Abstract
Cancer is one of the leading causes of death worldwide. During tumor development and progression, extracellular matrix is being intensively re-organized. One of the most abundant molecules in the extracellular matrix is the polysaccharide hyaluronan. Hyaluronan accumulation and high CD44 expression—the major hyaluronan cellular receptor—correlate with higher malignant states of cancer cells, increased incidence of metastases and poor prognosis of the patients in a wide array of tumor types. Thus, hyaluronan interaction with CD44 emerges as an important target for cancer treatment. In this chapter, recent efforts to exploit hyaluronan/CD44 network for tumor therapy are being discussed. Overall, there is a wide variety of tools available to target this system like anti-CD44 antibodies and peptides, gene therapies against CD44, nanotechnology and modified-hyaluronan. Initial in vivo evidence shows promising results, nominating hyaluronan/CD44 network targeting as a potent candidate to be introduced into clinical settings for tumor therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Cancer is one of the most common-occurring diseases and one of the leading causes of death worldwide. During cancer development, progression, and metastasis, the extracellular matrix is extensively re-organized (Theocharis et al. 2016; Larsen et al. 2006). One of the most abundant molecules in the extracellular matrix is the polysaccharide hyaluronan, which is differentially regulated during tumorigenesis. Physiologically, hyaluronan is found in many tissues such as the skin and the cartilage, where owing to its viscoelastic properties regulates several physiological characteristics of the tissues as well as proper cell function. In cancer, hyaluronan metabolism is altered compared to normal tissues and contributes to acquirement of tumor cell malignant properties (Skandalis et al. 2020; Karousou et al. 2017; Heldin et al. 2019). One of the main hyaluronan receptors, CD44, is considered a tumor promoter for most malignancies and plays significant roles in the regulation of cancer cell survival, proliferation, migration, invasion, metastasis, differentiation and drug resistance (Skandalis et al. 2019; Misra et al. 2011; Morath et al. 2016). Given the important roles of hyaluronan and its receptor CD44 in cancer, targeting this system opens a new avenue for the treatment of malignant diseases. Thereby, here we summarize the latest developments in the field of hyaluronan/CD44 network targeting for tumor therapy.
18.1.1 Hyaluronan Synthesis and Catabolism
Hyaluronan is a glycosaminoglycan consisting of repeating disaccharide units of N-acetyl-glucosamine (GlcNAc) and d-glucuronic acid (GlcUA), bound through alternate β1–3 and β1–4 linkages (Weigel 2015). Hyaluronan is synthesized in the plasma membrane by specific enzymes termed hyaluronan synthases (HASes). Three hyaluronan synthases have been described in the human genome, HAS1–3, which are encoded by distinct genes. HAS1 is found in 19q13.41 (www.genecards.org, GCID: GC19M054887), HAS2 in 8q24.13 (www.genecards.org, GCID: GC08M121594) and HAS3 in 16q22.1 (www.genecards.org, GCID: GC16P069105). Currently there is no information regarding their protein structure, but it is known that they have six transmembrane domains as well as UDP-sugar binding regions (Weigel 2015). Although the three hyaluronan synthases are structurally related they display different spatio-temporal expression and activities. For example, higher expression of HAS1 can be found in adipose tissue and the ovary, HAS2 is highly expressed in the adipose tissue, while HAS3 is expressed in urinary bladder, esophagus, and the lung (proteinatlas.org). Hyaluronan synthases synthesize hyaluronan of different molecular weights. Specifically, HAS2 synthesizes hyaluronan with molecular weight higher than 2 × 106 Da, in contrast to HAS1 and HAS3 which produce hyaluronan ranging from 2 × 105 to 2 × 106 Da (Itano et al. 1999). Furthermore, HAS1 displays the lowest catalytic activity compared to the other HASes, while HAS2 is less active than HAS3. Moreover, HAS1 exhibits higher Km values for both UDP-GlcUA and UDP-GlcNAc compared to HAS2 and HAS3 (Itano and Kimata 2002).
These membrane-embedded glycosyl-transferases utilize as substrates UDP-GlcUA and UDP-GlcNAc which are derived from various metabolic pathways inside the cells (Vigetti et al. 2012; Flores-Diaz et al. 1997). Accordingly, the availability of UDP-sugars is a major factor that regulates hyaluronan biosynthesis (Hascall et al. 2014; Rilla et al. 2013). This in turn suggests that specific microenvironment conditions regulate hyaluronan production, since it has been shown that high glucose upregulates hyaluronan synthesis (Wang et al. 2014). Moreover, the presence of Mg2+ is crucial for hyaluronan production (Weigel 2015). The enzymatic activity of HASes is also regulated by their sub-cellular localization and trafficking from and toward the plasma membrane, where hyaluronan is normally synthesized. HAS1 is mainly localized in Golgi, HAS3 in Golgi and membrane protrusions, while HAS2 is found in the endoplasmic reticulum. The fact that HASes mainly reside inside the cells suggests that there is a reservoir of enzymes ready to translocate in plasma membrane and produce hyaluronan upon stimulation (Torronen et al. 2014). Indicative of this notion is the finding that HAS3 synthesizes a pericellular hyaluronan stroma after its translocation to the plasma membrane (Deen et al. 2014). Furthermore, hyaluronan synthase activity is also regulated by post-translational modifications such as O-GlcNAcylation (Vigetti et al. 2012), poly- and mono-ubiquitinylation (Karousou et al. 2010; Mehic et al. 2017) and phosphorylation (Vigetti et al. 2011), while homo- and hetero-dimerization of HAS2 with any of the other synthases leads to concomitant increase in hyaluronan production (Karousou et al. 2010; Bart et al. 2015).
HAS gene regulation is another crucial factor that controls hyaluronan production by the cells in different tissues. Different transcription factors bind to the promoter of each HAS gene and control their expression. Specifically, HAS1 gene contains binding elements for SP1/3, SMAD, and E2F-myc. HAS2 is regulated by the binding of CREB, NF-κB, RAR, STAT3, YY1, ZEB1, E2F-myc, and SP1, while HAS2-AS1 encoding—a long non-coding RNA that also regulates hyaluronan production by HAS2—is controlled by NF-κB, SP1/3, SMAD and HIF1α. Finally, HAS3 expression is controlled by binding of ΔΝ-p63, NF-κB, C/EBP and SP1 in its promoter regions (Heldin et al. 2019).
Furthermore, hyaluronan synthesis is tightly regulated by several growth factors that by inducing intracellular signaling pathways control the expression of several HAS isoforms. The effect of each growth factor on hyaluronan synthesis is cell- and tissue-type-specific. Such growth factors include PDGF-BB, TGF-β, TNFα and IL-1, among others (Heldin et al. 2019).
Hyaluronan amount in the tissues is also modulated by catabolism from specific hyaluronan-degrading enzymes termed hyaluronidases (HYALs). In human, hyaluronan is recycled with high rates. Almost one-third of the total hyaluronan amount can be found in the skin and its half-life ranges from one to one and a half day (Pandey et al. 2008). Hyaluronan in the tissues has initial size about 1000–10,000 kDa and is degraded in the extracellular space in smaller fragments (10–100 kDa) (Fraser et al. 1997). Next, most of these generated fragments are drained through the lymphatic system and degraded in the lymph nodes. The remaining fragments enter the bloodstream and are finally removed by the liver, kidneys, and spleen (Pandey et al. 2008).
In the human genome, several hyaluronidase genes have been found and are encoded by different genes. HYAL-1 and HYAL-2 are widely expressed in several tissues.
HYAL-2 bears a glycosyl-phosphatidyl-inositol (GPI) tail which anchors the protein to the outside of plasma membrane mainly in lipid rafts, together with hyaluronan receptor CD44. HYAL-3 function despite its wide expression has not been fully deciphered (Shuttleworth et al. 2002; Flannery et al. 1998), HYAL-4 degrades chondroitin sulfate (CS) chains, while PHYAL-1 is a pseudogene and is not expressed in human. PH-20/SPAM1 is a hyaluronidase that is expressed in the testis and displays significant hyaluronan degrading activity (Cherr et al. 2001; Baba et al. 2002). Until recently, the widely recognized model for hyaluronan degradation suggested that hyaluronan residing in the extracellular space is degraded initially by HYAL-2 in 20 kDa fragments which then enter the cell by endocytosis through caveolae pathways in endosomes and transported to lysosomes for further degradation to disaccharides by HYAL-1 and exoglycosidases (Montanari et al. 2018). Recently, two new hyaluronidases that need to fit into the scheme of hyaluronan catabolism were discovered, HYBID/CEMIP/KIAA1199 and TMEM2. The transcription and translation of CEMIP/KIAA1199 gene produces a 153 kDa protein that contains a 30 amino acid N-terminal domain that is required for hyaluronan degradation (Yoshida et al. 2013b). Moreover, it contains seven N-glycosylation sites, one G8 domain, two GG, and four PbH1 regions. The GG regions seem to be implicated in the process of hyaluronan degradation, G8 domain in interaction with other proteins and PbH1 in poly-saccharide hydrolysis (Guo et al. 2006; He et al. 2006; Birkenkamp-Demtroder et al. 2011; Yoshida et al. 2013a). Hyaluronan degradation by HYBID is performed by endocytosis in clathrin-coated vesicles with acidic pH, while the resulting hyaluronan fragments are released in the extracellular space (Yoshida and Okada 2019). The TMEM2 gene product is a transmembrane protein of 154 kDa. The hyaluronidase TMEM2 contains one G8, one GG, and three PbH1 domains in the extracellular region, a transmembrane region, and a cytoplasmic tail (Yamaguchi et al. 2019). In contrast to HYAL-1/-2 which degrade hyaluronan in acidic pH, TMEM2 has optimal pH of enzymatic activity at 6–7. TMEM2 is located in plasma membrane and degrades extracellular hyaluronan in fragments of intermediate size, which then are endocytosed and degraded further in the lysosomes. The enzymatic activity of TMEM2 requires Ca2+ as a co-factor (Yamaguchi et al. 2019; Yamamoto et al. 2017). Finally, hyaluronan can be degraded by non-enzymatic ways by the action of reactive oxygen species (ROS) produced by diverse cellular metabolic pathways (Soltes et al. 2006; Agren et al. 1997).
18.1.2 CD44
Hyaluronan synthesized in the plasma membranes is subsequently extruded to the extracellular space where it can interact with several extracellular proteins, such as the proteoglycan family hyalectans, or plasma membrane receptors, and thus regulate several cell functional properties. The hyaluronan receptors discovered so far include CD44 (Cluster of Differentiation 44), RHAMM (Receptor for Hyaluronan-Mediated Motility), HARE/STAB2 (Hyaluronic Acid Receptor for Endocytosis/Stabilin-2), Laylin, Stabilin-1 and LYVE-1 (Lymphatic Vessel Endothelial Hyaluronic Acid Receptor 1).
The major and best characterized receptor for hyaluronan is CD44, which is expressed in various cell types and tissues. CD44 gene is located in 11p13 (genecards.org) and its transcript mRNA is subjected to alternative splicing which after translation leads to encoding of several different isoforms of the receptor. Human CD44 gene contains 19 exons, 10 standard (S1–10) and 9 variants (V2–10). The S1–10 exons are retained in all CD44 isoforms. CD44s (CD44 standard) isoform does not contain any of the V2–10 exons, while alternative splicing of V2–10 gives rise to CD44v (CD44 variants). From the mRNA translation the final product is a transmembrane protein with an extracellular region containing a LINK domain—which is responsible for hyaluronan/CD44 interactions—and a stalk-like domain—in which the exons V2–V10 are introduced—a transmembrane region, and a small cytoplasmic tail. The extracellular domain is modified by O- and N-glycosylations, while specific CD44 isoforms, such as CD44v3, bear covalently bound glycosaminoglycan chains (chondroitin sulfate or heparan sulfate chains), contributing further diversity to the resulting proteins. The cytoplasmic tail of CD44 despite not containing intrinsic kinase activity regulates several signaling pathways through interactions with cytoplasmic proteins such as Src, ERM (Ezrin, Radixin, Moesin) and IQGAP1 (Zoller 2011; Skandalis et al. 2010). Moreover, CD44 intracellular domain can be cleaved by γ-secretase and translocated to the nucleus where it regulates the expression of several genes (e.g., MMP9) (Miletti-Gonzalez et al. 2012). Apart from cellular signaling, CD44 can also participate in the endocytosis of hyaluronan, leading to its degradation (Thankamony and Knudson 2006; Skandalis et al. 2020).
18.2 Roles of Hyaluronan-CD44 Network in Tumors
Apart from the diverse roles of hyaluronan and its receptor CD44 in physiological processes like embryogenesis and cartilage function they play significant roles in tumor development and progression. In tumors, hyaluronan creates a highly hydrated extracellular matrix with specific physicochemical properties which allows cancer cells to proliferate and migrate. Moreover, hyaluronan synthesized by tumor stromal cells or cancer cells themselves, engages CD44 on the surface of cancer cells to regulate biological processes like growth/survival, epithelial-to-mesenchymal transition (EMT), differentiation, invasion, metastasis, drug resistance and cancer stem cell properties.
18.2.1 Growth/Survival
For tumors to successfully form, cancer cells need to deal with several stressful events, such as anchorage-independent growth, hypoxia and limited nutrient availability. Therefore, it is critical for cancer cells to take advantage of physiological molecular mechanisms and pathways allowing them to cope with such stressful events. One of the major cellular receptors correlated with survival and anti-apoptotic signaling in cancer cells is the hyaluronan receptor CD44. CD44 regulates the expression and activation of proteins involved in resistance to apoptosis and cell growth like Fas, caspase 3/9, Bcl-xl/Bak, Akt, pRb and Bcl-2 (Lakshman et al. 2004; Yasuda et al. 2001; Park et al. 2012b). For example, overexpression of CD44s, CD44v3–10, and CD44v8–10 in human colon cancer cells successfully attenuated etoposide-induced cell death (Lakshman et al. 2004). On the other hand, inhibition of CD44 expression in colon carcinoma cells reduced the expression of Bcl-2, Bcl-xL, while simultaneously increased the expression of apoptosis proteins Bax and caspase-3/8/9 (Park et al. 2012b). Furthermore, CD44 controls downstream activation of Akt, a major survival pathway, and cell cycle-regulating proteins p21 and pRb in many types of cancer cells, like breast, colon and lung cancer cells (Lakshman et al. 2004). In lung cancer cells, interaction of hyaluronan with CD44 reduced Fas expression and subsequent Fas-mediated apoptosis (Yasuda et al. 2001). In chronic lymphocytic leukemia (CLL) patients CD44 was found to promote cancer cell survival. The crucial importance of CD44 in CLL was further certified by the fact that CD44 knock-down reduced survival even in Akt-overexpressing cells. In that model, CD44 regulated the expression of MCL1 anti-apoptotic protein through Akt and Erk pathways (Fedorchenko et al. 2013). Hyaluronan engaged to CD44 also induced phosphorylation of FAK, which associates with PI3K to protect against apoptosis (Fujita et al. 2002). Furthermore, hyaluronan-CD44 interaction activated ErbB2 signaling through Hsp90, cdc37, p110 and p85 proteins (Chanmee et al. 2015).
18.2.2 Epithelial-to-Mesenchymal Transition (EMT) and Differentiation
Epithelial-to-mesenchymal transition is a dynamic process that cancer cells utilize in order to metastasize. During this process, hyaluronan synthase expression is induced and hyaluronan is synthesized to large amounts. Moreover, CD44 has been found to be overexpressed in mesenchymal cancer cells and its high expression correlates with a more undifferentiated phenotype (Misra et al. 2011; Heldin et al. 2014). In breast cancer, EMT correlates with poor prognosis and intriguingly, breast cancer cells with mesenchymal and more malignant phenotype express higher amounts of CD44, which also displays high hyaluronan-binding capacity (Bernert et al. 2011; Heldin et al. 1996). In breast epithelial cultures, TGF-β-induced EMT depends on the expression of HAS2. Specifically, TGF-β induces Smad and p38 MAPK pathways to upregulate HAS2 expression (Porsch et al. 2013). Moreover, HAS2 overexpression has been shown to promote the malignant phenotype via suppression of E-cadherin and translocation of β-catenin to the nucleus, signaling events that take place during EMT (Zoltan-Jones et al. 2003; Koyama et al. 2007). Interestingly, switching between CD44v isoforms to CD44s through alternative splicing promoted EMT by suppression of E-cadherin through PI3K/Akt pathways. Inhibition of splicing activity that produced CD44v occurred through downregulation of epithelial splicing regulatory protein 1 and 2 (ESRP1 and 2) by transcription factors Snail1, Zeb1, and Zeb2 (Reinke et al. 2012). Expression of CD44 and its interaction with hyaluronan also regulate differentiation of aggressive cancer cells like acute myeloid leukemia cells, thus downregulating their aggressive properties (Solis et al. 2012).
18.2.3 Invasion/Metastasis
Accumulation of hyaluronan as well as high CD44 expression in the cancerous tissues has been widely correlated with advanced incidence of invasion and metastasis of various types of cancer cells. CD44 interacts with several growth factor receptors like ErbB2 and PDGFR and its interaction with hyaluronan regulates their signaling activity (Bourguignon et al. 1997; Ghatak et al. 2005; Li et al. 2006). Binding of hyaluronan induces CD44 clustering, which activates downstream signaling pathways in a cell- and tissue-dependent manner. This clustering was critical for MMP9 activation and subsequent activation of TGF-β, which in turn induced cancer cell invasion and metastasis (Yu and Stamenkovic 1999). Moreover, CD44 interacted with MT1-MMP—a major metalloproteinase responsible for extracellular matrix degradation during invasion and metastasis—to enhance its activity. In turn, MT1-MMP enhanced the shedding of CD44 variants and promoted cancer cell invasiveness (Kajita et al. 2001; Stamenkovic and Yu 2009; Mori et al. 2002). Hyaluronan fragments also have a functional role during invasion and metastasis. Specifically, hyaluronan dodecasaccharides by engaging CD44 induced secretion of CXCL1, to enhance endothelial cell sprouting, which could be critical for tumor angiogenesis, a key process in metastasis (Takahashi et al. 2005). Hyaluronan is also synthesized by stromal cells. This stromal cell-derived hyaluronan engaged CD44v6 in the surface of colon tumor cells to sustain PI3K signaling in a positive CD44v6/PI3K loop and promote invasion (Misra et al. 2011).
18.2.4 Drug Resistance
One of the main properties that cancer cells acquire during tumor development is resistance to several drugs. Drug resistance in the tumors is thought to be acquired through different mechanisms. First, a subpopulation of cells with pre-existing potential for drug resistance could be present inside a tumor. Second, drugs utilized for cancer therapy can induce resistance by cancer cells. In both cases, cancer cells upregulate the expression of proteins, like multi-drug resistance proteins (MDRs) that allow them to evade drug-induced apoptosis. Intriguingly, there is extensive evidence correlating CD44 expression with resistance to radiotherapy or chemotherapy of various types of cancer cells (Yaghobi et al. 2021). CD44 physically interacts with P-glycoprotein to enhance drug resistance in cancer cells (Miletti-Gonzalez et al. 2005). In malignant cells, decrease in CD44 expression ameliorated drug resistance (Xu et al. 2015). Specifically, in hepatocellular carcinoma, inhibition of CD44 sensitized cancer cells to sorafenib (Fernando et al. 2015). Paclitaxel-resistant ovarian cells also showed increased expression of CD44 (Gao et al. 2015). It is important to note that interaction of high molecular weight hyaluronan with CD44 increased MDR expression and subsequently led to drug resistance, while low molecular weight hyaluronan-CD44 interaction led to MDR internalization, signifying that not only the presence of hyaluronan but also its size is critical for this process (Zoller 2011). Moreover, hyaluronan through CD44 binding activated PI3K signaling, which in turn induced MDR protein expression (Misra et al. 2005). In the context of CD44v, CD44v3 targeting in head and neck squamous cell carcinoma cells reduced resistance to cisplatin (Wang et al. 2007). Hyaluronan-CD44v3 interaction upregulates miRNA-302, enhanced Oct4-Sox2-NANOG signaling and increased expression of MDR1, leading to development of chemo-resistance (Bourguignon et al. 2012). CD44v3-hyaluronan interaction also regulated expression and activity of P300 which can in turn acetylate β-catenin and NF-κB-p65, resulting in upregulation of MDR1 expression (Zoller 2015). Interestingly, in prostate cancer, knocking down the expression of CD44v6 enhanced chemotherapy sensitivity (Ni et al. 2014).
18.2.5 Tumor Stem Cell Properties
In tumors, sub-populations of cancer cells, termed cancer stem cells (CSCs), have been shown to be responsible for tumor recurrence after chemotherapy or radiotherapy. Hyaluronan, like in normal stem cell niches, creates an ideal micro-environment for CSCs survival, self-renewal, and maintenance. Importantly, hyaluronan receptor CD44 is widely recognized as a stem cell marker (Skandalis et al. 2019). CD44HighCD24Low populations isolated from tumors of the breast showed stem cell properties, like self-renewal and tumor-initiating capacity (Shao et al. 2016; Li et al. 2017b; Wei et al. 2012). Mechanistically, ΔNp63 induced the expression of HAS3, HYAL-1, and CD44 to create a hyaluronan-rich environment that favored stemness of breast cancer cells (Gatti et al. 2018). The importance of hyaluronan and CD44 was further solidified by experiments in hyaluronan-based multilayer nanofilms, where pancreatic cells grown in such conditions upregulated CD44v6 and form colonies (Lee et al. 2018). In pancreatic tumor cells, the tumor suppressor KFL4 bound to CD44 promoter to block its expression and ameliorated cancer stem cell properties and metastasis (Yan et al. 2016). On the other hand, targeting hyaluronan with 4-methyl-umbelliferone—a widely utilized hyaluronan synthesis inhibitor—promoted phagocytosis of hepatocellular CSCs (Rodriguez et al. 2018). CD44 also activated Wnt/β-catenin pathway to upregulate FoxM1 and Twist, leading to enhanced stemness of lung adenocarcinoma cells (Su et al. 2016). Circulating oral squamous carcinoma cells that display CD44 expression were able to form spheres and displayed chemoresistance and self-renewal (Patel et al. 2016). For a more comprehensive analysis of hyaluronan/CD44 roles in cancer stem cell properties, refer to our previous review (Skandalis et al. 2019).
18.3 Hyaluronan/CD44 Network Targeting
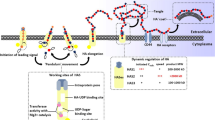
Given the important roles of hyaluronan/CD44 network in tumor development, progression, and metastasis, this system emerges as a potent pharmacological target. Therefore, several efforts have been concentrated toward this purpose, such as incorporation of hyaluronan on nanoparticles for specific delivery of drugs to tumor cells, antibodies or peptides blocking CD44 actions and interactions, chemically modified hyaluronan, and utilizing gene therapies (CRISPR/Cas9 or sh/siRNAs) against CD44 (Fig. 18.1). Below, we describe recent advances in pharmacological targeting of this system for cancer treatment (summarized in Table 18.1).
Hyaluronan/CD44 network targeting approaches. Incorporation of hyaluronan on nanoparticles for specific delivery of drugs to tumor cells, antibodies or peptides blocking CD44 actions and interactions, chemically modified hyaluronan and gene therapies targeting CD44 mRNA (CRISPR/Cas9 or sh/siRNAs against CD44)
18.3.1 Nanomedicine
Nanomedicine therapies utilize targeted drug delivery approaches to specifically target tumor cells and avoid adverse effects in normal tissues. Accordingly, there are two types of targeted drug delivery. First, passive targeting, which takes advantage of enhanced permeability and retention effect (EPR) in tumors. The EPR effect allows for accumulation of drugs and nanoparticles in tumors that have distinct architectural features from normal tissues, such as hyper-vasculogenesis, impaired lymphatic drainage, and different abnormal interstitial pressures (Fang et al. 2011). Second, active targeting, follows passive targeting to selectively deliver specific drugs to cancer cells overexpressing the desired receptor, taking advantage of receptor-substrate interactions (Danhier et al. 2010). Due to the important roles of hyaluronan/CD44 interactions and the correlation between CD44 expression and higher states of malignancy, hyaluronan is widely utilized in nanomedicine to specifically target CD44-expressing cancer cells and cancer stem cells. In blood circulation drug bioavailability and activity is altered by the body’s defense, through chemical modification or binding to serum factors. In the last years, a “3S” transition concept has emerged, which incorporates stability transition, surface transition and size transition to overcome barriers in the delivery process. Hyaluronan therefore is an excellent candidate to be incorporated to nanoparticles and drug carriers, since it fulfills all three criteria due to its biocompatibility, biodegradability, and specific targeting of CD44-expressing cancer cells (Zhong et al. 2020).
One of the main purposes of hyaluronan-coated nanoparticles is to specifically deliver encapsulated drugs in cancer cells to enhance anti-tumor efficacy and avoid adverse effects. Hyaluronan can decorate polyetheleneimine (PEI) biodegradable nanoparticles of poly(lactic-co-glycolic) acid (PLGA) to deliver docetaxel specifically to lung cancer cells and inhibit their proliferation (Maiolino et al. 2015). Tumor growth inhibition of lung cancer cells in vivo was also observed by using PLGA hyaluronan-coated docetaxel nanoparticles without PEI, verifying the anti-cancer efficacy of this strategy (Wu et al. 2017). In another study, docetaxel loaded in chitosan-coated hyaluronan nanoparticles was more effective than free docetaxel against CD44+ breast cancer cells (Shabani Ravari et al. 2016). Hyaluronan nanoparticles composed of branched cell-penetrating peptide B-mR9 could also successfully deliver methotrexate to CD44+ cells and exerted antitumor activity (Yoo et al. 2020). Curcumin and celecoxib loaded in hyaluronan-coated nanoparticles displayed in vitro toxicity and inhibited tumor growth, enhanced survival of mice, induced apoptosis, and abrogated the formation of lung metastasis of breast cancer cells (Liu et al. 2020). Tumor cells display higher levels of glutathione. Taking advantage of this, hyaluronan-coated redox-sensitive micelles were developed to aim CD44-expressing tumor cells and release the drugs inside the target cells (Du et al. 2020b). Furthermore, dextran-modified quercetin-Cu(II)/hyaluronan nanoparticles with a natural PARP inhibitor could induce synthetic lethality in triple-negative breast cancer cells and extended animal survival, without displaying any adverse effects on normal organs (Cheng et al. 2021). Similarly, in triple-negative breast cancer cells conjugation of resveratrol and a chalcone to hyaluronan enhanced their uptake (Shah et al. 2017). Doxorubicin was loaded in hyaluronan-super paramagnetic iron oxide nanoparticles to achieve better uptake and cytoplasmic release of the drug and subsequently enhanced apoptosis in triple-negative breast cancer cells (Vyas et al. 2015). In addition, hyaluronan could facilitate lipoplexes delivery in breast cancer cells that express CD44 (Surace et al. 2009). Recently, targeting mitochondrial pathways in cancer cells has emerged as a promising strategy for tumor therapy. Indeed, hyaluronan nanomedicine utilizing a berberine derivative and doxorubicin was able to target mitochondria and subsequently inhibited proliferation, migration, and enhanced apoptosis of triple-negative breast cancer cells. Importantly, lung metastasis in vivo was abrogated after this treatment. These effects were mediated by suppression of MMP-2/-9 activities and induction of mitochondrial apoptotic pathways (Lin et al. 2021). Cisplatin can be also loaded to hyaluronan-green tea catechin micellar nanocomplexes, to enhance its efficacy against ovarian cancer cells, without causing side effects in vivo (Bae et al. 2017). In glioblastoma cells, hyaluronan-conjugated liposome nanoparticles effectively delivered doxorubicin inside the cells and enhanced its anti-neoplastic function (Hayward et al. 2016). Paclitaxel is another drug that can be also delivered with hyaluronan-nanoparticle complexes to treat CD44-positive colon cancer cells in orthotopic mouse models (Zhu et al. 2018). Paclitaxel and curcumin encapsulated in poly (acrylamide-co-acrylonitrile-co-vinylimidazole-co-bis(2-methacryloyl) oxyethyl disulfide) (PAAVB) polymer-based intelligent platform coated with hyaluronan, were able to promote adaptive anti-tumor immunogenicity and inhibit immunosuppression of CD44-overexpressing breast tumor cells while simultaneously abrogating lung metastasis (Wang et al. 2021). Hyaluronan can also decorate serum albumin conjugate-based nanoparticles to enhance drug delivery and their cytotoxic effects on cancer cells expressing CD44 (Edelman et al. 2017). In addition, promising results have been obtained by using hyaluronan-modified nanoparticles for treatment of hematological tumors, where CD44 plays significant roles. Specifically, doxorubicin encapsulated in lipoic acid-crosslinked hyaluronic acid nanoparticles was used to inhibit tumor growth of multiple myeloma and acute myeloid leukemic cells in vivo (Zhong et al. 2017).
The size of hyaluronan that may be used to coat nanoparticles is apparently important. Specifically, hyaluronan nanoparticles coated with low molecular weight hyaluronan displayed low CD44-binding activity, while high binding affinity was displayed by high molecular weight hyaluronan-coated nanoparticles, suggesting that high molecular weight hyaluronan is more suitable as a coating substance (Mizrahy et al. 2011). Furthermore, high molecular weight hyaluronan-coated nanoparticles displayed enhanced circulation time and better tumor targeting specificity than low molecular weight hyaluronan-coated nanoparticles (Mizrahy et al. 2014).
As already mentioned, CD44 serves as a major cancer stem cell marker. Therefore, hyaluronan can be used to guide nanoparticle-drug conjugates to cancer stem cells. To target prostate cancer stem cells, hyaluronan was used to decorate cabazitaxel and silibinin co-encapsulated cationic liposomes to induce apoptosis and decrease cell migration (Mahira et al. 2019). Cis-dichlorodiamminoplatium (II) (CDDP) glyconanoparticles were also coated with hyaluronan to deliver the drug in vivo and suppress stem cell properties of prostate cancer cells (Jafari Malek et al. 2014). For breast cancer treatment, co-delivery of salinomycin and curcumin in hyaluronan-coated nanoparticles was achieved in CD44-expressing breast cancer stem cells, inducing cell cycle arrest in G1 and inhibiting EMT (Zhao et al. 2020). To target pancreatic cancer stem cells, hyaluronan was utilized to coat diethyldithiocarbamate-copper complex nanoparticles. These nanoparticles were able to impair sphere formation of pancreatic cancer stem cells, probably by inducing the generation of ROS (Marengo et al. 2019). Nanoparticles can be delivered also orally. Specifically, hyaluronan-decorated nanoparticles containing PTC209, a BMI-1 inhibitor, were able to target colon cancer stem cells and abrogate tumor growth in vivo (Xu et al. 2019).
Hyaluronan-coated nanoparticles can encapsulate phosphosensitizers, like Ce6, to deliver them in human colon cancer cells in vivo. After photodynamic therapy, tumor growth was attenuated, while no side effects were observed (Gao et al. 2017). Furthermore, co-administration of hyaluronidase with such nanoparticles induced their uptake from tumor cells and enhanced their anti-cancer efficacy, probably by increasing the enhanced permeability and retention (EPR) effect inside the tumor (Gong et al. 2016). For triple-negative breast cancer treatment, photothermally targeted hyaluronan-polyaniline (PANi)-imiquimod (R837, a TLR7 agonist) nanoparticles were used to induce immune responses against the tumor (Yasothamani et al. 2021). Interestingly, hyaluronan-decorated nanoparticles could be effectively used to encapsulate and deliver hyaluronidase enzymes specifically to the tumors together with doxorubicin to enhance its efficacy (Chen et al. 2018a). Gold-nanoclustered hyaluronan nano-assemblies were also used as a platform for photodynamic or photothermal cancer therapies, by delivering the photodynamic therapy agent verteporfin in cancer cells. These nanoparticles displayed excellent stability in the blood and were able to completely inhibit tumorigenesis while displaying 100% survival rate (Han et al. 2016). Hyaluronan-conjugated zinc protoporphyrin nanoprobes were also used for photodynamic therapy against colon cancer and fibrosarcoma cells (Gao et al. 2021).
Among others, hyaluronan-coated nanoparticles can carry siRNAs or shRNAs against specific proteins expressed in cancer cells. KRAS is a known oncogene with no available small molecule inhibitors. To target KRAS hyaluronan-decorated nanocarriers [poly(hexamethylene biguanide) and chitosan] bearing KRAS-si/shRNA have been designed to specifically silence KRAS expression in CD44-expressing cancer cells (Tirella et al. 2019). Moreover, chitosan nanoparticles were conjugated with hyaluronan to target bladder cancer cells that expressed high levels of CD44, to deliver siRNA against the Bcl-2 oncogene. These particles were able to target bladder cancer cells in vivo and successfully interfere with the Bcl-2 expression (Liang et al. 2021). A new anti-cancer siRNA delivery system named by the authors HPLR was manufactured with a siRNA-peptide core surrounded by lipid bilayer, thin hyaluronan coating, and EGFR-targeted peptides to target EGFR and CD44 overexpressing cells. This system was able to successfully deliver the siRNAs in subcutaneous liver tumors and inhibit their growth without displaying any significant toxicity (Liang et al. 2019). Additionally, hyaluronan-conjugated nanoparticles were utilized to deliver Gli1 siRNA in gastric cancer stem cells and subsequently reduced migration, invasion as well as tumor spheroid and colony formation (Yao et al. 2020).
Notably, nanoparticles can also be decorated with CD44-targeting peptides. Indeed, A6 anti-CD44 peptide conjugated to polymersomal epirubicin enhanced uptake and anti-cancer efficacy of epirubicin against multiple myeloma cells in vivo (Gu et al. 2019). Furthermore, nanoparticles can be decorated with anti-CD44 antibodies. Multifunctionalized iron oxide magnetic nanoparticles with CD44 antibodies were able to selectively target CD44-positive cancer cells (Aires et al. 2016). In breast cancer cells, conjugation of saporin, a ribosome-inactivating protein, with hyaluronan modified nanoparticles achieved intracellular release of saporin leading to enhanced apoptosis (Ding et al. 2018). Hyaluronan-coated nanoparticles have been also conjugated with AS1411 aptamer, to penetrate blood-brain barrier and deliver docetaxel to CD44- overexpressing glioma cells. Delivery of docetaxel through this pathway significantly attenuated the formation of spheroids and tumor growth in vivo (Wang et al. 2019).
18.3.2 Antibodies
Different monoclonal antibodies have been raised against CD44 standard and variant isoforms to be utilized for cancer treatment. One of the best-studied anti-CD44 monoclonal antibodies is RG7356 which binds to the constant region of CD44, abrogating hyaluronan binding. In chronic lymphocytic leukemia (CLL), interruption of hyaluronan/CD44 interaction induced caspase-dependent apoptosis, with the strongest effects being observed in ZAP-70+ CLL cells both in vitro and in vivo (D’arena et al. 2014). Mechanistically, RG7356 engages CD44 to induce its internalization in CLL cells, and subsequently decrease ZAP-70, which was found to be complexed with CD44 (Zhang et al. 2013). RG7356 is also effective against triple-negative breast cancer cells in mice xenografts by modifying the MAPK pathway (Weigand et al. 2012). Response to treatment with RG7356 in xenograft models and colorectal cancer patients depended on the presence of CD44s isoform, suggesting that only treatment in patients with CD44s expression could be effective (Birzele et al. 2015). RG7356 mechanism of function also involves activation of the immune system. Specifically, RG7356 treatment induced secretion of chemo-attractants responsible for recruitment of immune cells, such as macrophages, to the tumor, through activation of MAPK. Moreover, RG7356 activated antibody-dependent cellular phagocytosis (ADCP) of triple-negative breast cancer cells by macrophages (Maisel et al. 2016). In clinical trials, 89Zirconium-labeled RG7356 was taken up by several tissues such as the spleen, liver, bone marrow, lung, and kidney in a dose-dependent manner in patients (Jauw et al. 2018), while selectively targeting CD44+ breast and pancreatic cancer cells in monkeys (Vugts et al. 2014). Moreover, phase 1 clinical trials with RG7356 in patients with advanced, CD44-expressing solid tumors showed that it was well-tolerated, with most side effects being mild such as fever, headache, and fatigue. Its clinical efficacy was modest with 21% of patients experiencing disease stabilization (Menke-Van Der Houven Van Oordt et al. 2016). Furthermore, phase 1 studies in acute myeloid leukemia patients verified the mild adverse effects, although only 2 out of 44 patients showed response in the treatment suggesting that this antibody cannot be utilized as monotherapy (Vey et al. 2016).
Another popular anti-CD44 antibody is A3D8. On human acute myeloid leukemia (AML) cells, A3D8 treatment caused G0/G1 cell cycle arrest through induction of p21, p27 and reduction of pRb and CdK2/4 activities. Furthermore, JNK protein expression was reduced leading to reduction in c-Jun phosphorylation after A3D8 treatment (Zada et al. 2003; Gadhoum et al. 2004a, b; Li et al. 2016). A3D8, apart from inducing apoptosis and inhibiting cell proliferation, induced differentiation of the AML cells (Gadhoum et al. 2004a). This apoptotic effect was caused by A3D8-induced CD44s lipid raft clustering leading to Fas aggregation and subsequent caspase-8 activation (Qian et al. 2012). It is important to note that bone marrow stromal cells could protect acute myeloid leukemia cells from A3D8-induced apoptosis through activation of PI3K/Akt signaling to down-regulate p27 (Chen et al. 2015). In these studies, the effects of A3D8 were verified by another anti-CD44 antibody, H90. In chronic lymphocytic leukemia, A3D8 abrogated CLL cell viability and in vivo caused reduction of MCL1 protein and activation of caspases (Fedorchenko et al. 2013). In human erythroleukemia cells, A3D8 also evoked cell growth inhibition and caspase-independent apoptosis-like cell death, through disruption of mitochondrial membrane potential and release of AIF but not cytochrome c, which is typical for caspase-dependent apoptosis. This type of cell death involves activation of PARP and calpain, since their inhibition ameliorated A3D8-induced cell death (Artus et al. 2006). CD44 ligation by A3D8 was also able to arrest ovarian cancer stem cells in S phase and induced apoptosis (Du et al. 2013).
The monoclonal antibody F77 was developed to recognize glycolipids and O-glycosylation on prostate cancer cell proteins. F77 antigen was finally identified to be glycosylated CD44v10 isoform. On functional level, F77 induced apoptosis in prostate cancer cells. Moreover, this antibody can be utilized in ELISA assays to identify this glycosylated CD44v10 isoform in prostate cancer cells and serum of patients with prostate cancer (Chen et al. 2018b).
Hermes-1 is an anti-CD44-specific monoclonal antibody that specifically interrupts hyaluronan/CD44 interactions. When compared with other anti-CD44 antibodies, like Hermes-3, J173, and 50B4, Hermes-1 was the only one capable to completely inhibit binding of hyaluronan by colorectal carcinoma cells. It is important to note that Hermes-1, Hermes-3, and J173 inhibited the adhesion of the cells on laminin and collagen, while 50B4 did not have any effect (Ishii et al. 1993). Hermes-1 can be also utilized to investigate differences between metastatic cells and cells from the original tumor. Unexpectedly, utilizing Hermes-1, it was found that primary human colon carcinoma cells expressed higher amounts of CD44 on their cell surface, than the cells in lymph node metastases (Kubens and Zanker 1998).
Inhibition of hyaluronan/CD44 interactions has been also achieved with IM7 monoclonal antibody. Despite not displaying any effect on colon carcinoma cell growth in vitro, IM7 was able to inhibit liver metastasis in vivo, although tumor cell colonies were detected in all of the livers even in mice free of nodules, suggesting that IM7 probably delayed formation of metastases, rather than completely inhibiting them (Ogoshi et al. 1998). In glioma cells, which synthesize high levels of hyaluronan, IM7 decreased hyaluronan biosynthesis, and induced apoptosis (Wiranowska et al. 2010). IM7 can be also delivered with chitosan polylactic acid-coated nanoparticles to reduce its toxicity. This modified IM7 antibody was able to reduce proliferation of ovarian cancer cells and control the development and progression of ovarian cancer in vivo (Yang et al. 2017). It is known that hyaluronan fragments that engage CD44 can activate downstream NF-κB signaling in various tumor types, which in turn promotes inflammation and tumorigenesis. IM7 by inhibiting these interactions successfully reduced NF-κB activation in bladder cancer cells (Fitzgerald et al. 2000). IM7 was also able to block adhesion of melanoma cells on endothelial cells, a critical step during metastasis formation (Ota et al. 1995). IM7 can be also conjugated to ribosome inactivating protein saporin, to specifically target CD44-expressing cancer cells. These modified antibodies can release saporin in the target cell cytoplasm, which can then display its cytotoxic effects only on prostate cancer cells with high CD44 expression (Bostad et al. 2014). IM7 also enhanced natural killer cell activity (Tan et al. 1993), suggesting that CD44 targeting with antibodies can enhance activation of immune system against cancer cells. In the same study, different anti-CD44 antibodies were also studied, Hermes-1, S3 and S5, but the same effect was only observed with S5 antibody. The effect of CD44 ligation was also investigated with J173 and F10442 antibodies, where CD44 cross-linking upregulated CD16-mediated lysis. Moreover, CD44 blocking with these antibodies led to rapid increase of intracellular Ca2+ (Galandrini et al. 1994). Accordingly, J173 activated MAPK signaling to enhance the killing activity of peripheral mononuclear cells against cancer cells from Burkitt’s lymphoma and chronic myelogenous leukemia (Ishizuka et al. 2008).
Several other less studied anti-CD44 monoclonal antibodies have been developed. The HI44α antibody effects were investigated in acute myeloid leukemia cells derived from patients. Ligation of CD44 by HI44α was able to induce differentiation and apoptosis of AML cells, probably through inhibition of c-Myc expression (Song et al. 2004). In colon cancer cells, engagement of CD44 by a specific antibody reversed the resistance to anti-integrin antibody, altered cell morphology, and enhanced apoptosis (Bates et al. 1998). U36 is another antibody that has been raised to recognize CD44v6- expressing squamous cell-carcinomas (Van Hal et al. 1996), but whether it can be used for treatment of these tumors remains to be investigated. KMP1 is a CD44-specific antibody that shows significant anti-tumor effects against bladder cancer cells. Specifically, KMP1 inhibited the bladder cancer cell proliferation, migration, and adhesion in vivo, while suppressed tumor growth in xenograft models. Importantly, expression of the KMP1 epitope correlated with clinical severity and prognosis of bladder cancer (Chen et al. 2018c). Anti-CD44 antibody conjugated to oil liquid nanocapsules, aCD44O2LNCs, to target pancreatic cancer stem cells, has also been developed. This technology displayed high uptake of these nanoparticles by pancreatic cancer stem cells in vivo. Moreover, these nanoparticles when coupled with paclitaxel were able to enhance their anti-tumor efficacy (Navarro-Marchal et al. 2021). A defucosylated anti-CD44 antibody, 5-mG2a-f, also significantly reduced tumor development in oral squamous cell carcinoma xenograft models (Takei et al. 2020). In another study, the authors developed four anti-CD44 monoclonal antibodies, namely, P4G9, P3D2, P3A7 and P3G4 that recognized unglycosylated and conserved regions of CD44 ectodomain. P3D2 was able to inhibit breast cancer tumorigenesis in animal models (Lusche et al. 2021). Since CD44v7 is expressed in many human cancers, an antibody raised against CD44v7 is to be utilized as a diagnostic and therapeutic tool in clinical settings (Borgya et al. 1995). Whether this antibody is useful remains to be investigated. An antibody raised against CD44v9, mAb 44-IV was able to inhibit liver metastasis of human colon cancer cells by blocking their adhesion to the capillaries, a critical step in the metastatic cascade (Seki et al. 1997).
18.3.3 Peptides
Apart from antibodies, peptides have been also used to target CD44 and regulate hyaluronan binding capacity. Peptides can be used for detection of CD44- expressing tumor cells inside the tissues. Several peptides against CD44 have been described utilizing phage display libraries (Park et al. 2012a). For example, RP-1, a 12-mer peptide isolated from a phage peptide library, binds to CD44+ gastric cancer cells and allows their detection inside the tissues (Zhang et al. 2015). Importantly, RP1 peptide binding can predict prognosis of gastric cancer patients (Li et al. 2017a). Another 7-mer peptide also isolated from phage display library, termed CV-1, was able to detect CD44v3-v10 protein expression in gastric cancer cells and tissues (Zhang et al. 2016). Similarly, a phage displays 15 amino acid peptide PFT marked CD44v6-expressing prostate cancer stem cells (Peng et al. 2017). Polyvalent-directed peptide polymer (PDPP) specifically traced CD44-expressing breast cancer stem cells (Cho et al. 2015).
A widely studied peptide against CD44 is A6. A6 peptide is derived from uPA, but it does not bind to uPA receptor (uPAR) nor interferes with uPA/uPAR interactions. Importantly A6 did not cause any significant toxicity in animals. Moreover, A6 showed efficacy and exceptionally good safety profile in Phase 1a, 1b and 2 clinical trials. In chronic lymphocytic leukemia, A6 displayed significant toxicity against B-lymphocytes expressing ZAP-70 (Finlayson 2015). A6 peptides loaded with reduction-sensitive polymersomal vincristine sulfate targeted CD44-expressing acute myeloid leukemia cells and reduced the leukemia burden in the circulation, bone marrow, liver, and spleen, while extending survival of mice (Gu et al. 2021). A6 also inhibited migration of ovarian and breast cancer cells. Mechanistically, A6 regulated CD44-mediated adhesion to hyaluronan and the activation of downstream FAK and MAP/ERK signaling pathways. In vivo treating mice with A6 inhibited the metastasis of melanoma cells to the lung (Piotrowicz et al. 2011).
Peptides that bind CD44v have been also developed. A peptide mimicking a specific extracellular motif of CD44v6 reduced CD44v6-mediated activation of c-Met and VEGFR-2. Treatment with this peptide in vivo reduced angiogenesis in tumors, suggesting that targeting the co-receptor functions of CD44 is another viable possibility (Tremmel et al. 2009). Following the same notion, v6 peptide that interfered with CD44v6 co-receptor functions with these receptors, blocked tumor growth and metastasis in pancreatic tumors in mice, while extended their survival time. Of note, the v6 peptide achieved higher inhibition than c-Met or VEGFR-2 inhibitors (Matzke-Ogi et al. 2016). NLN and NEW are peptides that bind CD44v6, inducing its internalization to inhibit c-Met/Erk pathways, leading to reduction of cell migration, invasion, and metastasis. In vivo these peptides after conjugation with KLA pro-apoptotic peptides successfully killed tumor cells and impaired tumor growth and metastasis without displaying systemic side effects (Khan et al. 2021).
The FK506-binding protein-like (FKBPL) and a 24-amino acid-derived peptide AD-01 exerted anti-angiogenic effects and reduced tumor growth in vivo, effects that are dependent on the presence of CD44 (Valentine et al. 2011). Moreover, FKBPL and AD-01 bound CD44 and reduced breast cancer cell migration, through inhibition of Rac-1 activity, upregulation of RhoA and the actin-interacting proteins profilin and vinculin (Yakkundi et al. 2013). Moreover, AD-01 treatment inhibited mammosphere-forming capacity of breast cancer stem cells in vitro and reduced tumor initiation in vivo. Specifically, AD-01 induced differentiation of breast cancer stem cells, while reducing the expression of stem cell markers Nanog, Oct4, and Sox2. Importantly when combined with DAPT—a Notch inhibitor—it caused significant reduction of chemotherapy and radiotherapy resistance in breast cancer stem cells (McClements et al. 2013).
CD44, apart from binding hyaluronan, can also interact with other proteins like collagen. Consequently, there are also peptides taking advantage of collagen/CD44 interactions. Peptides derived from collagen type IV incorporated to liposomes bearing doxorubicin targeted CD44+ melanoma cells and reduced tumor size (Ndinguri et al. 2012). Moreover, taking advantage of pro-MMP9 and CD44 interactions, P3 and P6 peptide were prepared from PEX9 domain of pro-MMP9, and were shown to reduce chronic lymphocytic leukemia cell adhesion to pro-MMP9 and thus abrogated chemotaxis and transendothelial migration (Ugarte-Berzal et al. 2014). The peptide PCK3145 induced CD44 shedding by increasing MT1-MMP while decreasing MMP9 secretion. PCK3145 also abrogated adhesion of fibrosarcoma cells on hyaluronan, thus antagonizing tumor metastatic processes (Annabi et al. 2005). A5G27 peptides derived from laminin could also bind CD44v3 and CD44v6, and could be conjugated to particles bearing specific siRNAs. Introduction of such particles in vivo inhibited tumor growth by lung adenocarcinoma or ovarian carcinoma cells (Golan et al. 2016). A5G27 peptide blocked melanoma metastasis by inhibiting the binding of FGF2 in the heparan sulfate chains of CD44v3, and thus reducing bioactivity of FGF2 (Hibino et al. 2005). C21, a C-terminal peptide of thrombospondin-4 competed for osteopontin and hyaluronan binding to CD44, but its effect on tumor cells remains to be investigated (Sadvakassova et al. 2009). Another CD44-binding peptide (CD44BP) added to an engineered matrix showed significant anti-tumorigenic effect, as evidenced by reductions in tumor sphere formation in vitro (Yang et al. 2013).
Another interesting approach was synthesizing CD44 cytoplasmic tail peptides bearing phosphor-Ser325 which were conjugated to penetration sequences in order to enhance plasma membrane translocation. Such peptides blocked CD44-mediated cell migration without affecting hyaluronan binding or CD44 expression (Peck and Isacke 1998). CD44 could be used also as an antigen to be recognized by immune cells for cancer treatment. Specifically, CD44-derived peptides could function as immunogens to sensitize dendritic cells and enhance their anti-tumor activities against prostate cancer stem cells that expressed high CD44 levels in vitro and in vivo (Wang et al. 2020).
18.3.4 Chemically Modified Hyaluronan
Hyaluronan can be modified in several different chemical groups to inhibit binding to CD44, affect tissue architecture and to be utilized in hydrogels and as a scaffold for drug carriers. Sulfhydryl (-SH) modified hyaluronan is utilized to form hydrogels to be used as drug-loaded implant for chemotherapeutics, photosensitizer and photothermal reagent in chemotherapy, photodynamic and photothermal therapy against tumors (Xu et al. 2021). Hyaluronan modification with Au-Ag alloy can be used in nanoparticles that allow for the sensitization of breast cancer cells in radiotherapy, since ionizing radiation releases toxic Ag+ and enhances production of OH− in tumor sites (Chong et al. 2020). High molecular weight hyaluronan can be also modified with a hydrazide group and bisphosphonate (BP) for selective targeting of CD44 expressing cancer cells (Varghese et al. 2009). Notably, hyaluronan—a non-sulfated glycosaminoglycan—has been modified with sulfate groups which allowed simultaneous targeting of CD44 and P-selectin, to effectively target CD44+P-selectin+ cancer cells (Bhattacharya et al. 2020). Hyaluronan can be also modified with poly(lactic-co-glycolic acid) to produce highly stable and GSH sensitive micelles. Loading of these complexes with transferrin-targeted nanoformulated AUY922 eased their uptake by brain tumor cells and induced caspase-dependent cleavage of the apoptosis marker PARP followed by upregulation of p53. Moreover, this complex was drastic against tumor growth in vivo, without displaying any toxicity to other major organs (Debele et al. 2021). β-Cyclodextrin-modified hyaluronan with drug conjugates was able to exert significant toxicity against lung and prostate cancer cells with high expression of CD44, while on the other hand showed no toxicity against normal cells with low CD44 expression (Bai et al. 2020).
18.3.5 Gene Therapies
A promising strategy to target hyaluronan/CD44 network in tumors is to silence the expression of CD44 gene, with specific siRNA and/or shRNA or the most recently developed CRISPR/Cas9 system.
Introduction of a CD44-specific siRNA in lung cancer cells reduced the expression of the stem cell-related genes CXCR4 and POU5F genes after TGF-β1/TNFα treatments but failed to reverse EMT gene signature (Nurwidya et al. 2017). A recombinant adenovirus bearing CD44 shRNA was also able to inhibit cell proliferation, migration and invasion, while induced apoptosis of colon cancer cells. These adenoviral particles inhibited Akt and GSK-3β signaling pathways. Furthermore, the expression levels of anti-apoptotic proteins Bcl-2 and Bcl-xL were reduced, while the apoptotic proteins Bax, cleaved caspase-3/−9 and PARP were increased in colon cancer cells treated with the adenovirus (Lee et al. 2017). Targeted deletion of CD44v6 with specific shRNA in colon cancer cells also reduced the adenoma growth in vivo through interruption of hyaluronan/CD44v6/pErbB2/Cox-2 pathway (Misra et al. 2009). In triple-negative breast cancer cells, knock-down of CD44 with shRNA vectors significantly suppressed proliferation, colony formation, and invasion (Zhou et al. 2018). Doxorubicin resistance is a major caveat in the treatment of breast cancer. Targeting of breast cancer cells with CD44 siRNA in combination with doxorubicin treatment effectively limited tumor metastasis, proliferation, invasion, migration, and induced apoptosis in triple-negative breast cancer cells, signifying the importance of targeting CD44 to overcome drug resistance (Vahidian et al. 2020). Targeting of CD44-expressing breast cancer cells can be also performed with CD44-targeted aptamer Apt1. This aptamer conjugated to liposomes with encapsulated CD44 siRNA was able to successfully target CD44-expressing breast cancer cells to silence CD44 in vitro and in vivo (Alshaer et al. 2018). In another study, the authors utilized biodegradable poly d,l-lacticied-co-glycolide acid nanoparticles (PLGANPs) to deliver FAK and CD44 shRNAs in ovarian cancer cells in vivo, since both FAK and CD44 have active roles in tumor angiogenesis and cancer metastatic processes. Double knock-down of both FAK and CD44 reduced tumor size, inhibited angiogenesis, reduced proliferation and induced apoptosis (Zou et al. 2013).
CRISP/Cas9 technology in contrast to siRNA or shRNA systems offers silencing capacity of a specific gene by completely removing the target gene from the host genome. In osteosarcomas, high expression of CD44 predicts poor survival and higher incidence of metastases in patients. Targeting CD44 with CRISPR/Cas9 system in metastatic osteosarcoma cells abrogated the proliferation and spheroid formation in 3D cultures as well as migration and invasion (Liu et al. 2018). Furthermore, in multi-drug-resistant osteosarcoma cells, silencing of CD44 with CRISPR/Cas9 system could also enhance drug sensitivity (Xiao et al. 2018). Although CD44 seems to play an important role in tumor growth, CD44 silencing in vivo with CRISP/Cas9 in HAS3-overexpressing stromal fibroblasts or CD44 knock-down with shRNA in breast cancer cells was not able to inhibit tumor growth, in contrast with the reductions that were observed when hyaluronan production or accumulation was inhibited (Zhao et al. 2019). This rather contradicting study points out the context-dependent effects of hyaluronan and CD44 in several tumors. Given the fact that in most breast tumors HAS2 has been most extensively studied, while the role of HAS3 remains obscure, further studies are needed to clarify this discrepancy. CD44 can be also indirectly targeted, as evidenced by CRISPR/Cas9 silencing of Cosmc, an endoplasmic reticulum-localized chaperone that regulates protein O-glycosylation. Cosmc knock-out inhibited protein expression of CD44, confirming the notion that O-glycosylation is important for proper CD44 expression. Of note, reconstitution of CD44 reversed the effects of Cosmc disruption on MAPK signaling and breast cancer cell proliferation, verifying the important role of CD44 for breast tumor growth (Du et al. 2020a). In liver cancer stem cells, knock-out of CD44 utilizing CRISPR/Cas9 system resulted in less malignant and more differentiated tumors. Unexpectedly, CD44 silencing increased the expression of stem cell markers Oct4, Sox2, and Nanog. This contradictory finding warrants further investigation as in these cells CD44 was predominantly nuclear and bound in promoter regions of c-Myc and Sox2 (Han et al. 2015). The CRISPR/Cas9 system can be also utilized to target specific variants of CD44. In gastric cells, CD44 exon v6 was deleted, allowing for the rest of CD44 gene to be expressed. Removal of v6 exon from CD44 sensitized gastric cancer cells to cisplatin and abrogated their self-renewal (Lobo et al. 2020). In another study, utilizing CRISPR/Cas9 and overexpression approaches, chimeric antigen receptors (CARs) that specifically target CD44v6 from head and neck squamous cell carcinoma were expressed on T cells. Targeting CD44v6 demonstrated a direct correlation of CD44v6 expression and cytotoxic effects mediated from CAR T cells (Haist et al. 2021).
18.4 Conclusions
Conclusively, hyaluronan/CD44 network appears to offer an important target for translation into the clinic to treat tumors. However, given the omnipresent localization of hyaluronan in the body and ubiquitous expression of CD44 as well as their importance in physiological processes, targeting this system needs to be approached with caution. On the bright side, many of the hyaluronan effects depend on the expression of CD44v isoforms that are expressed specifically by tumor cells. Moreover, while many of the processes controlled by hyaluronan/CD44 network overlap with features of immune and inflammatory pathways, there are clear differences between tumor cells and physiologic events that can be exploited, like the size and interactions of hyaluronan found in cancerous tissues. Certainly, further research is needed to clarify the exact roles of hyaluronan and CD44 in tumorigenesis, but the evidence presented so far suggests that targeting of this system is a promising avenue for the development of safe and effective cancer treatment regiments in the future.
References
Agren UM, Tammi RH, Tammi MI (1997) Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med 23:996–1001
Aires A, Ocampo SM, Simoes BM, Josefa Rodriguez M, Cadenas JF, Couleaud P, Spence K, Latorre A, Miranda R, Somoza A, Clarke RB, Carrascosa JL, Cortajarena AL (2016) Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44- positive cancer cells. Nanotechnology 27:065103
Alshaer W, Hillaireau H, Vergnaud J, Mura S, Delomenie C, Sauvage F, Ismail S, Fattal E (2018) Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model. J Control Release 271:98–106
Annabi B, Bouzeghrane M, Currie JC, Hawkins R, Dulude H, Daigneault L, Ruiz M, Wisniewski J, Garde S, Rabbani SA, Panchal C, Wu JJ, Beliveau R (2005) A PSP94-derived peptide PCK3145 inhibits MMP-9 secretion and triggers CD44 cell surface shedding: implication in tumor metastasis. Clin Exp Metastasis 22:429–439
Artus C, Maquarre E, Moubarak RS, Delettre C, Jasmin C, Susin SA, Robert-Lezenes J (2006) CD44 ligation induces caspase-independent cell death via a novel calpain/AIF pathway in human erythroleukemia cells. Oncogene 25:5741–5751
Baba D, Kashiwabara S, Honda A, Yamagata K, Wu Q, Ikawa M, Okabe M, Baba T (2002) Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J Biol Chem 277:30310–30314
Bae KH, Tan S, Yamashita A, Ang WX, Gao SJ, Wang S, Chung JE, Kurisawa M (2017) Hyaluronic acid-green tea catechin micellar nanocomplexes: fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 148:41–53
Bai Y, Liu CP, Chen D, Liu CF, Zhuo LH, Li H, Wang C, Bu HT, Tian W (2020) beta-Cyclodextrin-modified hyaluronic acid-based supramolecular self-assemblies for pH- and esterase- dual-responsive drug delivery. Carbohydr Polym 246:116654
Bart G, Vico NO, Hassinen A, Pujol FM, Deen AJ, Ruusala A, Tammi RH, Squire A, Heldin P, Kellokumpu S, Tammi MI (2015) Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3. J Biol Chem 290:11479–11490
Bates RC, Elith CA, Thorne RF, Burns GF (1998) Engagement of variant CD44 confers resistance to anti-integrin antibody-mediated apoptosis in a colon carcinoma cell line. Cell Adhes Commun 6:21–38
Bernert B, Porsch H, Heldin P (2011) Hyaluronan synthase 2 (HAS2) promotes breast cancer cell invasion by suppression of tissue metalloproteinase inhibitor 1 (TIMP-1). J Biol Chem 286:42349–42359
Bhattacharya DS, Svechkarev D, Bapat A, Patil P, Hollingsworth MA, Mohs AM (2020) Sulfation modulates the targeting properties of hyaluronic acid to P-selectin and CD44. ACS Biomater Sci Eng 6:3585–3598
Birkenkamp-Demtroder K, Maghnouj A, Mansilla F, Thorsen K, Andersen CL, Oster B, Hahn S, Orntoft TF (2011) Repression of KIAA1199 attenuates Wnt-signalling and decreases the proliferation of colon cancer cells. Br J Cancer 105:552–561
Birzele F, Voss E, Nopora A, Honold K, Heil F, Lohmann S, Verheul H, Le Tourneau C, Delord JP, Van Herpen C, Mahalingam D, Coveler AL, Meresse V, Weigand S, Runza V, Cannarile M (2015) CD44 isoform status predicts response to treatment with anti-CD44 antibody in cancer patients. Clin Cancer Res 21:2753–2762
Borgya A, Woodman A, Sugiyama M, Donie F, Kopetzki E, Matsumura Y, Tarin D (1995) Isolation and characterisation of antibodies which specifically recognise the peptide encoded by exon 7 (v2) of the human CD44 gene. Clin Mol Pathol 48:M241–M250
Bostad M, Kausberg M, Weyergang A, Olsen CE, Berg K, Hogset A, Selbo PK (2014) Light-triggered, efficient cytosolic release of IM7-saporin targeting the putative cancer stem cell marker CD44 by photochemical internalization. Mol Pharm 11:2764–2776
Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC (1997) Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem 272:27913–27918
Bourguignon LY, Wong G, Earle C, Chen L (2012) Hyaluronan-CD44v3 interaction with Oct4- Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem 287:32800–32824
Chanmee T, Ontong P, Kimata K, Itano N (2015) Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol 5:180
Chen P, Huang H, Wu J, Lu R, Wu Y, Jiang X, Yuan Q, Chen Y (2015) Bone marrow stromal cells protect acute myeloid leukemia cells from anti-CD44 therapy partly through regulating PI3K/Akt-p27(Kip1) axis. Mol Carcinog 54:1678–1685
Chen L, Liu Z, Jin R, Yang X, Bai Y, Liu S, Chen X (2018a) Stepwise co-delivery of an enzyme and prodrug based on a multi-responsive nanoplatform for accurate tumor therapy. J Mater Chem B 6:6262–6268
Chen X, Nagai Y, Zhu Z, Ruan H, Peehl DM, Greene MI, Zhang H (2018b) A spliced form of CD44 expresses the unique glycan that is recognized by the prostate cancer specific antibody F77. Oncotarget 9:3631–3640
Chen Y, Wang H, Zuo Y, Li N, Ding M, Li C (2018c) A novel monoclonal antibody KMP1 has potential antitumor activity of bladder cancer by blocking CD44 in vivo and in vitro. Cancer Med 7:2064–2077
Cheng HW, Chiang CS, Ho HY, Chou SH, Lai YH, Shyu WC, Chen SY (2021) Dextran-modified Quercetin-Cu(II)/hyaluronic acid nanomedicine with natural poly(ADP-ribose) polymerase inhibitor and dual targeting for programmed synthetic lethal therapy in triple- negative breast cancer. J Control Release 329:136–147
Cherr GN, Yudin AI, Overstreet JW (2001) The dual functions of GPI-anchored PH-20: hyaluronidase and intracellular signaling. Matrix Biol 20:515–525
Cho JH, Lee SC, Ha NR, Lee SJ, Yoon MY (2015) A novel peptide-based recognition probe for the sensitive detection of CD44 on breast cancer stem cells. Mol Cell Probes 29:492–499
Chong Y, Huang J, Xu X, Yu C, Ning X, Fan S, Zhang Z (2020) Hyaluronic acid-modified au-ag alloy nanoparticles for radiation/nanozyme/ag(+) multimodal synergistically enhanced cancer therapy. Bioconjug Chem 31:1756–1765
Danhier F, Feron O, Preat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148:135–146
D’arena G, Calapai G, Deaglio S (2014) Anti-CD44 mAb for the treatment of B-cell chronic lymphocytic leukemia and other hematological malignancies: evaluation of WO2013063498. Expert Opin Ther Pat 24:821–828
Debele TA, Wu PC, Wei YF, Chuang JY, Chang KY, Tsai JH, Su WP (2021) Transferrin modified GSH sensitive hyaluronic acid derivative micelle to deliver HSP90 inhibitors to enhance the therapeutic efficacy of brain cancers. Cancers (Basel) 13:2375
Deen AJ, Rilla K, Oikari S, Karna R, Bart G, Hayrinen J, Bathina AR, Ropponen A, Makkonen K, Tammi RH, Tammi MI (2014) Rab10-mediated endocytosis of the hyaluronan synthase HAS3 regulates hyaluronan synthesis and cell adhesion to collagen. J Biol Chem 289:8375–8389
Ding L, Jiang Y, Zhang J, Klok HA, Zhong Z (2018) pH-sensitive coiled-coil peptide-cross-linked hyaluronic acid nanogels: synthesis and targeted intracellular protein delivery to CD44 positive cancer cells. Biomacromolecules 19:555–562
Du YR, Chen Y, Gao Y, Niu XL, Li YJ, Deng WM (2013) Effects and mechanisms of anti-CD44 monoclonal antibody A3D8 on proliferation and apoptosis of sphere-forming cells with stemness from human ovarian cancer. Int J Gynecol Cancer 23:1367–1375
Du T, Jia X, Dong X, Ru X, Li L, Wang Y, Liu J, Feng G, Wen T (2020a) Cosmc disruption-mediated aberrant O-glycosylation suppresses breast cancer cell growth via impairment of CD44. Cancer Manag Res 12:511–522
Du Y, Wang S, Zhang T, He D, Tu J, Shen Y (2020b) Enhanced cytotoxicity of a redox-sensitive hyaluronic acid-based nanomedicine toward different oncocytes via various internalization mechanisms. Drug Deliv 27:128–136
Edelman R, Assaraf YG, Levitzky I, Shahar T, Livney YD (2017) Hyaluronic acid-serum albumin conjugate-based nanoparticles for targeted cancer therapy. Oncotarget 8:24337–24353
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Fedorchenko O, Stiefelhagen M, Peer-Zada AA, Barthel R, Mayer P, Eckei L, Breuer A, Crispatzu G, Rosen N, Landwehr T, Lilienthal N, Mollmann M, Montesinos-Rongen M, Heukamp L, Durig J, Hallek M, Fingerle-Rowson G, Herling M (2013) CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood 121:4126–4136
Fernando J, Malfettone A, Cepeda EB, Vilarrasa-Blasi R, Bertran E, Raimondi G, Fabra A, Alvarez-Barrientos A, Fernandez-Salguero P, Fernandez-Rodriguez CM, Giannelli G, Sancho P, Fabregat I (2015) A mesenchymal-like phenotype and expression of CD44 predict lack of apoptotic response to sorafenib in liver tumor cells. Int J Cancer 136:E161–E172
Finlayson M (2015) Modulation of CD44 activity by A6-peptide. Front Immunol 6:135
Fitzgerald KA, Bowie AG, Skeffington BS, O’neill LA (2000) Ras, protein kinase C zeta, and I kappa B kinases 1 and 2 are downstream effectors of CD44 during the activation of NF-kappa B by hyaluronic acid fragments in T-24 carcinoma cells. J Immunol 164:2053–2063
Flannery CR, Little CB, Hughes CE, Caterson B (1998) Expression and activity of articular cartilage hyaluronidases. Biochem Biophys Res Commun 251:824–829
Flores-Diaz M, Alape-Giron A, Persson B, Pollesello P, Moos M, Von Eichel-Streiber C, Thelestam M, Florin I (1997) Cellular UDP-glucose deficiency caused by a single point mutation in the UDP-glucose pyrophosphorylase gene. J Biol Chem 272:23784–23791
Fraser JR, Laurent TC, Laurent UB (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242:27–33
Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, Kishi H, Hiwasa T, Koda K, Nakajima N, Harigaya K (2002) CD44 signaling through focal adhesion kinase and its anti- apoptotic effect. FEBS Lett 528:101–108
Gadhoum Z, Delaunay J, Maquarre E, Durand L, Lancereaux V, Qi J, Robert-Lezenes J, Chomienne C, Smadja-Joffe F (2004a) The effect of anti-CD44 monoclonal antibodies on differentiation and proliferation of human acute myeloid leukemia cells. Leuk Lymphoma 45:1501–1510
Gadhoum Z, Leibovitch MP, Qi J, Dumenil D, Durand L, Leibovitch S, Smadja-Joffe F (2004b) CD44: a new means to inhibit acute myeloid leukemia cell proliferation via p27Kip1. Blood 103:1059–1068
Galandrini R, De Maria R, Piccoli M, Frati L, Santoni A (1994) CD44 triggering enhances human NK cell cytotoxic functions. J Immunol 153:4399–4407
Gao Y, Foster R, Yang X, Feng Y, Shen JK, Mankin HJ, Hornicek FJ, Amiji MM, Duan Z (2015) Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 6:9313–9326
Gao S, Wang J, Tian R, Wang G, Zhang L, Li Y, Li L, Ma Q, Zhu L (2017) Construction and evaluation of a targeted hyaluronic acid nanoparticle/photosensitizer complex for cancer photodynamic therapy. ACS Appl Mater Interfaces 9:32509–32519
Gao S, Islam R, Fang J (2021) Tumor environment-responsive hyaluronan conjugated zinc protoporphyrin for targeted anticancer photodynamic therapy. J Pers Med 11:136
Gatti V, Fierro C, Compagnone M, Giangrazi F, Markert EK, Bongiorno-Borbone L, Melino G, Peschiaroli A (2018) DeltaNp63 regulates the expression of hyaluronic acid- related genes in breast cancer cells. Oncogenesis 7:65
Ghatak S, Misra S, Toole BP (2005) Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem 280:8875–8883
Golan M, Feinshtein V, Polyak D, Scomparin A, Satchi-Fainaro R, David A (2016) Inhibition of gene expression and cancer cell migration by CD44v3/6-targeted Polyion complexes. Bioconjug Chem 27:947–960
Gong H, Chao Y, Xiang J, Han X, Song G, Feng L, Liu J, Yang G, Chen Q, Liu Z (2016) Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett 16:2512–2521
Gu W, An J, Meng H, Yu N, Zhong Y, Meng F, Xu Y, Cornelissen J, Zhong Z (2019) CD44-specific A6 short peptide boosts targetability and anticancer efficacy of polymersomal epirubicin to orthotopic human multiple myeloma. Adv Mater 31:e1904742
Gu W, Liu T, Fan D, Zhang J, Xia Y, Meng F, Xu Y, Cornelissen J, Liu Z, Zhong Z (2021) A6 peptide-tagged, ultra-small and reduction-sensitive polymersomal vincristine sulfate as a smart and specific treatment for CD44+ acute myeloid leukemia. J Control Release 329:706–716
Guo J, Cheng H, Zhao S, Yu L (2006) GG: a domain involved in phage LTF apparatus and implicated in human MEB and non-syndromic hearing loss diseases. FEBS Lett 580:581–584
Haist C, Schulte E, Bartels N, Bister A, Poschinski Z, Ibach TC, Geipel K, Wiek C, Wagenmann M, Monzel C, Scheckenbach K, Hanenberg H (2021) CD44v6-targeted CAR T-cells specifically eliminate CD44 isoform 6 expressing head/neck squamous cell carcinoma cells. Oral Oncol 116:105259
Han S, Guo J, Liu Y, Zhang Z, He Q, Li P, Zhang M, Sun H, Li R, Li Y, Zeng W, Liu J, Lian L, Gao Y, Shen L (2015) Knock out CD44 in reprogrammed liver cancer cell C3A increases CSCs stemness and promotes differentiation. Oncotarget 6:44452–44465
Han HS, Choi KY, Lee H, Lee M, An JY, Shin S, Kwon S, Lee DS, Park JH (2016) Gold-nanoclustered hyaluronan nano-assemblies for photothermally maneuvered photodynamic tumor ablation. ACS Nano 10:10858–10868
Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, Vigetti D, Hanson RW, Hart GW (2014) The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol 35:14–17
Hayward SL, Wilson CL, Kidambi S (2016) Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 7:34158–34171
He QY, Liu XH, Li Q, Studholme DJ, Li XW, Liang SP (2006) G8: a novel domain associated with polycystic kidney disease and non-syndromic hearing loss. Bioinformatics 22:2189–2191
Heldin P, Delatorre M, Ytterberg D, Bergh J (1996) Differential synthesis and binding of hyaluronan by human breast cancer cell lines. Oncol Rep 3:1011–1016
Heldin P, Basu K, Kozlova I, Porsch H (2014) HAS2 and CD44 in breast tumorigenesis. Adv Cancer Res 123:211–229
Heldin P, Lin CY, Kolliopoulos C, Chen YH, Skandalis SS (2019) Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol 78-79:100–117
Hibino S, Shibuya M, Hoffman MP, Engbring JA, Hossain R, Mochizuki M, Kudoh S, Nomizu M, Kleinman HK (2005) Laminin alpha5 chain metastasis- and angiogenesis- inhibiting peptide blocks fibroblast growth factor 2 activity by binding to the heparan sulfate chains of CD44. Cancer Res 65:10494–10501
Ishii S, Ford R, Thomas P, Nachman A, Steele G Jr, Jessup JM (1993) CD44 participates in the adhesion of human colorectal carcinoma cells to laminin and type IV collagen. Surg Oncol 2:255–264
Ishizuka Y, Yokota A, Nishimura M, Saito Y, Nakaseko C (2008) Ligation of CD44 leads to killing activity in human peripheral mononuclear cells via MAP kinase and tyrosine kinases. Hematology 13:230–235
Itano N, Kimata K (2002) Mammalian hyaluronan synthases. IUBMB Life 54:195–199
Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274:25085–25092
Jafari Malek S, Khoshchehreh R, Goodarzi N, Khoshayand MR, Amini M, Atyabi F, Esfandyari-Manesh M, Tehrani S, Mohammad Jafari R, Maghazei MS, Alvandifar F, Ebrahimi M, Dinarvand R (2014) cis-Dichlorodiamminoplatinum (II) glyconanoparticles by drug-induced ionic gelation technique targeted to prostate cancer: preparation, optimization and in vitro characterization. Colloids Surf B Biointerfaces 122:350–358
Jauw YWS, Huisman MC, Nayak TK, Vugts DJ, Christen R, Naegelen VM, Ruettinger D, Heil F, Lammertsma AA, Verheul HMW, Hoekstra OS, Van Dongen G, Menke-Van Der Houven Van Oordt CW (2018) Assessment of target-mediated uptake with immuno-PET: analysis of a phase I clinical trial with an anti-CD44 antibody. EJNMMI Res 8:6
Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M (2001) Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol 153:893–904
Karousou E, Kamiryo M, Skandalis SS, Ruusala A, Asteriou T, Passi A, Yamashita H, Hellman U, Heldin CH, Heldin P (2010) The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J Biol Chem 285:23647–23654
Karousou E, Misra S, Ghatak S, Dobra K, Gotte M, Vigetti D, Passi A, Karamanos NK, Skandalis SS (2017) Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol 59:3–22
Khan F, Gurung S, Gunassekaran GR, Vadevoo SMP, Chi L, Permpoon U, Haque ME, Lee YK, Lee SW, Kim S, Lee B (2021) Identification of novel CD44v6-binding peptides that block CD44v6 and deliver a pro-apoptotic peptide to tumors to inhibit tumor growth and metastasis in mice. Theranostics 11:1326–1344
Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S, Itano N (2007) Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol 170:1086–1099
Kubens BS, Zanker KS (1998) Differences in the migration capacity of primary human colon carcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Cancer Lett 131:55–64
Lakshman M, Subramaniam V, Rubenthiran U, Jothy S (2004) CD44 promotes resistance to apoptosis in human colon cancer cells. Exp Mol Pathol 77:18–25
Larsen M, Artym VV, Green JA, Yamada KM (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 18:463–471
Lee SY, Kim KA, Kim CH, Kim YJ, Lee JH, Kim HR (2017) CD44-shRNA recombinant adenovirus inhibits cell proliferation, invasion, and migration, and promotes apoptosis in HCT116 colon cancer cells. Int J Oncol 50:329–336
Lee IC, Wu YC, Hung WS (2018) Hyaluronic acid-based multilayer films regulate hypoxic multicellular aggregation of pancreatic cancer cells with distinct cancer stem-cell-like properties. ACS Appl Mater Interfaces 10:38769–38779
Li L, Heldin CH, Heldin P (2006) Inhibition of platelet-derived growth factor-BB-induced receptor activation and fibroblast migration by hyaluronan activation of CD44. J Biol Chem 281:26512–26519
Li J, Yang J, Yuan J, Li Y, Wang RC, Wang SY, Hao HL (2016) Effect of anti-CD44 monoclonal antibody A3D8 on expression of AP-1 in HL-60 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 24:1360–1364
Li W, Jia H, Wang J, Guan H, Li Y, Zhang D, Tang Y, Wang TD, Lu S (2017a) A CD44-specific peptide, RP-1, exhibits capacities of assisting diagnosis and predicting prognosis of gastric cancer. Oncotarget 8:30063–30076
Li W, Ma H, Zhang J, Zhu L, Wang C, Yang Y (2017b) Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep 7:13856
Liang Y, Peng J, Li N, Yu-Wai-Man C, Wang Q, Xu Y, Wang H, Tagalakis AD, Du Z (2019) Smart nanoparticles assembled by endogenous molecules for siRNA delivery and cancer therapy via CD44 and EGFR dual-targeting. Nanomedicine 15:208–217
Liang Y, Wang Y, Wang L, Liang Z, Li D, Xu X, Chen Y, Yang X, Zhang H, Niu H (2021) Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact Mater 6:433–446
Lin C, Yang X, Li H, Zou Y, Mohammad IS, Rong H, Rao Y, Song J, Leung SSY, Hu H (2021) Self-assembled nanomedicine combining a berberine derivative and doxorubicin for enhanced antitumor and antimetastatic efficacy via mitochondrial pathways. Nanoscale 13:6605–6623
Liu T, Yan Z, Liu Y, Choy E, Hornicek FJ, Mankin H, Duan Z (2018) CRISPR-Cas9-mediated silencing of CD44 in human highly metastatic osteosarcoma cells. Cell Physiol Biochem 46:1218–1230
Liu J, Sun Y, Liu X, Yang Y, Widjaya AS, Long Z, Jiang Y (2020) Efficiency of different treatment regimens combining anti-tumor and anti-inflammatory liposomes for metastatic breast cancer. AAPS PharmSciTech 21:259
Lobo S, Pereira C, Oliveira C, Almeida GM (2020) Skipping exon-v6 from CD44v6-containing isoforms influences chemotherapy response and self-renewal capacity of gastric cancer cells. Cancers (Basel) 12:2378
Lusche DF, Wessels DJ, Reis RJ, Forrest CC, Thumann AR, Soll DR (2021) New monoclonal antibodies that recognize an unglycosylated, conserved, extracellular region of CD44 in vitro and in vivo, and can block tumorigenesis. PLoS One 16:e0250175
Mahira S, Kommineni N, Husain GM, Khan W (2019) Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer. Biomed Pharmacother 110:803–817
Maiolino S, Russo A, Pagliara V, Conte C, Ungaro F, Russo G, Quaglia F (2015) Biodegradable nanoparticles sequentially decorated with polyethyleneimine and hyaluronan for the targeted delivery of docetaxel to airway cancer cells. J Nanobiotechnology 13:29
Maisel D, Birzele F, Voss E, Nopora A, Bader S, Friess T, Goller B, Laifenfeld D, Weigand S, Runza V (2016) Targeting tumor cells with anti-CD44 antibody triggers macrophage-mediated immune modulatory effects in a cancer xenograft model. PLoS One 11:e0159716
Marengo A, Forciniti S, Dando I, Dalla Pozza E, Stella B, Tsapis N, Yagoubi N, Fanelli G, Fattal E, Heeschen C, Palmieri M, Arpicco S (2019) Pancreatic cancer stem cell proliferation is strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. Biochim Biophys Acta Gen Subj 1863:61–72
Matzke-Ogi A, Jannasch K, Shatirishvili M, Fuchs B, Chiblak S, Morton J, Tawk B, Lindner T, Sansom O, Alves F, Warth A, Schwager C, Mier W, Kleeff J, Ponta H, Abdollahi A, Orian-Rousseau V (2016) Inhibition of tumor growth and metastasis in pancreatic cancer models by interference with CD44v6 signaling. Gastroenterology 150:513–525 e10
McClements L, Yakkundi A, Papaspyropoulos A, Harrison H, Ablett MP, Jithesh PV, McKeen HD, Bennett R, Donley C, Kissenpfennig A, Mcintosh S, McCarthy HO, O’neill E, Clarke RB, Robson T (2013) Targeting treatment-resistant breast cancer stem cells with FKBPL and its peptide derivative, AD-01, via the CD44 pathway. Clin Cancer Res 19:3881–3893
Mehic M, De Sa VK, Hebestreit S, Heldin CH, Heldin P (2017) The deubiquitinating enzymes USP4 and USP17 target hyaluronan synthase 2 and differentially affect its function. Oncogenesis 6:e348
Menke-Van Der Houven Van Oordt CW, Gomez-Roca C, Van Herpen C, Coveler AL, Mahalingam D, Verheul HM, Van Der Graaf WT, Christen R, Ruttinger D, Weigand S, Cannarile MA, Heil F, Brewster M, Walz AC, Nayak TK, Guarin E, Meresse V, Le Tourneau C (2016) First-in-human phase I clinical trial of RG7356, an anti- CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget 7:80046–80058
Miletti-Gonzalez KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN, Rodriguez-Rodriguez L (2005) The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res 65:6660–6667
Miletti-Gonzalez KE, Murphy K, Kumaran MN, Ravindranath AK, Wernyj RP, Kaur S, Miles GD, Lim E, Chan R, Chekmareva M, Heller DS, Foran D, Chen W, Reiss M, Bandera EV, Scotto K, Rodriguez-Rodriguez L (2012) Identification of function for CD44 intracytoplasmic domain (CD44-ICD): modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem 287:18995–19007
Misra S, Ghatak S, Toole BP (2005) Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem 280:20310–20315
Misra S, Hascall VC, De Giovanni C, Markwald RR, Ghatak S (2009) Delivery of CD44 shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ MICE. J Biol Chem 284:12432–12446
Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S (2011) Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J 278:1429–1443
Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MG, Moghimi SM, Peer D (2011) Hyaluronan-coated nanoparticles: the influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J Control Release 156:231–238
Mizrahy S, Goldsmith M, Leviatan-Ben-Arye S, Kisin-Finfer E, Redy O, Srinivasan S, Shabat D, Godin B, Peer D (2014) Tumor targeting profiling of hyaluronan-coated lipid based-nanoparticles. Nanoscale 6:3742–3752
Montanari E, Di Meo C, Oates A, Coviello T, Matricardi P (2018) Pursuing intracellular pathogens with hyaluronan. From a ‘Pro-Infection’ polymer to a biomaterial for ‘Trojan Horse’ Systems. Molecules 23:939
Morath I, Hartmann TN, Orian-Rousseau V (2016) CD44: more than a mere stem cell marker. Int J Biochem Cell Biol 81:166–173
Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M (2002) CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J 21:3949–3959
Navarro-Marchal SA, Grinan-Lison C, Entrena JM, Ruiz-Alcala G, Tristan-Manzano M, Martin F, Perez-Victoria I, Peula-Garcia JM, Marchal JA (2021) Anti-CD44-conjugated olive oil liquid nanocapsules for targeting pancreatic cancer stem cells. Biomacromolecules 22:1374–1388
Ndinguri MW, Zheleznyak A, Lauer JL, Anderson CJ, Fields GB (2012) Application of collagen-model triple-helical peptide-amphiphiles for CD44-targeted drug delivery systems. J Drug Deliv 2012:592602
Ni J, Cozzi PJ, Hao JL, Beretov J, Chang L, Duan W, Shigdar S, Delprado WJ, Graham PH, Bucci J, Kearsley JH, Li Y (2014) CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 74:602–617
Nurwidya F, Takahashi F, Kato M, Baskoro H, Hidayat M, Wirawan A, Takahashi K (2017) CD44 silencing decreases the expression of stem cell-related factors induced by transforming growth factor beta1 and tumor necrosis factor alpha in lung cancer: preliminary findings. Bosn J Basic Med Sci 17:228–234
Ogoshi T, Ishii S, Mizoi T, Harada N, Sato W, Saito K, Ogawa H, Inabe K, Shiiba K, Matsuno S (1998) CD44H participates in the intrahepatic growth of murine colon 26 adenocarcinoma cells. Jpn J Cancer Res 89:1160–1168
Ota T, Matsui T, Kohno H, Maeda M, Tanino M, Odashima S (1995) CD44 participates in tumor cell adhesion to endothelial cells in the experimental metastatic process in B16BL6 melanoma cells. Anticancer Res 15:1215–1219
Pandey MS, Harris EN, Weigel JA, Weigel PH (2008) The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J Biol Chem 283:21453–21461
Park HY, Lee KJ, Lee SJ, Yoon MY (2012a) Screening of peptides bound to breast cancer stem cell specific surface marker CD44 by phage display. Mol Biotechnol 51:212–220
Park YS, Huh JW, Lee JH, Kim HR (2012b) shRNA against CD44 inhibits cell proliferation, invasion and migration, and promotes apoptosis of colon carcinoma cells. Oncol Rep 27:339–346
Patel S, Shah K, Mirza S, Shah K, Rawal R (2016) Circulating tumor stem like cells in oral squamous cell carcinoma: An unresolved paradox. Oral Oncol 62:139–146
Peck D, Isacke CM (1998) Hyaluronan-dependent cell migration can be blocked by a CD44 cytoplasmic domain peptide containing a phosphoserine at position 325. J Cell Sci 111(Pt 11):1595–1601
Peng Y, Prater AR, Deutscher SL (2017) Targeting aggressive prostate cancer-associated CD44v6 using phage display selected peptides. Oncotarget 8:86747–86768
Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M (2011) A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol Cancer Ther 10:2072–2082
Porsch H, Bernert B, Mehic M, Theocharis AD, Heldin CH, Heldin P (2013) Efficient TGFbeta-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2. Oncogene 32:4355–4365
Qian H, Xia L, Ling P, Waxman S, Jing Y (2012) CD44 ligation with A3D8 antibody induces apoptosis in acute myeloid leukemia cells through binding to CD44s and clustering lipid rafts. Cancer Biol Ther 13:1276–1283
Reinke LM, Xu Y, Cheng C (2012) Snail represses the splicing regulator epithelial splicing regulatory protein 1 to promote epithelial-mesenchymal transition. J Biol Chem 287:36435–36442
Rilla K, Oikari S, Jokela TA, Hyttinen JM, Karna R, Tammi RH, Tammi MI (2013) Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem 288:5973–5983
Rodriguez MM, Fiore E, Bayo J, Atorrasagasti C, Garcia M, Onorato A, Dominguez L, Malvicini M, Mazzolini G (2018) 4Mu decreases CD47 expression on hepatic cancer stem cells and primes a potent antitumor T cell response induced by interleukin-12. Mol Ther 26:2738–2750
Sadvakassova G, Dobocan MC, Congote LF (2009) Osteopontin and the C-terminal peptide of thrombospondin-4 compete for CD44 binding and have opposite effects on CD133+ cell colony formation. BMC Res Notes 2:215
Seki K, Yamaguchi A, Goi T, Nakagawara G, Matsukawa S, Urano T, Furukawa K (1997) Inhibition of liver metastasis formation by anti-CD44 variant exon 9 monoclonal antibody. Int J Oncol 11:1257–1261
Shabani Ravari N, Goodarzi N, Alvandifar F, Amini M, Souri E, Khoshayand MR, Hadavand Mirzaie Z, Atyabi F, Dinarvand R (2016) Fabrication and biological evaluation of chitosan coated hyaluronic acid-docetaxel conjugate nanoparticles in CD44(+) cancer cells. Daru 24:21
Shah KN, Ditto AJ, Crowder DC, Overmeyer JH, Tavana H, Maltese WA, Yun YH (2017) Receptor-mediated attachment and uptake of hyaluronan conjugates by breast cancer cells. Mol Pharm 14:3968–3977
Shao J, Fan W, Ma B, Wu Y (2016) Breast cancer stem cells expressing different stem cell markers exhibit distinct biological characteristics. Mol Med Rep 14:4991–4998
Shuttleworth TL, Wilson MD, Wicklow BA, Wilkins JA, Triggs-Raine BL (2002) Characterization of the murine hyaluronidase gene region reveals complex organization and cotranscription of Hyal1 with downstream genes, Fus2 and Hyal3. J Biol Chem 277:23008–23018
Skandalis SS, Kozlova I, Engstrom U, Hellman U, Heldin P (2010) Proteomic identification of CD44 interacting proteins. IUBMB Life 62:833–840
Skandalis SS, Karalis TT, Chatzopoulos A, Karamanos NK (2019) Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal 63:109377
Skandalis SS, Karalis T, Heldin P (2020) Intracellular hyaluronan: importance for cellular functions. Semin Cancer Biol 62:20–30
Solis MA, Chen YH, Wong TY, Bittencourt VZ, Lin YC, Huang LL (2012) Hyaluronan regulates cell behavior: a potential niche matrix for stem cells. Biochem Res Int 2012:346972
Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J (2006) Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7:659–668
Song G, Liao X, Zhou L, Wu L, Feng Y, Han ZC (2004) HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells. Leuk Res 28:1089–1096
Stamenkovic I, Yu Q (2009) Shedding light on proteolytic cleavage of CD44: the responsible sheddase and functional significance of shedding. J Invest Dermatol 129:1321–1324
Su J, Wu S, Wu H, Li L, Guo T (2016) CD44 is functionally crucial for driving lung cancer stem cells metastasis through Wnt/beta-catenin-FoxM1-twist signaling. Mol Carcinog 55:1962–1973
Surace C, Arpicco S, Dufay-Wojcicki A, Marsaud V, Bouclier C, Clay D, Cattel L, Renoir JM, Fattal E (2009) Lipoplexes targeting the CD44 hyaluronic acid receptor for efficient transfection of breast cancer cells. Mol Pharm 6:1062–1073
Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H, Heldin P (2005) Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1- dependent manner. J Biol Chem 280:24195–24204
Takei J, Kaneko MK, Ohishi T, Hosono H, Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, Harada H, Kato Y (2020) A defucosylated antiCD44 monoclonal antibody 5mG2af exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol Rep 44:1949–1960
Tan PH, Santos EB, Rossbach HC, Sandmaier BM (1993) Enhancement of natural killer activity by an antibody to CD44. J Immunol 150:812–820
Thankamony SP, Knudson W (2006) Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem 281:34601–34609
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 97:4–27
Tirella A, Kloc-Muniak K, Good L, Ridden J, Ashford M, PURI, S. & Tirelli, N. (2019) CD44 targeted delivery of siRNA by using HA-decorated nanotechnologies for KRAS silencing in cancer treatment. Int J Pharm 561:114–123
Torronen K, Nikunen K, Karna R, Tammi M, Tammi R, Rilla K (2014) Tissue distribution and subcellular localization of hyaluronan synthase isoenzymes. Histochem Cell Biol 141:17–31
Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, Christofori G, Heroult M, Augustin HG, Ponta H, Orian-Rousseau V (2009) A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood 114:5236–5244
Ugarte-Berzal E, Bailon E, Amigo-Jimenez I, Albar JP, Garcia-Marco JA, Garcia-Pardo A (2014) A novel CD44-binding peptide from the pro-matrix metalloproteinase-9 hemopexin domain impairs adhesion and migration of chronic lymphocytic leukemia (CLL) cells. J Biol Chem 289:15340–15349
Vahidian F, Safarzadeh E, Mohammadi A, Najjary S, Mansoori B, Majidi J, Babaloo Z, Aghanejad A, Shadbad MA, Mokhtarzadeh A, Baradaran B (2020) siRNA-mediated silencing of CD44 delivered by jet Pei enhanced doxorubicin chemo sensitivity and altered miRNA expression in human breast cancer cell line (MDA-MB468). Mol Biol Rep 47:9541–9551
Valentine A, O’rourke M, Yakkundi A, Worthington J, Hookham M, Bicknell R, McCarthy HO, McClelland K, McCallum L, Dyer H, McKeen H, Waugh DJ, Roberts J, McGregor J, Cotton G, James I, Harrison T, Hirst DG, Robson T (2011) FKBPL and peptide derivatives: novel biological agents that inhibit angiogenesis by a CD44- dependent mechanism. Clin Cancer Res 17:1044–1056
Van Hal NL, Van Dongen GA, Rood-Knippels EM, Van Der Valk P, Snow GB, Brakenhoff RH (1996) Monoclonal antibody U36, a suitable candidate for clinical immunotherapy of squamous-cell carcinoma, recognizes a CD44 isoform. Int J Cancer 68:520–527
Varghese OP, Sun W, Hilborn J, Ossipov DA (2009) In situ cross-linkable high molecular weight hyaluronan-bisphosphonate conjugate for localized delivery and cell-specific targeting: a hydrogel linked prodrug approach. J Am Chem Soc 131:8781–8783
Vey N, Delaunay J, Martinelli G, Fiedler W, Raffoux E, Prebet T, Gomez-Roca C, Papayannidis C, Kebenko M, Paschka P, Christen R, Guarin E, Broske AM, Baehner M, Brewster M, Walz AC, Michielin F, Runza V, Meresse V, Recher C (2016) Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget 7:32532–32542
Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P, Heldin P, Hascall VC, De Luca G, Passi A (2011) Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J Biol Chem 286:7917–7924
Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A (2012) Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287:35544–35555
Vugts DJ, Heuveling DA, Stigter-Van Walsum M, Weigand S, Bergstrom M, Van Dongen GA, Nayak TK (2014) Preclinical evaluation of 89Zr-labeled anti-CD44 monoclonal antibody RG7356 in mice and cynomolgus monkeys: prelude to phase 1 clinical studies. MAbs 6:567–575
Vyas D, Lopez-Hisijos N, Gandhi S, El-Dakdouki M, Basson MD, Walsh MF, Huang X, Vyas AK, Chaturvedi LS (2015) Doxorubicin-hyaluronan conjugated super-paramagnetic iron oxide nanoparticles (DOX-HA-SPION) enhanced cytoplasmic uptake of doxorubicin and modulated apoptosis, IL-6 release and NF-kappaB activity in human MDA-MB-231 breast cancer cells. J Nanosci Nanotechnol 15:6413–6422
Wang SJ, Wreesmann VB, Bourguignon LY (2007) Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck 29:550–558
Wang A, Midura RJ, Vasanji A, Wang AJ, Hascall VC (2014) Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes. J Biol Chem 289:11410–11420
Wang H, Zhu Z, Zhang G, Lin F, Liu Y, Zhang Y, Feng J, Chen W, Meng Q, Chen L (2019) AS1411 aptamer/hyaluronic acid-bifunctionalized microemulsion co-loading shikonin and docetaxel for enhanced antiglioma therapy. J Pharm Sci 108:3684–3694
Wang Z, Li Y, Wang Y, Wu D, Lau AHY, Zhao P, Zou C, Dai Y, Chan FL (2020) Targeting prostate cancer stem-like cells by an immunotherapeutic platform based on immunogenic peptide-sensitized dendritic cells-cytokine-induced killer cells. Stem Cell Res Ther 11:123
Wang Y, Gao D, Liu Y, Guo X, Chen S, Zeng L, Ma J, Zhang X, Tian Z, Yang Z (2021) Immunogenic-cell-killing and immunosuppression-inhibiting nanomedicine. Bioact Mater 6:1513–1527
Wei W, Hu H, Tan H, Chow LW, Yip AY, Loo WT (2012) Relationship of CD44+CD24−/low breast cancer stem cells and axillary lymph node metastasis. J Transl Med 10(Suppl 1):S6
Weigand S, Herting F, Maisel D, Nopora A, Voss E, Schaab C, Klammer M, Tebbe A (2012) Global quantitative phosphoproteome analysis of human tumor xenografts treated with a CD44 antagonist. Cancer Res 72:4329–4339
Weigel PH (2015) Hyaluronan synthase: the mechanism of initiation at the reducing end and a pendulum model for polysaccharide translocation to the cell exterior. Int J Cell Biol 2015:367579
Wiranowska M, Ladd S, Moscinski LC, Hill B, Haller E, Mikecz K, Plaas A (2010) Modulation of hyaluronan production by CD44 positive glioma cells. Int J Cancer 127:532–542
Wu J, Deng C, Meng F, Zhang J, Sun H, Zhong Z (2017) Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J Control Release 259:76–82
Xiao Z, Wan J, Nur AA, Dou P, Mankin H, Liu T, Ouyang Z (2018) Targeting CD44 by CRISPR-Cas9 in multi-drug resistant osteosarcoma cells. Cell Physiol Biochem 51:1879–1893
Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG, Wu K (2015) The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther 8:3783–3792
Xu J, Zhang Y, Xu J, Wang M, Liu G, Wang J, Zhao X, Qi Y, Shi J, Cheng K, Li Y, Qi S, Nie G (2019) Reversing tumor stemness via orally targeted nanoparticles achieves efficient colon cancer treatment. Biomaterials 216:119247
Xu K, Yao H, Fan D, Zhou L, Wei S (2021) Hyaluronic acid thiol modified injectable hydrogel: synthesis, characterization, drug release, cellular drug uptake and anticancer activity. Carbohydr Polym 254:117286
Yaghobi Z, Movassaghpour A, Talebi M, Abdoli Shadbad M, Hajiasgharzadeh K, Pourvahdani S, Baradaran B (2021) The role of CD44 in cancer chemoresistance: A concise review. Eur J Pharmacol 903:174147
Yakkundi A, McCallum L, O’kane A, Dyer H, Worthington J, McKeen HD, McClements L, Elliott C, McCarthy HO, Hirst DG, Robson T (2013) The anti-migratory effects of FKBPL and its peptide derivative, AD-01: regulation of CD44 and the cytoskeletal pathway. PLoS One 8:e55075
Yamaguchi Y, Yamamoto H, Tobisawa Y, Irie F (2019) TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol 78-79:139–146
Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y (2017) A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem 292:7304–7313
Yan Y, Li Z, Kong X, Jia Z, Zuo X, Gagea M, Huang S, Wei D, Xie K (2016) KLF4-mediated suppression of CD44 Signaling negatively impacts pancreatic cancer stemness and metastasis. Cancer Res 76:2419–2431
Yang X, Sarvestani SK, Moeinzadeh S, He X, Jabbari E (2013) Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PLoS One 8:e59147
Yang Y, Zhao X, Li X, Yan Z, Liu Z, Li Y (2017) Effects of anti-CD44 monoclonal antibody IM7 carried with chitosan polylactic acid-coated nano-particles on the treatment of ovarian cancer. Oncol Lett 13:99–104
Yao H, Sun L, Li J, Zhou X, Li R, Shao R, Zhang Y, Li L (2020) A novel therapeutic siRNA nanoparticle designed for dual-targeting CD44 and Gli1 of gastric cancer stem cells. Int J Nanomedicine 15:7013–7034
Yasothamani V, Karthikeyan L, Shyamsivappan S, Haldorai Y, Seetha D, Vivek R (2021) Synergistic effect of photothermally targeted NIR-responsive nanomedicine-induced immunogenic cell death for effective triple negative breast cancer therapy. Biomacromolecules 22:2472–2490
Yasuda M, Tanaka Y, Fujii K, Yasumoto K (2001) CD44 stimulation down-regulates Fas expression and Fas-mediated apoptosis of lung cancer cells. Int Immunol 13:1309–1319
Yoo J, Rejinold NS, Lee D, Noh I, Koh WG, Jon S, Kim YC (2020) CD44-mediated methotrexate delivery by hyaluronan-coated nanoparticles composed of a branched cell-penetrating peptide. ACS Biomater Sci Eng 6:494–504
Yoshida H, Okada Y (2019) Role of HYBID (hyaluronan binding protein involved in hyaluronan depolymerization), alias KIAA1199/CEMIP, in hyaluronan degradation in normal and photoaged skin. Int J Mol Sci 20:5804
Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S (2013a) KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci U S A 110:5612–5617
Yoshida H, Nagaoka A, Nakamura S, Sugiyama Y, Okada Y, Inoue S (2013b) Murine homologue of the human KIAA1199 is implicated in hyaluronan binding and depolymerization. FEBS Open Bio 3:352–356
Yu Q, Stamenkovic I (1999) Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 13:35–48
Zada AA, Singh SM, Reddy VA, Elsasser A, Meisel A, Haferlach T, Tenen DG, Hiddemann W, Behre G (2003) Downregulation of c-Jun expression and cell cycle regulatory molecules in acute myeloid leukemia cells upon CD44 ligation. Oncogene 22:2296–2308
Zhang S, Wu CC, Fecteau JF, Cui B, Chen L, Zhang L, Wu R, Rassenti L, Lao F, Weigand S, Kipps TJ (2013) Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc Natl Acad Sci U S A 110:6127–6132
Zhang D, Jia H, Wang Y, Li WM, Hou YC, Yin SW, Wang TD, He SX, Lu SY (2015) A CD44 specific peptide developed by phage display for targeting gastric cancer. Biotechnol Lett 37:2311–2320
Zhang D, Jia H, Li W, Hou Y, Lu S, He S (2016) Screening and identification of a phage display derived peptide that specifically binds to the CD44 protein region encoded by variable exons. J Biomol Screen 21:44–53
Zhao C, Thompson BJ, Chen K, Gao F, Blouw B, Marella M, Zimmerman S, Kimbler T, Garrovillo S, Bahn J, Huang L, Huang Z, Shepard HM, Rosengren S, Thanos CD, Maneval DC (2019) The growth of a xenograft breast cancer tumor model with engineered hyaluronan-accumulating stroma is dependent on hyaluronan and independent of CD44. Oncotarget 10:6561–6576
Zhao Y, Wang K, Zheng Y, Zeng X, Lim YC, Liu T (2020) Co-delivery of salinomycin and curcumin for cancer stem cell treatment by inhibition of cell proliferation, cell cycle arrest, and epithelial-mesenchymal transition. Front Chem 8:601649
Zhong Y, Meng F, Deng C, Mao X, Zhong Z (2017) Targeted inhibition of human hematological cancers in vivo by doxorubicin encapsulated in smart lipoic acid-crosslinked hyaluronic acid nanoparticles. Drug Deliv 24:1482–1490
Zhong W, Pang L, Feng H, Dong H, Wang S, Cong H, Shen Y, Bing Y (2020) Recent advantage of hyaluronic acid for anti-cancer application: a review of “3S” transition approach. Carbohydr Polym 238:116204
Zhou L, Sheng D, Deng Q, Wang D, Liu S (2018) Development of a novel method for rapid cloning of shRNA vectors, which successfully knocked down CD44 in mesenchymal triple-negative breast cancer cells. Cancer Commun (Lond) 38:57
Zhu C, Zhang H, Li W, Luo L, Guo X, Wang Z, Kong F, Li Q, Yang J, Du Y, You J (2018) Suppress orthotopic colon cancer and its metastasis through exact targeting and highly selective drug release by a smart nanomicelle. Biomaterials 161:144–153
Zoller M (2011) CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 11:254–267
Zoller M (2015) CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front Immunol 6:235
Zoltan-Jones A, Huang L, Ghatak S, Toole BP (2003) Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem 278:45801–45810
Zou L, Song X, Yi T, Li S, Deng H, Chen X, Li Z, Bai Y, Zhong Q, Wei Y, Zhao X (2013) Administration of PLGA nanoparticles carrying shRNA against focal adhesion kinase and CD44 results in enhanced antitumor effects against ovarian cancer. Cancer Gene Ther 20:242–250
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Karalis, T.T., Skandalis, S.S. (2022). Exploiting Hyaluronan-CD44 Network in Tumor Therapy. In: Kovalszky, I., Franchi, M., Alaniz, L.D. (eds) The Extracellular Matrix and the Tumor Microenvironment. Biology of Extracellular Matrix, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-99708-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-99708-3_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99707-6
Online ISBN: 978-3-030-99708-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)