Abstract
Boric acid and sodium borates have been considered as being “toxic to reproduction and development”, following results of animal studies with high doses. Experimentally, a NOAEL (no observed adverse effect level) of 17.5 mg B/kg-bw/day has been identified for the (male) reproductive effects of boron in a multigeneration study of rats, and a NOAEL for the developmental effects in rats was identified at 9.6 mg B/kg-bw/day. These values are being taken as the basis of current EU safety assessments. The present study was conducted to investigate the reproductive effects of boron exposure in workers employed in boric acid production plant in Bandırma, Turkey. In order to characterize the external and internal boron exposures, boron was determined in biological samples (blood, urine, semen), in workplace air, in food, and in water sources. Unfavorable effects of boron exposure on the reproductive toxicity indicators (concentration, motility, morphology of the sperm cells and blood levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and total testosterone) were not observed. The mean calculated daily boron exposure (DBE) of the highly exposed group was 14.45 ± 6.57 (3.32–35.62) mg/day. These human exposures represent worst-case exposure conditions to boric acid/borates in Turkey. These exposure levels are considerably lower than exposures, which have previously led to reproductive effects in experimental animals. In conclusion, this means that dose levels of boron associated with developmental and reproductive toxic effects in animals are by far not reachable for humans under conditions of normal handling and use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron-based compounds have reached widespread industrial and commercial use, and the demand is increasing rapidly. Turkey holds the world’s largest boron deposits and is one of the largest manufacturers of boric acid and sodium borates, world-wide. The boron industry is located in the South Marmara Region of Turkey. Occupational boron exposures are unavoidable for employees working in boron mining areas and in processing plants. Because of matters of geology, boron concentrations in the drinking water may be very high in residential areas close to mining areas. For instance, Sayli et al. (1998) have reported boron concentrations between 8.50 and 29.00 mg/L in the drinking water of some villages in the vicinity of Bigadiç, Turkey. People living in such boron-rich areas have been exposed to high amounts of boron for many years. Therefore, the determination of a safe daily boron intake level is of great importance for public health (Degen and Hengstler 2008).
Boron has recently been addressed to be an important nutrient in humans (Deviran and Volpe 2003; Nielsen 2008). A Tolerable Daily Intake (TDI) for humans was previously evaluated by WHO to be 0.4 mg/kg (IPCS 1998), in accordance with an earlier risk assessment of Murray (1995). Boron is an essential element for plants, and there is growing evidence that it is also essential for humans. Boron appears to be required for bone and joint functions, possibly via effects on the balance and absorption of calcium, magnesium, and phosphorus (McCoy et al. 1994). A boron deficiency may affect calcium and magnesium metabolism, influencing the composition, structure, and strength of the bone, and finally leading to changes similar to osteoporosis (Nielsen 1994).
Recommended daily intake levels have not been established for boron. However, based on adverse effects noted in animal studies, safe boron intake levels were evaluated by international organizations and official bodies such as IEHR, ECETOC, IPCS, WHO, NAS FNB, UK EVM, USEPA (Murray and Schlekat 2004, Jensen 2009). Although a common NOAEL (9.6 mg B/kg/day) was used as point of departure in the current health risk assessments of boron, the finally calculated safe daily boron intake values (0.16–0.4 mg B/kg-bw) show considerable variability (Murray and Schlekat 2004, Jensen 2009). This is mainly due to the choice of different chemical-specific adjustment factors. Therefore, the recommended safe daily boron intake values are not fully compatible internationally (Murray and Schlekat 2004). Recent epidemiological studies have provided additional data for the risk assessment of boron and for the evaluation of safe boron levels in humans (Yazbeck et al. 2005, Xing et al. 2008). Available epidemiological studies, notably from China, show that boron exposures had no adverse effect on reproductive toxicity biomarkers in occupationally boron-exposed workers (Xing et al. 2008, Robbins et al. 2010). These studies are consistent with, and supported by, the studies on environmentally exposed populations (Sayli et al. 1998, Sayli 1998, 2001, 2003).
The present study has been conducted to provide an independent data set concerning a possible relationship between chronic boron intake and reproductive toxicity outcomes in male workers, which is a major issue of current regulatory concern (ECHA 2008).
Materials and Methods
The project plan was reviewed by Ankara University and Hacettepe University, Ankara, Turkey, and was finally approved by the Ethics Committee of the School of Medicine, Hacettepe University, (HEK 08/167, 22.10.2008). All study subjects gave their informed consent prior to participation in the project.
Description of the study area
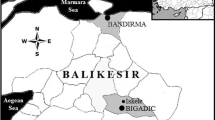
The study was conducted at the Boric Acid Plant, Bandırma, Turkey. Bandırma is located at the south coast of the Marmara Sea, distant from the boron mining areas. The Bandırma district serves as a production and exportation zone for boric acid and sodium borates. The boric acid production zone was the main study area of the present project. Boron-exposed workers and most of the control workers were recruited from this zone (Fig. 1). In addition, 28 control workers were from a sulfuric acid production plant located at the Marmara Sea. All workers from the boric acid/borax and sulfuric acid production plants worked for the same mining company. Therefore, the same shift schedule applied to all workers. Production continued 24 h per day and 7 days per week in both the boric acid and sulfuric acid plants. For the entire workforce, lunch and dinner was provided by the central cafeteria located in the boric acid production zone. Breakfast was not available in this central cafeteria. The meals (lunch and dinner) prepared in the central cafeteria were also transported to the sulfuric acid production plant, the route is indicated in map II of Fig. 1. Therefore, the sulfuric acid plant workers had been eating the same meals for lunch and dinner as the boric acid production workers.
I Boric acid production zone, Bandırma. 1 boric acid and borax production areas, 2 steam power plant. 3 DWP unit. 4 energy supply unit, laboratories, construction service. 5 mechanical workshop. 6 garage, steelyard. 7 administrative building (office). 8 infirmary. 9 central cafeteria. 10 sulfuric acid plant (map II). A static ambient air monitoring points (indoor sampling: 2A, 3A, 5A, 10A outdoor sampling: 4A, 6A, 7A, 9A). P personal air monitoring was performed for the workers working in this area. W tap water sampling points. II Bandırma, Bandırma port, and surrounding area. I boric acid production zone. II sulfuric acid plant. WS-I fresh water sampling point (Tatlısu fresh water source). WS-II fresh water sampling point (located at the center of Bandırma). WS-III tap water sampling point (guesthouse representative of the Bandırma city water). Black line Transportation road of the meals from the central cafeteria to the sulfuric acid plant
Description of the cohort
All workers, wishing to participate, were enrolled. They agreed to provide the biological samples and to complete a questionnaire with demographic, exposure, reproductive and general health information. The total number of workers (male) working in production of boron products was 428. One hundred and two of them participated in the study. The occupationally exposed workers consisted of boric acid [B(OH)3] production workers (n = 57), borax [Na2B4O7·10H2O] production workers (n = 31), “sodium perborate” production unit workers (n = 5), boric acid plus borax production workers [mixed area] (n = 5), laboratory workers (n = 2), a storage worker (n = 1), and a mechanic technician (n = 1). Actually, the sodium perborate production had been ceased in this plant 4 years ago. After technical adaptations, the sodium perborate production unit had been transformed into a borax production unit. Therefore, it was borax that was actually produced in this unit. Nevertheless, the traditional name of this transformed production unit had not been changed. In the packaging unit, workers were working for an independent contractor. These were seasonal workers, which were replaced every 3 months. These workers were not under the responsibility of the mining company. Therefore, they were not included in the study.
The total number of workers (male) working in the same zone, but outside of the production area of boron products and not occupationally exposed to boron, was 432. One hundred and two of them participated in the study as the control workers. These workers consisted of sulfuric acid workers (n = 28), steam power plant workers (n = 17), energy suppliers (n = 11), workers in mechanical workshop (n = 19), garage, and steelyard workers (n = 16), demineralized water production (DWP) workers (n = 2), construction workers (n = 3), laboratory technicians (n = 3), and officers (n = 3).
Biological sampling
Urine, blood, and semen samples were obtained from both occupationally boron-exposed and control workers. The details of specimen collection and of the analytical methods are given in the Electronic Supplementary Material (Annex I).
Briefly, post-shift urine samples were collected in polypropylene containers and kept at −20°C until urine and creatinine analysis. Blood was collected by venipuncture in suitable blood collection tubes. One tube was left to determine the boron concentrations in blood samples. The tubes containing clot activator (BD vacutainer, SST II Plus plastic tubes) were sent immediately to the Bandırma State Hospital for the determination of follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone, and prostate-specific antigen (PSA) levels. These parameters were determined using an Immulite 2000 Immunoassay Analyzer. Semen samples were collected in accordance with the recommendations of the World Health Organization (WHO 2003). The workers were informed about the importance of an abstinence for at least 48 h (but no longer than 7 days) prior to providing the semen samples. Sperm concentration and motility parameters were determined by using SQA-V Gold Sperm Quality Analyzer. Additionally sperm morphology was also analyzed as per the Kruger’s strict criteria (Kruger et al. 1986, 1988) by one experienced technician at the Research Center on Fertility, School of Medicine, University of Ankara.
Air, water, and food sampling
Personal air monitoring was performed using a personal air sampler (SKC, AirCheck 2000) for the exposed workers working in boric acid and borax production. The flow rate was 2 L/min, and the sampling time was 8 h. Low-ash PVC filters (SKC, 5 μm, 37 mm, pre-weighed) and SureSeal cassettes (SKC, 37 mm) were used in a closed-faced manner to assess the inhalable dust. The above-mentioned personal sampling devices and filters were also used for the measurement of the inhalable dust at the workplace (static sampling) of the control workers. The static sampling points are shown in map I and II of Fig. 1.
Boron concentrations in the main water sources of the Bandırma city were determined to assess the possible contribution of drinking water to the daily boron exposure. Fresh water samples from Tatlısu (a village in the vicinity of Bandırma), fresh water from Bandırma (city center), and Bandırma city water (tap water) were taken to determine the boron concentrations. Tap water samples were also taken from the central cafeteria and the infirmary located in the boric acid production zone. The water sampling points are shown in map I and II of Fig. 1. Boron concentrations determined in these water samples are shown in Table 1.
Food samples were taken from the central cafeteria located at the boric acid production zone. All common food types in the central cafeteria were sampled and analyzed. The boron concentrations determined in air, water, and foods were used to calculate the daily boron exposure (DBE) levels for the workers. The boron concentrations in sampled air and foods and the details about the calculation methodology of the DBE levels of the workers were described in the Electronic Supplementary Material (Annex II).
Boron analysis
Blood, semen, and food samples were analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) with a flow injection system. Urine, dust, and water samples were analyzed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Standard reference materials were used for quality control. The details of the method are described in the Electronic Supplementary Material (Annex I).
Statistical analysis
The data are presented separately for the four re-constituted groups (Table 2) and are summarized by the mean, the empirical standard deviation, and the range. Moreover, boxplots, Pearson’s correlation coefficient and linear regressions as shown in Figs. 2 and 3 display the empirical distributions and possible linear dependencies.
Calculated daily boron exposure (DBE), urine boron, and semen boron levels versus increasing blood boron levels. Box plot graphs; Chart A and B The mean DBE levels and the mean urine boron levels in low, medium, and high-exposure groups were significantly higher than the control (P < 0.05). Chart C The mean semen concentrations in medium and high-exposure groups were significantly higher than the control group (P < 0.05). Scatter plot graphs; Linear regression with 95% individual prediction interval. Chart I and II Blood boron levels show a reasonably high correlation with the calculated DBE levels (Pearson’s corr. coeff.: 0.635) and urine boron levels (Pearson’s corr. coeff.: 0.633) in workers. Chart III The correlation between blood boron concentration and semen boron concentration is weak (Pearson’s corr. coef.: 0.222)
Hormone levels versus increasing blood boron levels. Box plot graphs; open circle: outliers, filled circle: extremes. The mean FSH level in high exposed group is not significantly higher than the control group (P > 0.05). The mean LH level in high-exposure group is significantly higher than the medium exposure group (P < 0.05). The mean total testosterone levels in exposure groups are statistically not different from the control group (P > 0.05). Scatter plot graphs; Linear regression with 95% individual prediction interval. The Blood boron concentrations are weakly correlated with FSH (Pearson corr. coeff.: 0.143), LH (Pearson corr. coef.: 0.164), and total testosterone levels (Pearson corr. coeff.: −0.053) in workers
In order to investigate the global hypotheses that the means of the four groups are equal, a Kruskal–Wallis test was used for all variables in Tables 3, 4, 5, 6, 7, and 8 except for the categorical variables pH and cytoplasmic droplets. If the resulting P value was significant (P < 0.05), i.e., if the hypothesis of equal group means was rejected, the two-sided Mann–Whitney U test was applied to find distinct differences between each of the pairs of groups. The corresponding six P values were adjusted with the Bonferroni-Holm method for each variable in order to account for the problem of multiple testing (Holm 1979). These nonparametric tests were deliberately used as some of the variables show outliers. The dependence of the groups and the categorical variables (pH and cytoplasmic droplets) were analyzed with Fisher’s exact test. All statistical tests were performed with R, a language and environment for statistical computing, version 2.11.1 (R Development Core Team 2010). The local as well as the multiple significance levels of the tests were set at 0.05. For theoretical details on the nonparametric Kruskal–Wallis and Mann–Whitney U tests as well as Fisher’s exact test, see Conover (1999).
Results
The results of the present study represent the situation of boron exposure of workers working in the Bandırma boric acid production zone and sulfuric acid plant in 2009 (Fig. 1). When planning the study, the workers from the production area of boric acid and borax were addressed as “exposed workers”. The “control workers”, not occupationally exposed to boron, consisted of workers from the neighboring sulfuric acid plant, steam power plant, energy supply unit, demineralized water production unit (DWP), mechanical workshop, garage, steelyard, construction service, laboratory, and office. The locations of these working units and plants are shown in Fig. 1.
According to the preliminary results of this study, boron concentration was lower than the limit of quantification (LOQ) in Bandırma City and fresh water sources (Table 1). The water used in the boric acid production area was supplied by the Bandırma municipality, therefore representing the Bandırma City water. The surprising result of this investigation was that high concentrations of boron were present in the tap water taken from the central cafeteria (9.47 mg/L) and the infirmary (9.54 mg/L), both located close to the boric acid production area (Fig. 1, Table 1). The high boron concentration in water obviously reflected an environmental contamination within the boric acid production zone. Therefore, there was both occupational and environmental boron exposure of the workers assigned to the occupationally exposed group. More importantly, unexpected environmental boron exposure occurred also for the control workers (not occupationally exposed) through the cafeteria’s water and meals (lunch/dinner). The workers employed in the sulfuric acid plant (initially assigned as the remote control group) were also exposed to certain amounts of boron by lunch (Fig. 1). Therefore, all participating workers were re-classified both according to their calculated daily boron exposure levels and to the blood boron levels.
The mean DBE, blood, urine, and semen boron levels of the original control (n = 102) and exposed group (n = 102) are presented in the Electronic Supplementary Material (Annex I). The biological outcomes versus increasing calculated daily boron exposure levels were also evaluated and are comprehensively compiled in the Electronic Supplementary Material (Annex II). The biological outcomes of the re-classified workers versus the blood boron levels are evaluated in this main part of the report.
Blood boron levels reported in the earlier epidemiological studies were taken into consideration when re-constituting the low, medium, and high-exposure groups (Yazbeck et al. 2005, Xing et al. 2008). Workers with blood boron concentrations lower than the limit of quantification (LOQ) of the method (48.5 ng/g) were re-assigned as the control workers. The re-constituted control and exposure groups are shown in Table 2.
The mean ages of the re-constituted exposure groups are statistically not different (P > 0.05) from the mean age of the control group as shown in Table 3. The calculated mean DBE and mean urine boron concentrations of the workers in the low, medium, and high exposed groups are significantly higher (P < 0.05) than those of the control group. The significant increase of calculated DBE levels and urine boron concentrations versus increasing blood boron levels are presented in the related box plots and scatter plots of Fig. 2. Blood boron concentrations of the workers are correlated with the calculated DBE levels (Pearson corr. coeff.: 0.635) and urine boron concentrations (Pearson corr. coeff.: 0.633).
The mean semen boron concentrations of the exposed groups were also significantly higher (P < 0.05) than the control group (Table 3). However, the increase of the mean semen boron concentrations was not significant (P > 0.05) throughout the exposure groups. The correlation between semen boron concentrations and blood boron concentrations was very low (Pearson corr. coeff.: 0.222) (Fig. 2).
The mean FSH, LH, and total testosterone levels in the re-constituted exposure groups are statistically not different (P > 0.05) from those of the control group, and the blood boron concentrations were at most weakly correlated with the FSH (Pearson’s corr. coeff.: 0.143), LH (Pearson’s corr. coeff.: 0.164), and total testosterone levels (Pearson corr. coeff.: −0.053) of the workers (Table 4, Fig. 3). On the other hand, the mean LH level in the high-exposure group was significantly higher (P < 0.05) than that in the medium exposure group. As it is known, the testosterone level in men reaches its highest level in the morning between 08.00 and 10.00 a.m. However, blood was drawn from the workers at the end of the work shift in this study. Therefore, the mean total testosterone levels were compared also throughout the shifts (Table 5). The mean total testosterone levels were not statistically different (P > 0.05) between the shifts.
Some epidemiological (Barranco et al. 2007) and experimental (Barranco et al. 2004, 2006, 2009; Gallardo-Williams et al. 2004) findings indicate that boron may have a favorable effect regarding prostate cancer. Therefore, total PSA levels have been determined in all workers, resulting in normal findings. The mean total PSA levels in the re-constituted control and exposure groups, varying from 1.18 ± 0.62 to 1.25 ± 0.65 ng/mL, were not significantly different (Table 4).
Sperm concentration and motility parameters have been determined to investigate possible unfavorable influences of boron exposure on fertility. The sperm concentration parameters are compiled in Table 6. Statistically significant difference was not observed between the control and exposed groups (P > 0.05). The sperm motility parameters are shown in Table 7. A statistically significant difference in mean levels of the motility parameters was not observed throughout the control and exposure groups.
The morphology of the sperm cells was analyzed by an expert technician according to the Kruger’s strict criteria (Kruger et al. 1986, 1988). Accordingly head, neck/mid-piece, tail, and cytoplasmic abnormalities of sperm cells were scored by light microscopy, and the results were subjected to statistical analysis (Table 8). Statistically significant differences in mean percentages of these analyzed defects were not detected between the control and exposure groups (P > 0.05).
The percentage of the sperm cells with normal morphology was also determined in semen specimens as per Kruger’s criteria. Accordingly, 14% of the sperm cells with normal morphology were considered as the lower acceptable limit. The mean percentage of the sperm cells with normal morphology was higher than 14 in the low, medium, and high-exposure groups (Table 8). However, it was slightly lower than 14% in the control group. Additionally, the mean percentage of the sperm cells with normal morphology in high-exposure group was the highest (without statistical significance, P > 0.05) throughout the exposure groups (Table 8). These results could even suggest a beneficial effect of boron exposure on sperm morphology. However, the correlation between the percentages of the normal morphology and blood boron levels was very weak (Pearson corr. coeff.: 0.131).
Discussion
One of the reasons for the choice of Bandırma as the study area was its distance from the boron mineral deposits and an expected low environmental compared to possible occupational exposure. However, the unexpected finding of high boron concentrations in the central cafeteria’s drinking water (9.47 mg/L) required an assessment of both occupational and environmental exposures through food and water. Therefore, all subjects were re-classified according to the blood boron concentrations of the workers. The re-constituted control, low, medium, and high-exposure groups enabled to investigate a possible dose-dependence of the reproductive toxicity parameters.
Inhalable air sampling was performed by using a 37-mm closed-face cassette (CFC), because of simplicity in handling during field operations and the lower risks of contamination during handling (Demange et al. 2002). This is a commonly used aerosol sampler, despite its recognized low sampling efficiency for particles >30 μm (Li et al. 2000). Granulometric measurement has not been performed in this study, but the type of processing of boron materials was known to generate particles with aerodynamic diameters (D ae) of less than 30 μm (Culver et al. 1994). The efficiency of this aerosol sampler is comparable with the IOM sampler under these conditions, as reported in recent studies (Li et al. 2000; Demange et al. 2002).
The mean levels of inhaled boron (mg/8 h) in medium and high-exposure group were significantly higher (P < 0.05) than the control group. However, the difference between control and low exposure group was statistically not different (P > 0.05) (Table 3). The Pearson correlation coefficients were quite low between inhaled boron (mg/8 h)–blood boron (0.279), inhaled boron–semen boron (0.185), and inhaled boron–urine boron (0.106) levels. These relatively low values of correlation coefficients may reasonably be attributed to the influence of the additional environmental contribution by contamination of the cafeteria’s water supply.
As expected, the highest mean levels of the calculated DBE, urine boron, and semen boron were determined in the high-exposure group. The difference of mean urine boron and mean DBE level was statistically significant between the exposure groups both indicating an increase with increasing exposure (Table 3, Fig. 2). However, the same steady increase was not observed for the semen boron levels. The mean boron concentrations of semen samples in the medium and high-exposure groups were significantly higher than in the control group. However, the mean boron concentrations in the medium and high-exposure groups were statistically not different from each other (Table 3, Fig. 2).
The boron concentrations determined in semen samples of the workers were ~20, ~12, and ~8 times higher than the mean blood boron concentrations in low, medium, and high exposed groups, respectively (Table 3). This points to an accumulation of boron in human semen over blood levels. In spite of these high boron concentrations in seminal fluid, the percentage of sperm cells with “normal morphology” was highest in the high-exposure group (Table 8). An accumulation of boron in seminal fluid was also reported by Robbins et al. (2010). This finding may be of relevance for studies on a preventive role of boric acid on prostate cancer, as boric acid has been reported to reduce the risk of prostate cancer, tumor growth, and prostate cancer cell proliferation in humans (Barranco and Eckhert 2006, Barranco et al. 2009).
In a study on 898 Turkish volunteers from the Kozlu Zonguldak area, participating in a prostate cancer screening program, the mean total PSA levels increased from 1.6 ng/mL (SD ± 1.8; median 1.0, 0.2–7.6) in 30–39-year-old men to 4.3 ng/mL (SD ± 5.7; median 2.0 ng/mL, 0–42.7) in 60–69-year-old men with benign findings (Mungan et al. 2007). A smaller study on 255 Turkish volunteers from a small village in the Aegean region of Turkey, also participating in prostate cancer screening, provided mean total PSA levels from 1.45 ng/mL (95% CI 0.66–2.55; median 0.74) in the 40–49-year-old men to 1.64 ng/mL in the 60–69-year-old men (95% CI 1.16–2.11; median 1.00) (Müezzinoglu et al. 2005). In these two studies and in the present study, as expected, PSA levels increased with age (total PSA = 0.022 × age [years] + 0.3067). The total PSA levels in the present study are comparable with the above-mentioned second study. In spite of the different results of the two Turkish studies, it must be considered that PSA levels are difficult to compare because the reference values are differing from manufacture to manufacture of the reagent (Kort et al. 2006) and may differ with ethnicity (Kehinde et al. 2005). Furthermore, PSA levels may be affected by other factors. For example, lower PSA levels were reported in obese white non-Hispanic men, compared to those with a normal body mass index (OR 0.54; 95% CI 0.31–0.91). Nevertheless, this effect was not observed in African-American or Hispanic men (Culp and Porter 2009). Therefore, the most robust information from the determined total PSA levels in this study is that total PSA levels did not significantly differ between the control, low, medium, and high-exposure groups.
The calculated DBE and urine boron levels of the workers show a reasonably high correlation (Pearson’s corr. coef.: 0.635 and 0.633, respectively) with the blood boron levels (Fig. 2). The weakest correlation was found between the semen boron concentrations and blood boron concentrations of the workers (Pearson’s corr. coeff. = 0.222). A comparable correlation (Pearson’s corr. coeff.: 0.85) between DBE and boron levels in blood samples has also been reported in occupationally boron-exposed Chinese workers (Xing et al. 2008). Thus, our results are in general compatible with the results of the Chinese study.
Effects of boric acid on the endocrine regulation of the reproductive system have been studied in experimental animals (Ku et al. 1993, Fail et al. 1998). Decreases in testosterone and increases in FSH and LH levels have been reported in rats under continuous oral exposure (9,000 ppm in the diet). Increases in FSH levels in rats have been reported with 4,500 ppm boric acid in the diet. These studies in experimental animals show that boric acid could be toxic to endocrine system at very high concentrations (Ku et al. 1993). Such effects of boron compounds (boric acid and/or sodium borates) on the human endocrine system have not been studied so far. In the present study, total testosterone, FSH, and LH values of the workers have been determined and evaluated statistically versus increasing blood boron levels (Fig. 3, Table 4). The only statistically significant difference (P < 0.05) was observed for the mean LH levels between medium and high-exposure groups (Table 4). However, a steady increase in mean LH levels was not observed throughout the exposure groups (Fig. 3). In spite of the presence of the person with the highest LH value (20 mIU/mL) in the high-exposure group, the difference of mean LH values between control and high-exposure group was statistically not significant (P > 0.05). Additionally, the correlation between the LH levels and blood boron levels was very low (Pearson corr. coeff.: 0.164).
Based on animal experiments, reproductive toxicity effects of boron are pivotal for setting regulatory exposure limits for boron. Depressed testicular weight and testes atrophy (linked with an effect on spermatogenesis) have been reported as the most sensitive endpoints of reproductive toxicity in rats (Ku et al. 1993). However, these toxic effects of boric acid have not been found in reliable human studies so far. The first comprehensive study on semen parameters has been published by Robbins et al. (2010) in highly boron-exposed Chinese workers. According to the results of this study, the semen parameters (total sperm count, sperm concentration, motility, morphology, DNA breakage, apoptosis, and aneuploidy) of the boron-exposed workers were not adversely affected with increasing boron exposure. In spite of extremely high average blood boron levels (mean: 499.2 ppb [ng/g]), statistically significant correlations between blood boron and adverse effects on semen parameters were not reported (Xing et al. 2008, Robbins et al. 2010). The present study, independent of the first, is the second to evaluate semen parameters versus increasing blood boron levels in boron-exposed workers. The results of the quantitative data are shown in Table 6 and Table 7. Statistically significant differences in mean levels of sperm concentration (Table 6) and sperm motility parameters (Table 7) could not be determined between the control and exposure groups. In spite of the lack of a statistical significance, the motility parameters are tending to increase with increasing blood boron levels. In addition, the mean percentage of immotility was the lowest in the high-exposure group, but without a statistical significance (P > 0.05).
Sperm morphology has also been evaluated as per Kruger’s strict criteria. Accordingly, a sperm cell without defects (head, neck, midpiece, tail, cytoplasmic droplests) was considered as “normal” (Table 8). The percentage of the sperm cells with normal morphology and morphological defects are shown in Table 8. Statistically significant differences in the percentages of head, neck/midpeace, tail defects, and cytoplasmic droplets were not determined between the control and exposure groups (P > 0.05). On the other hand, when comparing the mean percentages of the sperm cells with normal morphology between exposure groups, it was the highest in the high-exposure group without a statistical significance (P > 0.05). This result may even suggest a beneficial effect of certain level of boron exposure on maintaining the normal sperm morphology. However, the correlation between blood boron levels and percentages of the sperm cells with normal morphology was very weak (Pearson’s corr. coeff.: 0.131).
All these results indicate that boron had no adverse effects on FSH levels, LH levels, total testosterone levels, sperm concentration parameters, motility parameters, and sperm morphology of workers, under the exposure conditions prevailing in Bandırma.
Studies in experimental animals have been fundamental to classify boric acid and sodium borates as being toxic to male fertility and development. However, the results of the published environmental and occupational studies do not support the outcomes of the animal studies. The doses tested in animal studies are possibly the major reason of this discrepancy. For instance, mild inhibited spermiation, followed by atrophy of the testes in rats was seen after administration of boric acid in the feed for up to 9 weeks at daily doses of 26 mg boron/kg/day and 52 mg boron/kg/day, respectively (Ku et al. 1993). These daily boron intake levels are not reached by boron-exposed humans, even under extreme worst-case conditions (Xing et al. 2008, Robbins et al. 2010).
In contrast to animal experiments, an association between boron exposure and toxic effects on reproduction and/or development have not been identified in environmental and occupational epidemiology studies so far (Xing et al. 2008, Robbins et al. 2010, Scialli et al. 2010, Sayli et al. 1998, Sayli 1998, 2001, and 2003). These studies consistently show that it was not possible to exceed safe blood boron concentrations during normal activities and even under exaggerated exposure conditions for humans (Culver et al. 1994, Yazbeck et al. 2005, Xing et al. 2008, Robbins et al. 2010). In this context, our study is in agreement with the previously published studies. Consequently, it appears that the doses associated with developmental and reproductive toxic effects in animals are by far not reachable for humans under normal handling and use conditions.
References
Barranco WT, Eckhert CD (2004) Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett 216(1):21–29
Barranco WT, Eckhert CD (2006) Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br J Cancer 94:884–890
Barranco WT, Hudak PF, Eckhert CD (2007) Evaluation of ecological and in vitro effects of boron on prostate cancer risk (United States). Cancer Causes Control 18(1):71–77. Erratum in: Cancer Causes Control. 2007 18(5):583–584
Barranco WT, Kim DH, Stella SL Jr, Eckhert CD (2009) Boric acid inhibits stored Ca2 + release in DU-145 prostate cancer cells. Cell Biol Toxicol 25(4):309–320
Conover WJ (1999) Practical nonparametric statistics, 3rd edn. John Wiley, New York
Culp S, Porter M (2009) The effect of obesity and lower serum prostate-specific antigen levels on prostate-cancer screening results in American men. BJU Int 104(10):1457–1461
Culver BD, Shen PT, Taylor TH, Lee-Feldstein A, Anton-Culver H, Strong PL (1994) The relationship of blood and urine boron to boron exposure in borax workers and the usefulness of urine-boron as an exposure marker. Environ Health Perspect 102(7):133–137
Degen GH, Hengstler JG (2008) Developments in industrial and occupational toxicology: REACH, toxicogenomics, mycotoxins, lead, asbestos, boron, bitumen, deletion polymorphisms and SNP interactions. Meeting report of the 16th EUROTOX Training and Discussion Session. Arch Toxicol 82:483–487
Demange M, Görner P, Elcabache JM, Wrobel R (2002) Field comparison of 37 mm closed-faced cassettes and IOM samplers. Appl Occup Environ Hyg 17(3):200–208
Deviran TA, Volpe SL (2003) The physiological effects of dietary boron. Crit Rev Food Sci Nutr 43:219–231
ECHA [European Chemicals Agency] (2008) Annex XV Transitional reports: boric acid. Available online: http://echa.europa.eu/doc/trd_substances/boric_acid/ann_xv_trd/trd_austria_boric_acid.pdf (accessed 28.07.2010)
Fail PA, Chapin RE, Price CJ, Heindel JJ (1998) General, reproductive, developmental, and endocrine toxicity of boronated compounds. Reprod Toxicol 12(1):1–18
Gallardo-Williams MT, Chapin RE, King PE, Moser GJ, Goldsworthy TL, Morrison JP, Maronpot RR (2004) Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol 32(1):73–78
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
IPCS (1998) Envieonmenntal health criteria 204; boron. International Programme on Chemical Safety. World Health Organization (WHO), Geneva
Jensen AA (2009) Risk assessment of boron in glass wool insulation. Environ Sci Pollut Res 16:73–78
Kehinde EO, Mojiminiyi OA, Sheikh M, Al-Awadi KA, Daar AS, Al-Hunayan A, Anim JT, Al-Sumait AA (2005) Age-specific reference levels of serum prostate-specific antigen and prostate volume in healthy Arab men. BJU Int 96(3):308–312
Kort SA, Martens F, Vanpoucke H, van Duijnhoven HL, Blankenstein MA (2006) Comparison of 6 automated assays for total and free prostate-specific antigen with special reference to their reactivity toward the WHO 96/670 reference preparation. Clin Chem 52(8):1568–1574
Kruger TF, Menkveld R, Stander FSH, Lombard CJ, Van der Merwe JP, Van Zyl JA, Smith K (1986) Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril 46:1118–1123
Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S (1988) Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 49:112–117
Ku WW, Chapin RE, Wine RN, Gladen BC (1993) Testicular toxicity of boric acid (BA): Relationship of dose to lesion development and recovery in F344 rat. Reprod Toxicol 7:305–319
Li SN, Lundgren DA, Rovell-Rix D (2000) Evaluation of six inhalable aerosol samplers. Am Ind Hyg Assoc J 61:506–516
McCoy H, Kenney MA, Montgomery C, Irwin A, Williams L, Orrell R (1994) Relation of boron to the composition and mechanical properties of bone. Environ Health Perspect 102(7):49–53
Müezzinoğlu T, Lekili M, Eser E, Uyanik BS, Büyüksu C (2005) Population standards of prostate specific antigen values in men over 40: community based study in Turkey. Int Urol Nephrol 37(2):299–304
Mungan AG, Erol B, Akduman B, Bozdogan G, Kiran S, Yesilli C, Mungan NA (2007) Values for free/total prostate-specific antigen ratio as a function of age: necessity of reference validation in a Turkish population. Clin Chem Lab Med 45(7):912–916. Erratum in: Clin Chem Lab Med 45(11):1570
Murray FJ (1995) A human health risk assessment of boron (boric acid and borax) in drinking water. Reg Toxicol Pharmacol 22:221–230
Murray FJ, Schlekat CE (2004) Comparison of risk assessments of boron: alternate approaches to chemical-specific adjustment factors. Hum Ecol Risk Assess 10:57–58
Nielsen FH (1994) Biochemical and physiologic consequences of boron deprivation in humans. Environ Health Perspect 102(7):59–63
Nielsen FH (2008) Is boron nutritionally relevant? Nutr Rev 66:183–191
Robbins WA, Xun L, Jia J, Kennedy N, Elashoff DA, Ping L (2010) Chronic boron exposure and human semen parameters. Reprod Toxicol 29:184–190
Sayli BS (1998) An assessment of fertility in boron-exposed Turkish subpopulations, 2. Evidence that boron has no effect on human reproduction. Biol Trace Elem Res 66:409–422
Sayli BS (2001) Assessment of fertility among sibs and in “borate families”. Biol Trace Elem Res 81:255–267
Sayli BS (2003) Low frequency of infertility among workers in a borate processing facility. Biol Trace Elem Res 93:19–29
Sayli BS, Tüccar E, Elhan AH (1998) An assessment of fertility in boron-exposed Turkish subpopulations. Reprod Toxicol 12(3):297–304
Scialli AR, Bonde JP, Brüske-Hohlfeld I, Culver BD, Li Y, Sullivan FM (2010) An overview of male reproductive studies of boron with an emphasis on studies of highly exposed Chinese workers. Reprod Toxicol 29:10–24
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
WHO [World Health Organization] (2003) Laboratory manual for the examination and processing of human semen and sperm, 4th edn. WHO Press, Geneva
Xing X, Wu G, Wei F, Liu P, Wei H, Wang C, Xu Y, Xun L, Jia J, Kennedy N, Elashoff D, Robbins W (2008) Biomarkers of environmental and workplace boron exposure. J Occup Environ Hyg 5:141–147
Yazbeck C, Kloppmann W, Cottier R, Sahuquillo J, Debotte G, Huel G (2005) Health impact evaluation of boron in drinking water: a geographical risk assessment in Northern France. Environ Geochem Health 27:419–427
Acknowledgments
This project was funded by National Boron Research Institute (BOREN) and Eti Mine Works General Management (2008–G0207). The outline of the study and the processing of the raw data were reviewed by an external expert panel. The authors thank the reviewers, Professors G. Assennato, Bari, and M. Guillemin, Lausanne, for their scrutiny and dedicated effort.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duydu, Y., Başaran, N., Üstündağ, A. et al. Reproductive toxicity parameters and biological monitoring in occupationally and environmentally boron-exposed persons in Bandırma, Turkey. Arch Toxicol 85, 589–600 (2011). https://doi.org/10.1007/s00204-011-0692-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0692-3