Abstract
Boric acid and sodium borates are currently classified as being toxic to reproduction under “Category 1B” with the hazard statement of “H360 FD” in the European CLP regulation. This has prompted studies on boron-mediated reprotoxic effects in male workers in boron mining areas and boric acid production plants. By contrast, studies on boron-mediated developmental effects in females are scarce. The present study was designed to fill this gap. Hundred and ninety nine females residing in Bandirma and Bigadic participated in this study investigating pregnancy outcomes. The participants constituted a study group covering blood boron from low (< 100 ng B/g blood, n = 143) to high (> 150 ng B/g blood, n = 27) concentrations. The mean blood boron concentration and the mean estimated daily boron exposure of the high exposure group was 274.58 (151.81–975.66) ng B/g blood and 24.67 (10.47–57.86) mg B/day, respectively. In spite of the high level of daily boron exposure, boron-mediated adverse effects on induced abortion, spontaneous abortion (miscarriage), stillbirth, infant death, neonatal death, early neonatal death, preterm birth, congenital anomalies, sex ratio and birth weight of newborns were not observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron is an ubiquitous element within the earth crust, present in the form of inorganic borates. Boric acid, borax, borax pentahydrate and anhydrous borax are commercially important inorganic boron compounds, at a constantly increasing demand. In parallel, numbers of scientific studies on biological effects of boron compounds show a steady increase. This includes studies on human safety aspects, which are triggered by regulatory issues (Bolt et al. 2017; Khaliq et al. 2018).
While there is increasing scientific evidence for a physiological role of boron (Khaliq et al. 2018), a major regulatory focus is on the reproductive toxicity of boric acid and its salts. Experimentally, decreased foetal body weights and increased incidences of short rib XIII in rats had been reported (Price et al. 1996). The NOAEL for this effect was 9.6 mg B/kg/day, based on test doses between 3.3 and 25 mg B/kg/day. The NOAEL for reproductive effects in male rats was reported as 17.5 mg B/kg/day in a multi-generation study. In the European Regulation on Classification, Labelling and Packaging of Substances and Mixtures, dating 2007, boric acid and sodium borates have been classified as being toxic to reproduction (Category 1B, hazard statement H360 FD). Epidemiological studies were initiated in China and Turkey to investigate possible effects of boron on human reproduction. In exposed male workers, boron-mediated reprotoxic effects could not be found, even under extreme conditions of occupational exposure (Robbins et al. 2010; Duydu et al. 2011, 2016).
Epidemiological studies on reproductive boron effects in females are scarce. The present study was designed to fill this gap, investigating possible boron-mediated developmental effects in females environmentally exposed in boron-reach areas. The field study was performed in the areas of Bandirma and Bigadic (Western Anatolia, Turkey) between 2014 and 2017. Bigadic is known as a boron-rich area with high boron levels in drinking water (Sayli 1998, 2001, 2003; Korkmaz et al. 2007), while boron concentrations in the public water supply in Bandirma are relatively low, as evidenced in our previous male reproduction study (Duydu et al. 2011). It was expected that the combination of female participants from these two locations of the same geographical area would result in a broad spectrum of individual boron exposures that could allow estimations of dose–response.
The present study assessed the pregnancy outcomes in females, dependent on their individual boron exposure. A special focus was on the birth weight of newborns.
The principal determinant of individual boron exposure in the present study is the boron blood level. To characterise the exposure pathway more in detail, measurements of urinary boron excretion and assessments of individual dietary exposures were included.
Because of the high pKa of boric acid, regardless of the form of inorganic borate incorporated (boric acid, borax, boron associated with animal or plant tissues), the uptake, distribution, and excretion of inorganic borates in the urine occurs almost exclusively in the form of non-dissociated boric acid. Because of the absence of further metabolism, the toxicokinetics of boric acid are also very much comparable across species, including humans (Bolt et al. 2017).
Materials and methods
This study was approved by the Ethics committee of the Ankara University School of Medicine (no. 20-853-14, dated 08/12/2014). All participants gave their informed written consent prior to participation in the project.
Study areas
The study was conducted in the Bandirma and Bigadic districts of Balikesir. Geographically, both districts belong to the Marmara Region of Turkey (Fig. 1).
Bandirma
Bandirma is located in the south cost of the Marmara Sea, with a population of 152,480. Of these, 75,349 were females (data of 17 Oct 2017; http://www.yerelnet.org.tr/belediyeler/belediye.php?belediyeid=129256). Bandirma is a production and export zone for boric acid and for specified borates but is distant from the boron deposits and mining areas. Although significant boron exposure occurs in employees of the local boric acid production plant and the commercial port of Bandirma, environmental boron exposure is negligible for the general population living in Bandirma (see Duydu et al. 2011, 2012a, b). Therefore, females from Bandirma represent a relatively low exposed population. Hundred females from Bandirma voluntarily participated in our study. These women lived in this area since they were born.
Bigadic
The Bigadic district of Balikesir is located about 130 km south of Bandirma, at an altitude of 165 metres above sea level. This area has the largest known boron deposits in Turkey, which are specifically located around the small village of Iskele. The local boron deposits contain colemanite (2CaO·3B2O3·5H2O) and ulexite (Na2O·2CaO·5B2O3·16H2O).
Boron concentration in some drinking water sources of Iskele are much higher (12.2 mg B/L) compared to limits in current drinking water guidelines of both EU (1 mg B/L) and WHO (2.4 mg B/L) (EU Drinking Water Directive 1998; WHO 2011). This means that the residents of this village are chronically exposed to high boron via drinking water and food (Table 2).
Iskele has a total population of 1516, among these 776 females (Türkiye Nüfusu, Balikesir population 2017). Of these, 99 females voluntarily participated in our study. The participating females lived in this area since they were born.
All participating females, both in Badirma and in Bigadic, accepted to provide biological samples (blood and urine) and specimens of food from their meals (breakfast, lunch, dinner), as well as drinking water samples.
Questionnaire
Demographic information and information on pregnancy outcomes were obtained by a questionnaire survey. Included items were age, duration of marriage, preterm birth, numbers of children, birth weights of newborns, congenital anomalies, abortions, miscarriage, stillbirth, early neonatal death, neonatal death and infant death. Information on possible confounders (alcohol consumption, smoking, pesticide application) was also obtained.
This study did not include pregnant women, as pregnancy monitoring was not within the scope of the project.
Individual boron exposures were strongly dependent on the residential area of the participants. Boron concentrations in drinking water sources were periodically monitored in the study areas, at least since 20 years, by official institutions (according to records of Eti Mine Works) and universities (Sayli 1998, 2003; Korkmaz et al. 2007, 2011; Duydu et al. 2011, 2012a, b, 2016). Significant differences between earlier and actual boron concentrations in drinking water sources of both areas, Bandirma and Bigadic, were not apparent. Therefore, a major change in daily boron exposure (DBE) levels with time was not expected for the study areas. Therefore, the study was based on the assumption that individuals were chronically exposed to their area-specific level of boron.
Sampling of biological fluids, foods and drinking water
Due to the local circumstances, the sampling organisation was slightly different in Bandirma and Bigadic. In Bandirma, sampling was performed on pre-scheduled dates in the guesthouse of Eti Mine Works General Management that is located distant from both the local boric acid production plant and the commercial port. The participants were asked to bring samples of their actual meals (breakfast, lunch, dinner) and of their drinking water. Containers for food and water were provided. After completing the questionnaire, blood and urine samples were taken and stored.
In Bigadic, sampling was performed by visiting the participants at home. Again, after completing the questionnaire, blood, urine, drinking water and meal samples (breakfast, lunch and dinner) were stored.
Vein blood samples were drawn into Vacutainer® collection tubes containing heparin and stored at 4 °C for subsequent boron determination. Spot urine samples of all volunteers were collected in polypropylene containers and kept at − 20 °C until analysis of boron and creatinine. Creatinine analysis was performed using the creatinine assay kit of Cayman Chem. Corp.
Drinking water and food samples were stored in polypropylene containers at − 20 °C until boron analysis.
Air samples
Air sampling was performed at two and five different sites of Bandirma and Bigadic, respectively. The sampling sites were representative of the appropriate study area. Static air sampling was performed using IOM samplers and personal air sampling pumps (SKC, AirCheck 2000). The flow rate was 2 L/min, and the sampling time was 8 h. SKC (GLA-5000), 5-µm and 25-mm filtres, were used to sample inhalable dust.
Estimation of daily boron exposure (DBE)
Boron concentrations in air samples taken from Bandirma and Bigadic were lower than the limit of quantitation (LOQ). Therefore, environmental boron exposure by inhalation was not taken into account when estimating DBE levels. The major and relevant sources of boron exposure, in both Bandirma and Bigadic, were drinking water and food.
The daily drinking water consumption of all participating females was assumed to be 2 L/day.
The daily boron exposure via food was estimated using the “double plate method” for both lunch and dinner (i.e. the provided food samples from lunch and dinner menus were equal to the amounts actually consumed). Local bread, cheese, eggs and olives were mostly consumed for breakfast. Boron concentration in these food samples was negligible. Therefore, boron exposure via breakfast was neglected. Accordingly, the DBE was estimated by:
Bw: B exposure via drinking water: boron concentration (mg/L) in drinking water samples × 2 L. Bf: B exposure via food: [boron concentration in lunch] + [boron concentration in dinner].
Boron analysis
Blood and urine samples were digested using a closed vessel microwave system (MARS 6, CEM, Germany) at 200 °C for 20 min. The digestion solutions were transferred into a sample vial and diluted with 3 mL water. Subsequently, the concentration of B was quantified using ICP-MS (Agilent 8800 ICP-QQQ, Agilent Technologies, Germany) in the “no gas” mode. For quality assurance, calibration blanks and re-calibration check points were analysed periodically, together with certified reference material (Seronorm Trace Elements Whole Blood L-1 and Seronorm Trace Elements Urine L-1), which was processed accordingly. The determinations of boron in blood and urine were performed at the Institute of Nutritional Science, University of Potsdam, Germany.
Food and air (filtres) samples were digested using the Anton Paar Multiwave 3000 Microwave Digestion System (Rotor type 8SXF100). Subsequently, the boron concentrations were quantified using ICP-OES (Perkin Elmer Optima 4300 DV). Boron concentrations in water samples were determined using ICP-OES (Perkin Elmer Optima 4300 DV) and/or ICP-MS (Perkin Elmer Sciex ELAN DRCII). Determinations of boron in food, water and air samples (filtres) were performed by the Central Laboratory of Middle East Technical University, Ankara, Turkey.
The limit of detection (LOD) for boron was 2.08 ng/g in whole blood, 2.1 µg/L in urine, 0.3 µg/L in water, 0.4 mg/kg in food, and 0.3 µg/filtre in air samples. The limits of quantification (LOQ) for boron were 6.55 ng/g in whole blood, 6.6 µg/L in urine, 0.9 µg/L in water 1.2 mg/kg in food, and 0.9 µg/filtre in air samples. Boron concentrations in eight blood samples were below the LOQ. In the statistical analysis, these were replaced by LOQ/2.
Classification of exposure groups
The study participants were classified into low, medium and high exposure groups, according to their blood boron concentrations. Females with blood boron concentrations lower than 100 ng B/g blood were classified as “low exposure group” (n = 143). Blood boron concentrations of 29 females ranged between 100 and 150 ng B/g blood; these were classified as “medium exposure group”. Females (n = 27) with concentrations higher than 150 ng B/g blood were classified as “high exposure group” (Table 1). These classification limits were identical with those of our preceding study in males (Duydu et al. 2011).
Statistics
The different exposure groups were characterised by the mean, the empirical standard deviation, the range and the 5th and 95th quantile of B in blood. Moreover, boxplots (Chart A and B of Fig. 2), Pearson’s correlation coefficient, linear regression (Chart C of Fig. 2) and spline regression (Fig. 4; Table 5) display the empirical distribution and possible linear dependencies. Spline regression is a piecewise regression method, which is more flexible than linear regression and particularly suited to show within class effects, i.e. the effects in our three exposure groups.
To investigate the global hypothesis that the means of the three groups are equal, a Kruskal–Wallis test is used for all variables in Tables 1 and 4. If the resulting p value was significant (p < 0.05), i.e. if the hypothesis of equal group means was rejected, the two-sided Wilcoxon–Mann–Whitney test as a post hoc test was applied to find distinct differences between each of the pairs of groups. The corresponding three p values were adjusted with the Bonferroni–Holm method for each variable to account for the problem of multiple testing (Holm 1979). For the comparison of the reproductive outcomes (Table 3) the χ2 test was used. These nonparametric tests were deliberately employed as some of the variables showed outliers.
All statistical tests were performed with R, version 3.4.1 (R Core Team 2017). The local as well as multiple significance levels of the tests were set at 0.05. For theoretical details of the nonparametric Kruskal–Wallis, as well as the Wilcoxon–Mann–Whitney test, see Conover (1999); for theoretical details of spline regression, see Fahrmeir et al. (2009).
Results
The study covers 199 voluntarily participating females with 326 children (162 girls and 164 boys), living in the South Marmara region of Turkey. The specific sampling areas of this study were Bandirma and Bigadic (Iskele) in the Balikesir district, as shown in Fig. 1.
The study aimed at assessing possible developmental effects mediated by environmental boron exposure in a population that included high environmental exposures to B in drinking water and food (Table 2). In total, individual blood boron concentrations ranged from 3.28 to 975.66 ng B/g blood (Table 1).
The distribution of boron concentrations in biological fluids and the calculated DBE levels in the low, medium and high exposure groups are shown in Charts A and B of Fig. 2. As anticipated, the DBE levels in the high exposure group were significantly higher (p < 0.05) than those in the low exposure group (Table 1). The correlation between blood boron and DBE was statistically significant (p < 0.05) as shown in Chart C of Fig. 2. This correlation between blood boron and DBE confirmed our general methodology.
The calculated DBE levels vary between 2.26 and 57.86 mg B/day in entire the study cohort. The mean DBE levels for the low and high exposure groups were 9.73 ± 5.29 and 24.67 ± 11.39 mg B/day, respectively, and the difference was statistically significant (p < 0.05), as shown in Table 1.
Mean boron concentrations in water and food samples are presented in Table 2.
There were 56 females with blood boron concentrations higher than 100 ng B/g blood. These were sub-divided into medium (> 100–150 ng B/g blood) and high exposure (> 150 ng B/g blood). Induced abortion, spontaneous abortion (miscarriage), stillbirth, infant death, neonatal death, early neonatal death, preterm birth, congenital anomalies, sex ratio and birth weight of newborns were recorded as the pregnancy outcomes.
Boron concentrations in urine were determined as supplementary information. However, the pregnancy outcomes were basically evaluated based on blood boron concentrations as the preferred bio-indicator. Data on urinary boron concentrations, the distribution of urine boron concentrations within the exposure groups and the relation between urine boron concentrations, blood boron and estimated DBE are provided as “Electronic Supplementary Material”.
The numbers of spontaneous and induced abortions were 33 and 23, respectively. This equals 10.1 and 7.1% (per 100 live births) in our study cohort. Also, two stillbirths and four infant deaths were reported in our study cohort. The number of preterm births was 17, and 12 of them were reported in the low exposure group. The total number of congenital anomalies was eight in the study cohort, and six of them were reported in the low exposure group. In total, significant boron-mediated effects on rates and numbers of induced abortion, spontaneous abortion (miscarriage), stillbirth, infant death, preterm birth and congenital anomalies were not found (p > 0.05, χ2 test) in our study cohort, as shown in Table 3. Also, early neonatal death and neonatal death were not reported.
The number of childless women was 15, one of them belonging to the medium exposure group. The rest of childless women belonged to the low exposure group and six of them were presently pregnant. One out of the remaining nine childless women was not able to get pregnant due to the low sperm count of her husband. The remaining eight women indicated that childlessness was their own decision, mostly due to economic reasons.
The sex ratio was 1.01 (male/female = 164/162 = 1.01) for the whole study population, and there was no statistically significant difference between the low, medium and high exposure groups (p > 0.05, χ2 test), as shown in Table 3.
The birth weight of newborns was also assessed. We analysed birth weights in two different ways. (1) We handled the birth weights as continuous variables (mean ± standard deviation). (2) We described the birth weights as discontinuous variables by determining the numbers of infants with low birth weight (LBW, < 2500 g) and very low birth weight (VLBW, < 1500 g) as the second way.
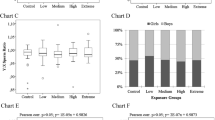
Birth weights of newborns (girls, boys, girls + boys) were statistically not different between low, medium and high exposure groups (p < 0.05), as shown in Table 4 and Fig. 3. The boron-mediated effects on the birth weights of newborns were also analysed using linear spline regression models with two knots at 100 and 150 ng B/g blood (Fig. 4). A statistically significant association between birth weights of newborns and blood boron concentrations has not been determined, as shown in Table 5.
The numbers of newborns with LBW and VLBW were also compared between the low, medium and high exposure groups, and statistically significant differences (p > 0.05) were not visible, as shown in Table 3.
Influences of alcohol consumption, age, smoking, smoking during pregnancy and pesticide application on the birth weight of newborns were assessed as possible confounders. The impact of alcohol consumption, age, smoking and pesticide application on the birth weight of newborns was not statistically significant. As to be expected, the birth weights of newborns were negatively affected by smoking during pregnancy, and this association was statistically significant (p < 0.05), as shown in Table 6.
Discussion
The present field study in females residing in Bandirma and Bigadic was conducted between 2014 and 2017. As mentioned in the “Materials and methods” section, the boron drinking water concentrations in these areas had been periodically monitored, and major changes in local boron concentrations with time were not reported. Therefore, it was anticipated that the females were chronically exposed to an area-specific level of boron, at least since water monitoring started in this region about 20 years ago.
Our present study has limitations. The first limitation is the low sample size to study boron-mediated effects on stillbirth, infant death, neonatal death, early neonatal death, preterm birth and congenital anomalies. However, this is common in many epidemiological studies investigating rare events. The second limitation is the time gap between the time of birth and the time of sampling. The study was not performed in pregnant women. As described, the birth weights of the newborns were based on retrospective information from the mothers.
The estimated individual DBE within the entire cohort ranged between 2.26 and 57.86 mg B/day (Table 1). The boron concentrations in blood samples were significantly correlated with the DBE levels. This was a further argument to use boron in blood as preferred exposure biomarker when assessing pregnancy outcomes (Chart C of Fig. 2). Therefore, possible boron-mediated developmental effects were compared between females with low, medium and high blood boron concentrations, as specified above and in Table 3. In detail, spontaneous abortion (miscarriage), induced abortion, stillbirth, infant death, neonatal death, early neonatal death, preterm birth, congenital anomalies, sex ratio and birth weight of newborns were assessed.
Induced and spontaneous abortion (miscarriage)
Many known confounding factors lead to minor suitability of miscarriage as an endpoint when assessing developmental toxicity in epidemiological studies (Hood 2006). Nevertheless, the numbers of spontaneous and induced abortions were determined in our study population by the questionnaire survey (Table 3). The numbers of spontaneous and induced abortions were 33 and 23, respectively. These numbers correspond to 10.1 and 7.1% (per 100 live births) in our study cohort. The average percentages of spontaneous and induced abortions across the country in 2013 were reported as 23 and 14%, respectively (Turkey Demographic and Health Survey—2013, “TDHS-2013”). Although the percentages of induced abortion vary between geographical regions, it was reported to be highest in the West Marmara region (the region that includes the study area), at 19.7% (TDHS-2013). Nevertheless, the spontaneous and induced abortion rates were lower in our study cohort than the average rates of the country and the average rates of the West Marmara region. A statistically significant boron-mediated increase in spontaneous and induced abortion rates between low, medium and high exposure groups was not visible (p > 0.05, χ2 test) as shown in Table 3.
Stillbirth
The major drawback of using stillbirth, infant death, neonatal death, and early neonatal death in epidemiological studies is the low numbers encountered, especially in developed areas. In Turkey, according to the records in TDHS-2013, there were 0.9 stillbirths per 100 pregnancies. The percentage of stillbirth in our cohort was determined as 0.61 per 100 pregnancies (live births + stillbirths) and there was no significant (p > 0.05, χ2 test) boron-associated increase in stillbirth rates between the low, medium and high exposure groups (Table 3).
Infant death, neonatal death, early neonatal death
The infant mortality rate in Turkey was 13 deaths per 1000 live births, and the neonatal mortality rate was seven deaths per 1000 live births (TDHS-2013). Any early neonatal death or neonatal death was not observed in our study cohort (probably due to the low sample size), while the infant death rate was 1.2 per 100 live births (Table 3). The infant mortality rate of our cohort was lower than the average rate of Turkey, and a boron-associated statistically significant (p > 0.05, χ2 test) increase with exposure was not visible.
Preterm birth
According to the UN World Health Statistics-(2014), the preterm birth rate per 100 live births in Turkey was 12. The preterm birth rate in our study cohort was 5.2 per 100 live births (Table 3) and a boron-associated statistically significant (p > 0.05, χ2 test) increase with exposure groups was not visible.
Congenital anomalies
The prevalence of major congenital anomalies in Europe has been reported as 23.9 per 1000 births for 2003–2007 (Dolk et al. 2010). Unfortunately, figures on congenital anomalies covering all regions of Turkey are not available. However, the rate of congenital anomalies recorded for our study area (2.4 per 100 birth) was in line with the results of Europe, and boron-mediated statistically significant differences (p > 0.05, χ2 test) between low, medium and high exposure groups were not apparent (Table 3).
Sex ratio at birth
Some earlier published studies reported an increase in the percentage of female offspring in boron-exposed populations, but without statistical significance (Whorton et al. 1994a, b; Sayli 1998; Chang et al. 2006). According to the report of Turkey Demographics Profile (2017), the sex ratio at birth in Turkey is 1.05 males/female. Within our study population, the mothers had 326 children, and 164 being male. Accordingly, the male/female sex ratio of 1.01 in our study population is in line with the report of Turkey Demographics Profile. The sex ratio in the high exposure group was 1.25. This ratio does not support a tendency in sex ratio at birth towards females, as claimed in earlier studies for a similarly boron-exposed population in China (Roobins et al. 2008). This conclusion is in line with Yazbeck et al. (2005). Their study was conducted in Northern France on 180 healthy individuals, and there was no statistical difference in the male/female sex ratios between zones of different boron concentrations in drinking water (Yazbeck et al. 2005).
Birth weight of newborns
LBW is an important marker of risk for neonatal and child health, and is defined as a live birth weight less than 2500 g (WHO 1992). LBW infants are 25–30 times more at risks of neonatal death than normal birth weight (NBW) infants (Podja and Kelley 2000). VLBW is defined as a live birth of less than 1500 g, and VLBW is associated with the death of the infants at a rate 40 times higher than those with NBW (Levene and Vries 2006). The prevalence of LBW and VLBW was 34 and 4, respectively, in our cohort and a statistically significant boron-associated adverse effect on these parameters could not be seen, even in the high exposure group (Table 3). The birth weights were also assessed as continuous variables, and the mean birth weights of infants (girls, boys and girls + boys) were compared between the exposure groups as shown in Fig. 3 and Table 4. The mean birth weights of newborns were statistically not different (p > 0.05) between the exposure groups.
Possible boron-mediated effects on the birth weights of newborns were also analysed using linear spline regression models with two knots at 100 and 150 ng B/g blood (Fig. 4). The association between birth weights and blood boron concentrations was negative between blood boron concentrations of 100 and 150 ng/g. An increase of 1 ng B/g blood was associated with a reduction in birth weight of 4.1 g between these blood boron concentrations. However, this association was statistically not significant, as shown in Table 5.
Foetal body weight is the major study item in reproductive toxicity investigations of boric acid (Heindel et al. 1992; Price et al. 1996). Reduced birth weight was not reported in epidemiological studies until a study by Igra et al. (2016), which was the first investigating boron-mediated developmental effects in pregnant women. It was reported that the birth weight was adversely affected with elevated serum boron concentrations in pregnant women residing in Salta, Northern Argentina. However, there are two major points of weakness, owing to the high altitude of the locality and the complexity of the environmental exposure conditions. (1) The sampling area of this study was located at very high altitude (3180–4070 m above sea level). High altitude is a major factor leading to reduced birth weight (Jensen and Moore 1997; Krampl 2002), most probably due to decreased oxygen supply. In a study conducted in Colorado (970–3550 m above the sea level) birth weights declined by 121 g for every 1000 m altitude gain, when the effect of altitude was considered alone (Jensen and Moore 1997). This negative effect of the high altitude on the weight of newborns has not been taken into account by Igra et al. (2016). (2) The same study group (Harari et al. 2015) also reported a high level of lithium exposure (5–1600 µg/L) via drinking water in the identical study area. The conclusion was that elevated environmental lithium exposures during pregnancy were inversely associated with foetal size (Harari et al. 2015). Actually, the same population and the same participating persons (co-exposed to arsenic, lithium and boron) were studied in both publications. In one publication, decreased foetal size was attributed to the environmental lithium exposure (Harari et al. 2015); in the second publication, the decreased birth size was attributed to environmental boron exposure (Igra et al. 2016). Therefore, the suggested inverse association between environmental boron exposure during pregnancy and birth size is not persuasive. Our results do not support the claims of Igra et al. (2016).
Final considerations
In animal experiments, decreased foetal body weights and increased incidences of short rib XIII were found in rats, which was assessed as the critical toxic effects for boric acid, as mentioned in the Introduction (EGVM 2003; EFSA 2004). The NOAEL for these developmental effects was 9.6 mg B/kg/day (Price et al. 1996). Associated blood boron concentrations in pregnant rats fed boric acid throughout gestation were provided by Price et al. (1997). The blood boron concentration corresponding to the NOAEL (9.6 mg B/kg/day) in female rats was 1270 ng B/g blood. In humans, the highest blood boron concentration that we found in females was 975.7 ng B/g blood, and the mean blood boron concentration in the high exposure group was 274.6 ng B/g blood (Table 1). Boron-mediated adverse effects on pregnancy outcomes, including birth weights of newborns, could not be determined in the medium and high exposure groups of our cohort. As far as the human exposure levels are concerned, blood boron concentrations in our study were clearly below those corresponding to the NOAEL in female rats. Therefore, a lack of boron-mediated adverse effects on pregnancy outcomes and birth weight of newborns is plausible. As far as the levels of human boron exposure are concerned, the data of the present study in environmentally exposed females in a boron-rich area are comparable to those of our previous data in occupationally exposed males in Turkey (Duydu et al. 2011, 2012a, b, 2015). In occupationally exposed males in California, mean blood levels were 260 ng B/g blood, with extreme values up to 330 ng B/g blood (Culver et al. 1994). In occupationally exposed males in Turkey, blood levels in the high exposure group amounted to 223.89 ± 69.49 ng B/g blood (range: 152.82–454.02 ng B/g blood; Duydu et al. 2011). This demonstrates that the environmental boron exposure in our highly exposed female subgroup was comparable to reported high occupational exposures to boron in borate processing plants.
Under conditions of normal handling and use of boron compounds, it is very unlikely that women could exceed blood boron concentrations corresponding to the NOAEL for reproductive toxicity in female rats. This is in line with similar conclusions on male fertility effects (Duydu et al. 2011; Bolt et al. 2012, 2017; Basaran et al. 2012). In essence, such effects appear to be irrelevant for humans.
References
Basaran N, Duydu Y, Bolt HM (2012) Reproductive toxicity in boron exposed workers in Bandirma, Turkey. J Trace Elem Med Biol 26(2–3):165–167
Bolt HM, Basaran N, Duydu Y (2012) Human environmental and occupational exposures to boric acid: reconciliation with experimental reproductive toxicity data. J Toxicol Environ Health A 75(8–10):508–514
Bolt HM, Duydu Y, Basaran N, Golka K (2017) Boron and its compounds: current biological research activities. Arch Toxicol 91:2719–2722
Chang BL, Robbins WA, Wei F, Xun L, Wu G, Li N, Elashoff DA (2006) Boron workers in China, exploring work and lifestyle factors related to boron exposure. AAOHN J 54(10):435–443
Conover WJ (1999) Practical nonparametric statistics, 3rd edn. Wiley, New York
Culver BD, Shen PT, Taylor TH, Lee-Feldstein A, Anton-Culver H, Strong PL (1994) The relationship of blood- and urine-boron to boron exposure in borax workers and the usefulness of urine-boron as an exposure marker. Environ Health Perspect 102(Suppl 7):133–137
Dolk H, Loane M, Garne E (2010) The prevalence of congenital anomalies in Europe. In: Posada de la Paz M, Groft S (eds) Rare diseases epidemiology. Advances in experimental medicine and biology, vol 686. Springer, Dordrecht, pp 349–364. https://doi.org/10.1007/978-90-481-9485-8$420
Duydu Y, Basaran N, Ustundag A, Aydin S, Undeger U et al (2011) Reproductive toxicity parameters and biological monitoring in occupationally and environmentally boron-exposed persons in Bandirma, Turkey. Arch Toxicol 85(6):589–600
Duydu Y, Basaran N, Bolt HM (2012a) Exposure assessment of boron in Bandirma boric acid production plant. J Trace Elem Med Biol 26(2–3):161–164
Duydu Y, Basaran N, Ustundag A, Aydin S, Undeger et al (2012b) Assessment of DNA integrity (COMET assay) in sperm cells of boron exposed workers. Arch Toxicol 86(1):27–35
Duydu Y, Basaran N, Bolt HM (2015) Risk assessment of borates in occupational settings, 1st edn., Elsevier, Amterdam, pp 65–105
Duydu Y, Basaran N, Ustundag A, Aydin S, Undeger U et al (2016) Is boric acid toxic to reproduction in humans? Assessment of the animal reproductive toxicity data and epidemiological study results. Curr Drug Deliv 13(3):324–329
EFSA [European Food Safety Authority] (2004) Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the tolerable upper intake level of boron (sodium borate and boric acid). EFSA J 80:1–22
EGVM [Expert Group on Vitamins and Minerals] (2003) Safe upper levels for vitamins and minerals. https://cot.food.gov.uk/committee/committee-on-toxicity/cotreports/cotjointreps/evmreport. Accessed 19 Mar 2018
EU Drinking Water Directive (1998) Council Directive 98/83/EC, of 3 November 1998, on the quality of water intended for human consumption
Fahrmeir L, Kneib T, Lang S (2009) Regression. Springer, New York
Harari F, Langeen M, Casimiro E, Bottai M, Palm B, Nordqvist H, Vahter M (2015) Environmental exposure to lithium during pregnancy and fetal size: a longitudinal study in the Argentinean Andes. Environ Int 77:48–54
Heindel JJ, Price CJ, Field EA, Marr MC, Myers CB, Morrissey RE, Schwetz BA (1992) Developmental toxicity of boric acid in mice and rats. Fund Appl Toxicol 18:266–277
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Hood RD (2006) Developmental and reproductive toxicology, a practical approach, 2nd edn. CRC press, Boca Raton, pp 804–806
Igra AM, Harrari F, Lu Y, Casimiro E, Vahter M (2016) Boron exposure through drinking water during pregnancy and birth size. Environ Int 95:54–60
Jensen GM, Moore LG (1997) The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Pub Health 87(6):1003–1007
Khaliq H, Juming Z, Me-Mei P (2018) The physiological role of boron on health. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1284-3
Korkmaz M, Sayli U, Sayli BS, Bakirdere S, Titretir S, Ataman OY, Keskin S (2007) Estimation of human daily boron exposure in a boron-rich area. Br J Nutr 98:571–575
Korkmaz M, Yenigun M, Bakirdere S, Ataman OY, Keskin S, Muezzinoglu T, Lekili M (2011) Effects of chronic boron exposure on semen profile. Biol Trace Elem Res 143(2):738–750
Krampl E (2002) Pregnancy at high altitude. Ultrasound Obstet Gynecol 19:535–539
Levene MI, Vries LD (2006) Epidemiology and perinatal services. In: Martin RJ, Fanaroff AA, Walsh MC Fanaroff and Martin Neonatal–Perinatal Medicine. Disease of the Fetus and Infant, 8th edn. Elsevier, Philadelphia, pp 19–24
Podja J, Kelley L (2000) Administrative Committee on Coordination/Sub-Committee on Nutrition. The UN Systems Forum for Nutrition. Nutrition Policy Paper # 8. http://lib.riskreductionafrica.org/bitstream/handle/123456789/1325/154.Low%20Birthweight.%20Report%20of%20a%20Meeting%20(Dhaka%20Bangladesh%2014-17%20June%201999).pdf?sequence=1. Accessed 24 Apr 2018
Price CJ, Strong PL, Marr MC, Myers CB, Murray FJ (1996) Developmental toxicity NOAEL and postnatal recovery in rats fed boric acid during gestation. Fund Appl Toxicol 32:179–193
Price CJ, Strong PL, Murray FJ, Goldberg MM (1997) Blood boron concentrations in pregnant rats fed boric acid throughout gestation. Rep Toxicol 11(6):833–842
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Robbins WA, Xun L, Jia J, Kennedy N, Elashoff DA, Ping L (2010) Chronic boron exposure and human semen parameters. Reprod Toxicol 29:184–190
Roobins WA, Wei F, Elashoff DA, Wu G, Xun L, Jia J (2008) Y:X sperm ratio in boron-exposed men. J Andrology 29(1):115–121
Sayli BS (1998) An assessment of fertility in boron-exposed Turkish subpopulations, 2. evidence that boron has no effect on human reproduction. Biol Trace Elem Res 66(1–3):409–422
Sayli BS (2001) Assessment of fertility and infertility in boron-exposed Turkish subpopulations, 3. evaluation of fertility among sibs and in “borate families”. Biol Trace Elem Res 81(3):255–267
Sayli BS (2003) Low frequency of fertility among workers in a borate processing facility. Biol Trace Elem Res 93(1–3):19–28
TDHS (2013) Turkey demographic and health survey (2013) “TDHS-2013”
Turkey Demographics Profile (2017) https://www.indexmundi.com/turkey/demographics _profile.html Accessed 12 Dec 2017
Türkiye Nüfusu (2017) https://www.nufusu.com/ilce/bandirma_balikesir-nufusu. Accessed 17 Oct 2017
UN World Health Statistics (2014) https://knoema.com/UNWHODATA2014/un-world-health-statistics-2014?location=1001780-turkey&variable=1002040-preterm-birth-rate-per-100-live-births/. Accessed 6 Nov 2017
WHO (1992) Low birth weight: a tabulation of available information, WHO/MCH/92.2. World Health Organization, Geneva
WHO (2011) Guidelines for drinking water quality, fourth edition, 2011. WHO Press, World Health Organization, Geneva. http://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/. Accessed 19 Mar 2018
Whorton D, Haas J, Trent L (1994a) Reproductive effects of inorganic borates on male employees: birth rate assessment. Environ Health Perspect 102(7):129–132
Whorton MD, Haas JL, Trent L, Wong O (1994b) Reproductive effects of sodium borates on male employees: birth rate assessment. Occup Environ Med 51(11):761–767
Yazbeck C, Kloppmann W, Cottier R, Sahuquillo J, Debotte G, Huel G (2005) Health impact evaluation of boron in drinking water: a geographical risk assessment in Northern France. Environ Geochem Health 27(5–6):419–427
Acknowledgements
Funds for the present cooperative “Boron Project II” were provided by the Eti Mine Works General Management (2014-2017).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duydu, Y., Başaran, N., Üstündağ, A. et al. Birth weights of newborns and pregnancy outcomes of environmentally boron-exposed females in Turkey. Arch Toxicol 92, 2475–2485 (2018). https://doi.org/10.1007/s00204-018-2238-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2238-4