expressed on human head and neck carcinoma cells. It turned out that U36 is also specific for CD44v6 (Schrijvers et al. 1993; Van Hal et al. 1996). Interestingly, the epitopes recognized by the two mAbs overlap and differ only by two amino acids (Van Hal et al. 1997).
Both mAbs were radiolabelled and showed selective tumor targeting and high tumor uptake in HNSCC patients undergoing surgery (Colnot et al. 2000; de Bree et al. 1995; Stroomer et al. 2000; Van Hal et al. 1996; Verel et al. 2002). Since the BIWA antibody induced an immune response in patients, it was humanized to give BIWA4 (bivatuzumab) that was then used for further clinical studies (Colnot et al. 2003; Stroomer et al. 2000). A radio-immune therapy study (RIT) with 186Re-labeled bivatuzumab in HNSCC patients gave promising anti-tumor effects with consistent stable disease at higher radioactivity dosage (Borjesson et al. 2003; Postema et al. 2003). The U36 antibody was also tested in several RIT studies with promising outcomes especially in adjuvant settings (Colnot et al. 2000, 2002).
Based on these results, the non-radioactive cytotoxic drug mertansine, an anti-microtubule agent, was coupled with bivatuzumab (Sauter et al. 2007). Indeed, bivatuzumab could direct mertansine activity to CD44v6-expressing tumor cells. Pharmacokinetics, immunogenicity and safety of the antibody drug conjugate were evaluated. In a phase I escalation study with 31 HNSCC patients, no immune response was observed with bivatuzumab–mertansine (Sauter et al. 2007), and in a phase I trial, 12 HNSCC patients were treated with the maximal tolerated dose (Riechelmann et al. 2008). Unexpectedly, the binding of the conjugate to skin keratinocytes mediated serious skin toxicity and had a fatal outcome, and therefore, the study was terminated. However, in three patients, a partial response with a disappearance of tumor infiltration could be observed. The tumor regression lasted for 4–8 months under continued drug treatment (Riechelmann et al. 2008).
In a parallel study, seven HNSCC patients received bivatuzumab–mertansine for 23 weeks. With the highest dose, one patient developed toxic epidermal necrolysis and died. The risk–benefit assessment turned out to be negative mainly for skin-related adverse events although the majority of skin reactions were reversible. Further clinical development was discontinued (Tijink et al. 2006).
Despite the clinical drawbacks with bivatuzumab–mertansine CD44v6 mAbs were further developed for targeting CD44v6 in HNSCC tumors. The U36 antibody was labeled with 111In using the chelator CHXA”-DTPA and the chimeric molecule showed promising results regarding bio-distribution and tumor uptake in HNSCC bearing nude mice (Sandstrom et al. 2008). F(ab′)2 and Fab′ fragments of U36 were even superior to the mAb regarding bio-distribution and accumulation in the tumor (Sandstrom et al. 2012). Although the tumor uptake of 125I-F(ab′)2 was lower as compared to the 125I-labeled mAb U36, a higher tumor-to-blood ratio was observed.
Bivatuzumab was also used in bio-distribution and safety studies in other tumors (breast cancer (Koppe et al. 2004); thyroid cancer (Fortin et al. 2007) and for tumor imaging (Vermeulen et al. 2013)). It was additionally used for the detection of lymph node metastases (Borjesson et al. 2006). Most recently, a fully human Fab fragment obtained from a synthetic Fab library and selected for binding to CD44v6 isoforms was assessed for tumor imaging. A comparison between 111In- and 125I-labeled Fab fragments revealed that both had a high tumor targeting capacity but the 111In-labeled had a higher tumor-to-blood ratio and could discriminate better between high and moderate expression of CD44v6 in HNSCC xenografts (Haylock et al. 2014).
A very interesting and often successful approach is the targeting of tumor antigens by adoptive cell therapy. For this approach, T cells were genetically engineered to express receptors directed against the tumor antigen. CD44v6 was used as such an antigen. The antigen-recognizing determinant of bivatuzumab was fused to the signaling domains of CD28 and CD3ζ. The chimeric genes were introduced by means of retroviral gene transfer into human T cells, which then displayed anti-CD44v6 effector functions. They eliminated CD44v6 positive acute myeloid leukemia and multiple myeloma cells in murine xenografts (Casucci et al. 2013). The drawback of this approach is the off-tumor/on-target toxicity due to the expression of the tumor antigen on normal tissue. For CD44v6, these tissues are mainly keratinocytes and circulating monocytes. The elimination of these monocytes in particular would lead to long-term monocytopenia, a disease that is life threatening. To avoid side effects, the authors have incorporated a suicide gene into the engineered T cells namely, the non-immunogenic, quick acting and inducible iCasp9 (Straathof et al. 2005). iCasp9 refers to a Casp9 gene fused to a dimerization sequence. This dimerization sequence can be addressed by treatment with a small molecule that exclusively drives iCasp9 dimerization and activation in the transfected T cells thereby leading to their elimination.
Other antibodies against CD44
A study with pancreatic cancer patients that underwent surgery demonstrated that patients with higher levels of panCD44 in the pancreatic adenocarcinoma had a worse prognosis for survival when compared to patients with lower levels (Li et al. 2014) suggesting CD44 as a therapeutic target. Indeed, a panCD44 Ab reduced growth, metastasis and post-radiation recurrence of pancreatic xenograft tumors. The antibody also reduced the number of tumor initiating cells (TICs or CSC, cancer stem cells) in cultured pancreatic cancer cells and xenograft tumors. The elimination of theses TICs is most likely due to the down-regulation of stem cell self-renewal markers as well as inhibition of the survival factor STAT. Interestingly, the RTK Met appears to be also a marker of pancreatic cancer stem cells and its inhibition by specific inhibitors or its down-regulation by shRNA also reduced the population of CSCs (Li et al. 2011).
Targeting of CD44 also eradicated human acute myeloid leukemic stem cells (AML-LSCs) (Jin et al. 2006). Administration of a pan CD44-specific monoclonal antibody into immunosuppressed mice transplanted with AML-LSCs drastically decreased the leukemic population. The trafficking of LSCs to the bone marrow niche and its engraftment in this supportive microenvironment were drastically reduced. An interesting point to note concerning the pan CD44 mAb used in this study is that it is an activating Ab that is thought to induce ligation of CD44, thereby reverting the differentiation of immature AML blasts.
Targeting CD44 with a humanized monoclonal antibody resulted in the complete clearance of engrafted human mammary carcinoma cells or human chronic lymphocytic leukemia (CLL) cells in immune-deficient mice (Weigand et al. 2012; Zhang et al. 2013). In CLL cells, but not in normal B cells, the zeta-associated protein of 70 kDa (ZAP-70), a survival factor that inhibits spontaneous or drug-induced apoptosis, seems to be up-regulated and is found in a complex with CD44. The treatment with already low doses of CD44-specific Ab leads to internalization of the complex resulting in down-modulation of ZAP-70 thereby impairing BCR-dependent survival signaling. This might explain the cytotoxic effect of the CD44 mAb. Preclinical evaluation of the 89Zr-labeled mAb in mice and in cynomolgus monkeys transplanted with CD44 positive human carcinoma cells or CD44 negative tumor cells revealed a selective targeting of the CD44 positive tumors (Vugts et al. 2014).
Other strategies against CD44
Aptamers
Aptamers are small synthetic molecules (either DNA, RNA or peptides) that have high binding affinity and specificity (similar to mAbs) for target proteins thereby inhibiting their functions (Cox and Ellington 2001). DNA aptamers were isolated based on their binding to exon v10 of CD44 by SELEX technology (Iida et al. 2014). In breast cancer cells, CD44v10 proteins appear to form complexes with the surface protein EphA2, which accounts for the migratory ability of the cells (Iida et al. 2014). This complex formation is impaired by the treatment of the breast cancer cells with the v10-specific aptamers, and consequently, the migration of the breast cancer cells is inhibited. This suggests a potential therapeutic use of the aptamers that should now be tested in vivo.
Peptide-based strategies
The understanding of the molecular mechanism of action of CD44v6 in the activation of RTKs such as Met and VEGFR-2 led to the identification of CD44v6 peptides that inhibit both RTKs (Matzke et al. 2005; Tremmel et al. 2009). Mutational analysis of CD44v6 revealed that three amino acids in the exon v6 region are absolutely required for its co-receptor function for Met and VEGFR-2. Peptides with the minimal length of five amino acids containing these critical amino acids interfere with the co-receptor function of CD44v6 and inhibit vascularization of pancreatic tumors (Matzke et al. 2005; Tremmel et al. 2009) (Fig. 2b). These observations are a further hint for the functional relevance of the co-receptor function of CD44v6 in tumor growth and metastasis and are the basis for the development of therapeutic tools for treatment of pancreatic cancers (http://amcure.com/).
Interestingly, a peptide comprising eight amino acids and derived from human urokinase plasminogen activator (A6) acts in an uPA-independent pathway to inhibit migration, invasion and metastasis of cancer cells (Boyd et al. 2003). This peptide turned out to bind specifically to CD44 (Piotrowicz et al. 2011) and is now examined in a trial phase II study for its efficacy in the treatment of human ovarian cancer (Ghamande et al. 2008; Gold et al. 2012). Another CD44 binding peptide, was identified in an indirect way, namely in a screen for overlapping synthetic peptides from the laminin α5 globular domain. One of the peptides that inhibited tumor growth and lung colonization of B16-F10 mouse melanoma cells was shown to target CD44 (Hibino et al. 2004).
The use of peptides was also proposed to fight against CLL (Ugarte-Berzal et al. 2012, 2014). Advanced stages of CLL and poor survival of patients correlate with elevated levels of (pro)MMP9. This (pro)MMP9 is localized at the membrane of CLL cells and forms a complex with the α4β1 integrin and an isoform of CD44 that accounts for cell survival, cell adhesion and transendothelial migration. The hemopexin domain of (pro)MMP9 contains binding sites for CD44 and the α4β1 integrin. Blocking of the binding sites with specific peptides unraveled the independent contribution of CD44 and α4β1 to the pathogenesis of CLL. However, interference with each binding site independently had only partial therapeutic effects (Ugarte-Berzal et al. 2012). Interestingly, one peptide was able to prevent the binding of both, CD44 and α4β1, and is thus a promising candidate for therapeutic approaches (Ugarte-Berzal et al. 2014). A therapeutic approach for CLL using CD44-specific antibodies has been described in chapter II,2,b.
Another peptide of 24 amino acids was obtained from the sequence of the FKBLP protein, a Hsp90 co-chaperone with anti-angiogenic activity. Similar to the protein, the peptide was anti-angiogenic and inhibited tumor cell migration and tumor growth in two human tumor xenograft models (Valentine et al. 2011). Interestingly, this peptide conferred this inhibition only if the cells express CD44 and prevented HA-induced signal transduction by CD44.
As mentioned in a previous chapter, a 42 amino acid peptide containing three BX7B HA-binding motifs found also in CD44 induced apoptosis of melanoma cells and thereby inhibited tumor growth in vivo (Xu et al. 2003).
Inhibition of CD44 expression
Instead of interfering with the function of CD44 proteins (e.g., by antibody treatment), the inhibition of their expression in tumor cells is an alternative. A powerful method for such an approach is the delivery or the expression of siRNA in tumor cells. In tumor cell lines, the treatment with siRNA or the transfection of expression vectors for shRNA is a standard tool to examine the relevance of proteins in the transformation process. The involvement of CD44 isoforms in tumor growth and metastatic spreading has been confirmed by such approaches. For therapeutic use, the problem of tumor-specific delivery and/or expression has to be solved.
One example is the down-regulation of CD44v6 by siRNA in a tumor-specific manner (Misra et al. 2009). It is based on nanoparticles coated with transferrin (Tf), an iron-transporting protein binding to Tf-receptors (Bellocq et al. 2003). Such receptors are highly expressed on tumor cells and mediate the tumor-specific targeting of the nanoparticles. Binding of the nanoparticles to Tf-R induces up-take of the particles into dividing and non-dividing tumor cells via endocytosis. The nanoparticles carried a CD44v6-specific shRNA generator plasmid that is silenced by interrupting sequences. These sequences can be eliminated by expression of the Cre recombinase in a promoter-specific manner. In ApcMin/+ mice that develop spontaneously colorectal carcinoma with high expression of CD44 variant isoforms, the tissue-specific delivery of siRNA was performed using a colon-specific promoter. The expression of CD44v6-specific shRNA led to the reduction of tumors in these mice (Misra et al. 2009). This approach is highly flexible since the use of specific shRNA and tissue-specific promoters for expression of the Cre recombinase allow its application for several tumor types and target genes.
A direct delivery of CD44-specific siRNA was mediated by a dendrimer-based nanoparticle used for treatment of ovarian cancer (Shah et al. 2013). The system is based on a polypropylenimine dendrimer as a carrier for the cell death inducing drug paclitaxel, a synthetic analog of the luteinizing hormone-releasing hormone for tumor targeting and CD44-specific siRNA. The efficiency of these combinatorial particles for treatment of ovarian carcinoma was demonstrated in vitro on cells isolated from patients and in vivo in murine xenograft models.
A very efficient means to control gene expression is the use of microRNAs (miRNAs). These RNAs bind to specific sequences in the 3’UTR region of RNAs and either repress or enhance their translation. These miRNAs have pleiotropic actions since binding sites for these miRNAs are not only found on one RNA species but on several RNAs that are most often involved in the regulation of common cellular programs. Importantly, several miRNAs are aberrantly expressed in tumors and metastasis (Nicoloso et al. 2009; Sotiropoulou et al. 2009; Ventura and Jacks 2009) and expression profiles of miRNAs even predict the clinical outcome of neoplasias (Calin and Croce 2006). Interestingly, the majority of miRNAs affected in tumors are down-regulated and function as bona fide tumor suppressor genes. This holds also true for several miRNAs that affect CD44 expression. Examples are miR-34a (Liu et al. 2011), miR-328 (Chen et al. 2014), miR-143 (Ma et al. 2013) and miR-199a-3p (Henry et al. 2010).
miRNA34a is one of the best characterized tumor suppressor within the miRNAs. Ectopic expression of miRNA34a induces cell cycle arrest, apoptosis and inhibits cancer proliferation, migration and metastasis in a variety of cancer types (Hermeking 2010). This miRNA regulates several target RNAs involved in cell proliferation, survival and migration among which the cyclin-dependent kinases, the RTK Met, Bcl-2, Myc and CD44. The miRNA34a gene was cloned into an expression plasmid driven by a breast cancer-specific promoter to allow expression in breast cancer cell lines (Li et al. 2012). Transfection of several breast cancer cell lines with this expression vector resulted in growth arrest and apoptosis in vitro. Most importantly, in an orthotopic mouse model of human breast cancer, the injection of liposomal complexes of the miRNA34a expression plasmid resulted in reduced tumor size and extended life span of the animals with only minor side effects (Li et al. 2012). Similarly, the systemic application of miR-34a complexed with a lipid-based delivery agent in orthotopic tumor models of prostate cancer in mice resulted in inhibition of tumor growth and metastasis and an extended life span (Liu et al. 2011).
CD44 as a stem cell marker
Many studies within the last years have identified cells in tumors with stem-like characteristics, so-called CSCs or TICs. These cells have self-renewal capacity; the potential to give rise to several cell types within a tumor; and account for tumor initiation, tumor recurrence, tumor metastasis and the resistance of tumors to chemo-or radiotherapy. These CSCs should be the target of efficient tumor therapies. In many tumors including breast cancer HNSCC and colorectal cancer, CD44 have been identified as a marker on CSCs although in most cases the specific isoforms are not known (reviewed in Trapasso and Allegra 2012; Williams et al. 2013; Woodward and Sulman 2008). The role of CD44 in cancer stemcellness is, however, not yet unraveled. Interestingly, in HNSCC and pancreatic cancer cells, Met has also been identified as a marker of CSCs. Met positive HNSCCs have self-renewal capacity, form spherical colonies, are highly chemo-resistant and their transplantation into immunosuppressed mice leads to metastasis (Sun and Wang 2011). Pancreatic cancer cells that express Met and CD44 have the capability of self-renewal and show the highest tumorigenic potential of all cell populations (Li et al. 2011).
CD44v6 isoforms have been identified as markers of CSCs in colon cancer and account for the metastatic propensity of the tumors (Todaro et al. 2014), suggesting that CD44v6 targeting in colon cancer is a promising therapeutic approach. Indeed, CD44 variant isoforms (the one tested was CD44v4–10) but not CD44s in the intestinal stem cells in the crypts of ApcMin/+ mice, that are prone to develop colorectal cancer, account for tumor formation and relapse controlling the balance between cell survival and apoptosis (Zeilstra et al. 2008, 2014). Indeed, a therapeutic approach targeting CD44v6 by means of shRNA in ApcMin/+ mice inhibited the development of colorectal cancer and is described in chapter III). In human gastro intestinal cancer cells, however, a CD44v8–10 variant isoform seems to be characteristic for CSCs rendering the tumor cells resistant to chemo- or radiotherapy by a mechanism that regulates the redox status of the cells (Ishimoto et al. 2011). It is most probable that the various therapeutic approaches that we have discussed in this review target CSCs in the tumors even if this has not been directly proven and are therefore efficient.
Outlook
Since the discovery of CD44 and in particular of CD44v6 isoforms as prognostic markers in a variety of cancers, several approaches have been developed to target them. Although only few approaches have made it so far to clinical trials, the scientific progress in the last years suggests strong perspectives in anti-CD44 therapies. This, on the one hand, is due to the identification of CD44 isoforms (including CD44v6) as functional markers for CSCs in several human tumors and on the other hand to the molecular functions of CD44 isoforms as multidomain platforms integrating extracellular cues with growth factors, cytokines and metalloproteinases. These findings suggest that CD44 is one of the main players in tumor growth and in the most life-threatening steps of cancer, namely metastasis. The detection of CD44 on CSCs was most of the times performed with antibodies that recognized all CD44 isoforms. Since CD44s is expressed ubiquitously in tissues, there is an urgent need to define which CD44-specific isoforms are present on these CSCs. Only then will the specific strategies be directed more selectively against tumor cells.
References
Ahrens T, Sleeman JP, Schempp CM et al (2001) Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene 20(26):3399–3408
Akima K, Ito H, Iwata Y et al (1996) Evaluation of antitumor activities of hyaluronate binding antitumor drugs: synthesis, characterization and antitumor activity. J Drug Target 4(1):1–8. doi:10.3109/10611869609046255
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61(7):1303–1313
Avigdor A, Goichberg P, Shivtiel S et al (2004) CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood 103(8):2981–2989
Bartolazzi A, Peach R, Aruffo A, Stamenkovic I (1994) Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med 180(1):53–66
Bellocq NC, Pun SH, Jensen GS, Davis ME (2003) Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem 14(6):1122–1132. doi:10.1021/bc034125f
Bennett KL, Jackson DG, Simon JC et al (1995) CD44 isoforms containing exon v3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol 128:687–698
Borjesson PK, Postema EJ, Roos JC et al (2003) Phase I therapy study with (186)Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin Cancer Res 9(10 Pt 2):3961S–3972S
Borjesson PK, Jauw YW, Boellaard R et al (2006) Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin Cancer Res 12(7 Pt 1):2133–2140. doi:10.1158/1078-0432.CCR-05-2137
Boyd DD, Kim SJ, Wang H, Jones TR, Gallick GE (2003) A urokinase-derived peptide (A6) increases survival of mice bearing orthotopically grown prostate cancer and reduces lymph node metastasis. Am J Pathol 162(2):619–626. doi:10.1016/S0002-9440(10)63855-2
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866. doi:10.1038/nrc1997
Casucci M, Nicolis di Robilant B, Falcone L et al (2013) CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 122(20):3461–3472. doi:10.1182/blood-2013-04-493361
Chen CH, Cheng CY, Chen YC et al (2014) MicroRNA-328 inhibits renal tubular cell epithelial-to-mesenchymal transition by targeting the CD44 in pressure-induced renal fibrosis. PLoS One 9(6):e99802. doi:10.1371/journal.pone.0099802
Choi KY, Saravanakumar G, Park JH, Park K (2012) Hyaluronic acid-based nanocarriers for intracellular targeting: interfacial interactions with proteins in cancer. Coll Surf B Biointerfaces 99:82–94. doi:10.1016/j.colsurfb.2011.10.029
Colnot DR, Quak JJ, Roos JC et al (2000) Phase I therapy study of 186Re-labeled chimeric monoclonal antibody U36 in patients with squamous cell carcinoma of the head and neck. J Nucl Med 41(12):1999–2010
Colnot DR, Ossenkoppele GJ, Roos JC et al (2002) Reinfusion of unprocessed, granulocyte colony-stimulating factor-stimulated whole blood allows dose escalation of 186Relabeled chimeric monoclonal antibody U36 radioimmunotherapy in a phase I dose escalation study. Clin Cancer Res 8(11):3401–3406
Colnot DR, Roos JC, De Bree R et al (2003) Safety, biodistribution, pharmacokinetics, and immunogenicity of (99 m)Tc-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 52:576–582
Cox JC, Ellington AD (2001) Automated selection of anti-protein aptamers. Bioorg Med Chem 9(10):2525–2531
Culty M, Nguyen HA, Underhill CB (1992) The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol 116(4):1055–1062
Culty M, Shizari M, Thompson EW, Underhill CB (1994) Binding and degradation of hyaluronan by human breast cancer cell lines expressing different forms of CD44: correlation with invasive potential. J Cell Physiol 160(2):275–286
de Bree R, Roos JC, Quak JJ, den Hollander W, Snow GB, van Dongen GA (1995) Radioimmunoscintigraphy and biodistribution of technetium-99 m-labeled monoclonal antibody U36 in patients with head and neck cancer. Clin Cancer Res 1(6):591–598
DeGrendele HC, Estess P, Picker LJ, Siegelman MH (1996) CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte–endothelial cell primary adhesion pathway. J Exp Med 183(3):1119–1130
DeGrendele HC, Estess P, Siegelman MH (1997) Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 278(5338):672–675
Eliaz RE, Szoka FC Jr (2001) Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res 61(6):2592–2601
Eliaz RE, Nir S, Marty C, Szoka FC Jr (2004) Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res 64(2):711–718
Fortin MA, Salnikov AV, Nestor M, Heldin NE, Rubin K, Lundqvist H (2007) Immuno-PET of undifferentiated thyroid carcinoma with radioiodine-labelled antibody cMAb U36: application to antibody tumour uptake studies. Eur J Nucl Med Mol Imaging 34(9):1376–1387. doi:10.1007/s00259-006-0346-5
Fuchs K, Hippe A, Schmaus A, Homey B, Sleeman JP, Orian-Rousseau V (2013) Opposing effects of high- and low-molecular weight hyaluronan on CXCL12-induced CXCR4 signaling depend on CD44. Cell Death Dis 4:e819. doi:10.1038/cddis.2013.364
Gately CL, Muul LM, Greenwood MA et al (1984) In vitro studies on the cell-mediated immune response to human brain tumors. II. Leukocyte-induced coats of glycosaminoglycan increase the resistance of glioma cells to cellular immune attack. J Immunol 133(6):3387–3395
Ghamande SA, Silverman MH, Huh W et al (2008) A phase 2, randomized, double-blind, placebo-controlled trial of clinical activity and safety of subcutaneous A6 in women with asymptomatic CA125 progression after first-line chemotherapy of epithelial ovarian cancer. Gynecol Oncol 111(1):89–94. doi:10.1016/j.ygyno.2008.06.028
Ghatak S, Misra S, Toole BP (2002) Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem 277(41):38013–38020. doi:10.1074/jbc.M202404200
Ghosh SC, Neslihan Alpay S, Klostergaard J (2012) CD44: a validated target for improved delivery of cancer therapeutics. Expert opinion on therapeutic targets 16(7):635–650. doi:10.1517/14728222.2012.687374
Goetinck PF, Stirpe NS, Tsonis PA, Carlone D (1987) The tandemly repeated sequences of cartilage link protein contain the sites for interaction with hyaluronic acid. J Cell Biol 105(5):2403–2408
Gold MA, Brady WE, Lankes HA et al (2012) A phase II study of a urokinase-derived peptide (A6) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 125(3):635–639. doi:10.1016/j.ygyno.2012.03.023
Goldstein LA, Zhou DF, Picker LJ et al (1989) A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell 56(6):1063–1072
Gunthert U, Hofmann M, Rudy W et al (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65(1):13–24. doi:10.1016/0092-8674(91)90403-L
Haylock AK, Spiegelberg D, Nilvebrant J, Sandstrom K, Nestor M (2014) In vivo characterization of the novel CD44v6-targeting Fab fragment AbD15179 for molecular imaging of squamous cell carcinoma: a dual-isotope study. EJNMMI Res 4(1):11. doi:10.1186/2191-219X-4-11
Heider KH, Sproll M, Susani S et al (1996) Characterization of a high-affinity monoclonal antibody specific for CD44v6 as candidate for immunotherapy of squamous cell carcinomas. Cancer Immunol Immunother 43(4):245–253
Henry JC, Park JK, Jiang J et al (2010) miR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell lines. Biochem Biophys Res Commun 403(1):120–125. doi:10.1016/j.bbrc.2010.10.130
Hermeking H (2010) The miR-34 family in cancer and apoptosis. Cell Death Differ 17(2):193–199. doi:10.1038/cdd.2009.56
Herrera MB, Bussolati B, Bruno S et al (2007) Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int 72(4):430–441. doi:10.1038/sj.ki.5002334
Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK (2004) Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res 64(14):4810–4816
Hofmann M, Rudy W, Zoller M et al (1991) CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res 51(19):5292–5297
Iida J, Clancy R, Dorchak J et al (2014) DNA aptamers against exon v10 of CD44 inhibit breast cancer cell migration. PLoS ONE 9(2):e88712. doi:10.1371/journal.pone.0088712
Ishimoto T, Nagano O, Yae T et al (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19(3):387–400. doi:10.1016/j.ccr.2011.01.038
Jackson DG, Bell JI, Dickinson R, Timans J, Shields J, Whittle N (1995) Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol 128(4):673–685
Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC (1987) Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95 kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 105:983–990
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE (2006) Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12(10):1167–1174
Jung T, Castellana D, Klingbeil P et al (2009) CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 11(10):1093–1105
Jung T, Gross W, Zoller M (2011) CD44v6 coordinates tumor matrix-triggered motility and apoptosis resistance. J Biol Chem 286(18):15862–15874. doi:10.1074/jbc.M110.208421
Knudson W, Biswas C, Toole BP (1984) Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A 81(21):6767–6771
Koppe M, Schaijk F, Roos J et al (2004) Safety, pharmacokinetics, immunogenicity, and biodistribution of (186)Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with early-stage breast cancer. Cancer Biother Radiopharm 19(6):720–729. doi:10.1089/cbr.2004.19.720
Laurent TC, Fraser JR (1992) Hyaluronan. Faseb J 6(7):2397–2404
Lesley J, Hyman R (1992) CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur J Immunol 22(10):2719–2723
Li C, Wu JJ, Hynes M et al (2011) c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141(6):2218–2227. doi:10.1053/j.gastro.2011.08.009
Li L, Xie X, Luo J et al (2012) Targeted expression of miR-34a using the T-VISA system suppresses breast cancer cell growth and invasion. Mol Ther 20(12):2326–2334. doi:10.1038/mt.2012.201
Li L, Hao X, Qin J et al (2014) Antibody against CD44 s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology 146(4):1108–1118. doi:10.1053/j.gastro.2013.12.035
Liu C, Kelnar K, Liu B et al (2011) The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 17(2):211–215. doi:10.1038/nm.2284
Luo Y, Prestwich GD (1999) Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug Chem 10(5):755–763. doi:10.1021/bc9900338
Luo Y, Kirker KR, Prestwich GD (2000) Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release 69(1):169–184
Ma Q, Jiang Q, Pu Q et al (2013) MicroRNA-143 inhibits migration and invasion of human non-small-cell lung cancer and its relative mechanism. Int J Biol Sci 9(7):680–692. doi:10.7150/ijbs.6623
Matzke A, Herrlich P, Ponta H, Orian-Rousseau V (2005) A five-amino-acid peptide blocks Met- and Ron-dependent cell migration. Cancer Res 65(14):6105–6110. doi:10.1158/0008-5472.CAN-05-0207
McBride WH, Bard JB (1979) Hyaluronidase-sensitive halos around adherent cells. Their role in blocking lymphocyte-mediated cytolysis. J Exp Med 149(2):507–515
Mielgo A, van Driel M, Bloem A, Landmann L, Gunthert U (2005) A novel antiapoptotic mechanism based on interference of Fas signaling by CD44 variant isoforms. Cell Death Differ
Misra S, Hascall VC, De Giovanni C, Markwald RR, Ghatak S (2009) Delivery of CD44shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ mice. J Biol Chem. doi:10.1074/jbc.M806772200
Misra S, Heldin P, Hascall VC et al (2011) Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J 278(9):1429–1443. doi:10.1111/j.1742-4658.2011.08071.x
Mizrahy S, Goldsmith M, Leviatan-Ben-Arye S et al (2014) Tumor targeting profiling of hyaluronan-coated lipid based-nanoparticles. Nanoscale 6(7):3742–3752. doi:10.1039/c3nr06102g
Naor D, Sionov RV, Ish-Shalom D (1997) CD44: structure, function and association with the malignant process. In: VandeWoude GF, Klein G (eds) Advances in cancer research, vol 71. Elsevier, San-Diego, pp 243–318
Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y (2002) CD44 in cancer. Crit Rev Clin Lab Sci 39(6):527–579
Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA (2009) MicroRNAs—the micro steering wheel of tumour metastases. Nat Rev Cancer 9(4):293–302. doi:10.1038/nrc2619
O’Neill HC (1989) Antibody which defines a subset of bone marrow cells that can migrate to thymus. Immunology 68(1):59–65
Oppenheimer-Marks N, Davis LS, Lipsky PE (1990) Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol 145(1):140–148
Orian-Rousseau V (2010) CD44, a therapeutic target for metastasising tumours. Eur J Cancer 46(7):1271–1277. doi:10.1016/j.ejca.2010.02.024
Orian-Rousseau V, Sleeman J (2014) CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv Cancer Res 123:231–254. doi:10.1016/B978-0-12-800092-2.00009-5
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H (2002) CD44 is required for two consecutive steps in HGF/c-met signaling. Genes Dev 16(23):3074–3086
Orian-Rousseau V, Morrison H, Matzke A et al (2007) Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell 18(1):76–83
Pals ST, Hogervorst F, Keizer GD, Thepen T, Horst E, Figdor CC (1989) Identification of a widely distributed 90-kDa glycoprotein that is homologous to the Hermes-1 human lymphocyte homing receptor. J Immunol 143(3):851–857
Peach RJ, Hollenbaugh D, Stamenkovic I, Aruffo A (1993) Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol 122(1):257–264
Peer D, Margalit R (2004) Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer 108(5):780–789. doi:10.1002/ijc.11615
Peterson RM, Yu Q, Stamenkovic I, Toole BP (2000) Perturbation of hyaluronan interactions by soluble CD44 inhibits growth of murine mammary carcinoma cells in ascites. Am J Pathol 156(6):2159–2167
Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M (2011) A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol Cancer Ther 10(11):2072–2082. doi:10.1158/1535-7163.MCT-11-0351
Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4(1):33–45
Postema EJ, Borjesson PK, Buijs WC et al (2003) Dosimetric analysis of radioimmunotherapy with 186Re-labeled bivatuzumab in patients with head and neck cancer. J Nucl Med 44(10):1690–1699
Pouyani T, Prestwich GD (1994) Functionalized derivatives of hyaluronic acid oligosaccharides: drug carriers and novel biomaterials. Bioconjug Chem 5(4):339–347
Qiu L, Li Z, Qiao M et al (2014) Self-assembled pH-responsive hyaluronic acid-g-poly[(l)-histidine] copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater 10(5):2024–2035. doi:10.1016/j.actbio.2013.12.025
Riechelmann H, Sauter A, Golze W et al (2008) Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol 44(9):823–829. doi:10.1016/j.oraloncology.2007.10.009
Rudy W, Hofmann M, Schwartz-Albiez R et al (1993) The two major CD44 proteins expressed on a metastatic rat tumor cell line are derived from different splice variants: each one individually suffices to confer metastatic behavior. Cancer Res 53(6):1262–1268
Sandstrom K, Nestor M, Ekberg T, Engstrom M, Anniko M, Lundqvist H (2008) Targeting CD44v6 expressed in head and neck squamous cell carcinoma: preclinical characterization of an 111In-labeled monoclonal antibody. Tumour Biol 29(3):137–144. doi:10.1159/000143399
Sandstrom K, Haylock AK, Spiegelberg D, Qvarnstrom F, Wester K, Nestor M (2012) A novel CD44v6 targeting antibody fragment with improved tumor-to-blood ratio. Int J Oncol 40(5):1525–1532. doi:10.3892/ijo.2012.1352
Sauter A, Kloft C, Gronau S et al (2007) Pharmacokinetics, immunogenicity and safety of bivatuzumab mertansine, a novel CD44v6-targeting immunoconjugate, in patients with squamous cell carcinoma of the head and neck. Int J Oncol 30(4):927–935
Schmitt M, Metzger M, Gradl D, Davidson G, Orian-Rousseau V (2014) CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ. doi:10.1038/cdd.2014.156
Schrijvers AH, Quak JJ, Uyterlinde AM et al (1993) MAb U36, a novel monoclonal antibody successful in immunotargeting of squamous cell carcinoma of the head and neck. Cancer Res 53(18):4383–4390
Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, Minko T (2013) Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug. Clin Cancer Res 19(22):6193–6204. doi:10.1158/1078-0432.CCR-13-1536
Song S, Chen F, Qi H et al (2014a) Multifunctional tumor-targeting nanocarriers based on hyaluronic acid-mediated and pH-sensitive properties for efficient delivery of docetaxel. Pharm Res 31(4):1032–1045. doi:10.1007/s11095-013-1225-y
Song S, Qi H, Xu J et al (2014b) Hyaluronan-based nanocarriers with CD44-overexpressed cancer cell targeting. Pharm Res. doi:10.1007/s11095-014-1393-4
Sotiropoulou G, Pampalakis G, Lianidou E, Mourelatos Z (2009) Emerging roles of microRNAs as molecular switches in the integrated circuit of the cancer cell. RNA 15(8):1443–1461. doi:10.1261/rna.1534709
Straathof KC, Pule MA, Yotnda P et al (2005) An inducible caspase 9 safety switch for T-cell therapy. Blood 105(11):4247–4254. doi:10.1182/blood-2004-11-4564
Stroomer JW, Roos JC, Sproll M et al (2000) Safety and biodistribution of 99mTechnetium-labeled anti-CD44v6 monoclonal antibody BIWA 1 in head and neck cancer patients. Clin Cancer Res 6(8):3046–3055
Sun S, Wang Z (2011) Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer 129(10):2337–2348. doi:10.1002/ijc.25927
Tijink BM, Buter J, de Bree R et al (2006) A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res 12(20 Pt 1):6064–6072
Todaro M, Gaggianesi M, Catalano V et al (2014) CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 14(3):342–356. doi:10.1016/j.stem.2014.01.009
Trapasso S, Allegra E (2012) Role of CD44 as a marker of cancer stem cells in head and neck cancer. Biologics 6:379–383. doi:10.2147/BTT.S37906
Tremmel M, Matzke A, Albrecht I et al (2009) A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood 114(25):5236–5244. doi:10.1182/blood-2009-04-219204
Turley EA, Tretiak M (1985) Glycosaminoglycan production by murine melanoma variants in vivo and in vitro. Cancer Res 45(10):5098–5105
Ugarte-Berzal E, Bailon E, Amigo-Jimenez I et al (2012) A 17-residue sequence from the matrix metalloproteinase-9 (MMP-9) hemopexin domain binds alpha4beta1 integrin and inhibits MMP-9-induced functions in chronic lymphocytic leukemia B cells. J Biol Chem 287(33):27601–27613. doi:10.1074/jbc.M112.354670
Ugarte-Berzal E, Bailon E, Amigo-Jimenez I, Albar JP, Garcia-Marco JA, Garcia-Pardo A (2014) A novel CD44-binding peptide from the pro-matrix metalloproteinase-9 hemopexin domain impairs adhesion and migration of chronic lymphocytic leukemia (CLL) cells. J Biol Chem 289(22):15340–15349. doi:10.1074/jbc.M114.559187
Valentine A, O’Rourke M, Yakkundi A et al (2011) FKBPL and peptide derivatives: novel biological agents that inhibit angiogenesis by a CD44-dependent mechanism. Clin Cancer Res 17(5):1044–1056. doi:10.1158/1078-0432.CCR-10-2241
Van Hal NL, Van Dongen GA, Rood-Knippels EM, Van Der Valk P, Snow GB, Brakenhoff RH (1996) Monoclonal antibody U36, a suitable candidate for clinical immunotherapy of squamous-cell carcinoma, recognizes a CD44 isoform. Int J Cancer 68(4):520–527
Van Hal NL, Van Dongen GA, Ten Brink CB, Herron JN, Snow GB, Brakenhoff RH (1997) Sequence variation in the monoclonal-antibody-U36-defined CD44v6 epitope. Cancer Immunol Immunother 45(2):88–92
Ventura A, Jacks T (2009) MicroRNAs and cancer: short RNAs go a long way. Cell 136(4):586–591. doi:10.1016/j.cell.2009.02.005
Verel I, Heider KH, Siegmund M et al (2002) Tumor targeting properties of monoclonal antibodies with different affinity for target antigen CD44V6 in nude mice bearing head-and-neck cancer xenografts. Int J Cancer 99(3):396–402
Vermeulen JF, van Brussel AS, Adams A et al (2013) Near-infrared fluorescence molecular imaging of ductal carcinoma in situ with CD44v6-specific antibodies in mice: a preclinical study. Mol Imaging Biol 15(3):290–298. doi:10.1007/s11307-012-0605-8
Vugts DJ, Heuveling DA, Stigter-van Walsum M et al (2014) Preclinical evaluation of 89Zr-labeled anti-CD44 monoclonal antibody RG7356 in mice and cynomolgus monkeys: prelude to Phase 1 clinical studies. MAbs 6(2):567–575. doi:10.4161/mabs.27415
Weigand S, Herting F, Maisel D et al (2012) Global quantitative phosphoproteome analysis of human tumor xenografts treated with a CD44 antagonist. Cancer Res 72(17):4329–4339. doi:10.1158/0008-5472.CAN-12-0136
Williams K, Motiani K, Giridhar PV, Kasper S (2013) CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med 238(3):324–338. doi:10.1177/1535370213480714
Woodward WA, Sulman EP (2008) Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev 27(3):459–470. doi:10.1007/s10555-008-9130-2
Xu XM, Chen Y, Chen J et al (2003) A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res 63(18):5685–5690
Yadav AK, Mishra P, Jain S, Mishra AK, Agrawal GP (2008) Preparation and characterization of HA–PEG–PCL intelligent core-corona nanoparticles for delivery of doxorubicin. J Drug Target 16(6):464–478. doi:10.1080/10611860802095494
Yang B, Yang BL, Savani RC, Turley EA (1994) Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J 13(2):286–296
Yu Q, Stamenkovic I (1999) Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 13(1):35–48
Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14(2):163–176
Yu Q, Stamenkovic I (2004) Transforming growth factor-beta facilitates breast carcinoma metastasis by promoting tumor cell survival. Clin Exp Metastasis 21(3):235–242
Yu Q, Toole BP, Stamenkovic I (1997) Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med 186(12):1985–1996
Yu WH, Woessner JF Jr, McNeish JD, Stamenkovic I (2002) CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 16(3):307–323
Zeilstra J, Joosten SP, Dokter M, Verwiel E, Spaargaren M, Pals ST (2008) Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res 68(10):3655–3661
Zeilstra J, Joosten SP, van Andel H et al (2014) Stem cell CD44v isoforms promote intestinal cancer formation in apc(min) mice downstream of wnt signaling. Oncogene 33(5):665–670. doi:10.1038/onc.2012.611
Zhang L, Underhill CB, Chen L (1995) Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Cancer Res 55(2):428–433
Zhang S, Wu CC, Fecteau JF et al (2013) Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc Natl Acad 110(15):6127–6132. doi:10.1073/pnas.1221841110
Acknowledgments
Special thanks to David Koschut for the help with the figures. The group of Véronique Orian-Rousseau is supported by the Deutsche Forschungsgemeinschaft and the Mildred Scheel Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orian-Rousseau, V., Ponta, H. Perspectives of CD44 targeting therapies. Arch Toxicol 89, 3–14 (2015). https://doi.org/10.1007/s00204-014-1424-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1424-2