Abstract
The occurrence of multidrug-resistant pathogenic bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Acinetobacter baumannii (MDRAB), extended-spectrum β-lactamase (ESBL) Escherichia coli, and Pseudomonas aeruginosa, has become a serious problem in animals and public. The objective of this study was to identify and isolate lactic acid bacterial (LAB) strains from the intestinal tracts of pigs and feces of dogs and then characterize them as potential probiotics with antimicrobial activity against multidrug-resistant pathogenic bacteria. In a preliminary isolation screening, 45 of 1167 isolated LAB strains were found to have anti-S. aureus ATCC 27,735 activity. Using 16S rDNA and 16S-23S rDNA intergenic spacer region (ISR) sequences, five of these isolates were further identified as Lactobacillus animalis 30a-2, Lactobacillus reuteri 4-12E, Weissella cibaria C34, Lactococcus lactis 5-12H, and Lactococcus lactis 6-3H. Antimicrobial substance assays suggest that the L. lactis 5-12H, L. lactis 6-3H, L. animalis 30a-2, L. reuteri 4-12E, and W. cibaria C34 strains might produce bacteriocins and hydrogen peroxide (H2O2) as antimicrobial substances. The L. animalis 30a-2 and W. cibaria C34 strains were further characterized for probiotic properties and shown to have high acid and bile salt tolerance. Additionally, they have broad antimicrobial spectra, and can significantly repress the growth of all of the tested strains of MRSA isolates, some MDRAB, ESBL E. coli, and P. aeruginosa isolates, along with food-borne pathogenic bacteria such as Bacillus cereus ATCC 11778, Listeria monocytogens ATCC 19111, Salmonella spp., Shigella spp., and Yersinia enterocolitica BCRC 12986. This is the first report of H2O2-producing L. animalis 30a-2 and W. cibaria C34 isolated from the intestinal tracts of pigs and feces of dogs that have good antimicrobial activity against multidrug-resistant and food-borne pathogenic bacteria and have excellent probiotic properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
African swine fever keeps spreading across Asia, causing Asia countries lose hundreds of millions of pigs last 2 years. Therefore, health of pig has received increasing attention. The gut microbiome has been linked closely to the health of its host, leading to gut microbiota studies of animals which have been performed progressively (Kim et al. 2017; Crespo-Piazuelo et al. 2018; Schmidt et al. 2018; Gresse et al. 2019). Bacterial infection occurs regularly in animals, such as pigs and dogs. Especially, the occurrence of multidrug-resistant pathogen infection causes important economic losses in livestock and threat of infectious disease transmission in public (Otero and Nader-Macias 2006; Espeche et al. 2009; Brunel and Guery 2017).
Multidrug-resistant (MDR) bacteria in the Gram-negative group, such as Acinetobacter baumannii, extended-spectrum β-lactamase (ESBL) E. coli, and Pseudomonas aeruginosa, and in the Gram-positive group, such as Staphylococcus aureus and Enterococcus spp., have been regarded as serious threats to humans and animals during recent decades (Rice 2006; Cerceo et al. 2016; Maraolo et al. 2017; Satlin and Walsh, 2017). Bacteria are able to develop different types of resistance mechanisms against antibiotics (Fernandez and Hancock 2012; Cerceo et al. 2016); for example, they can use special outer membranes with characteristics of low permeability or constitutive efflux pumps to prevent or exclude antimicrobial agents. They may acquire new genetic material, such as DNA that encodes drug-degrading enzymes capable of inactivating antibiotics (Cerceo et al. 2016). Additionally, biofilms formed by MDR bacteria create a physical barrier that makes them more resistant to antibiotics and tolerant of the environment (Fleming and Rumbaugh 2017; Kumar et al. 2017). Biofilms have become a key factor for the development of infection, especially the nosocomial infections frequently seen in hospital intensive care and burn units (van Duin and Paterson 2016; Geisinger and Isberg 2017).
Substantial efforts have been undertaken to fight drug-resistant bacteria, such as the development of new antibiotics, antibodies, and signaling molecules that inhibit pathogen growth, neutralize infectious agents, and interfere with quorum sensing signaling (Brunel and Guery 2017). Additionally, probiotics, beneficial microorganisms such as Lactobacillus species, have been recognized as promising biological therapeutics that can effectively repress the growth of several pathogens, including human and food-borne pathogens (Kumar et al. 2016; Brunel and Guery 2017; Vieco-Saiz et al. 2019). Probiotics can produce organic acids, bacteriocins, H2O2, and small antimicrobial compounds; for example, Lactobacillus johnsonii has been found to produce organic acid and bacteriocin, as well as H2O2, as antimicrobial substances against several pathogens (Fayol-Messaoudi et al. 2005; Sgouras et al. 2005; Pridmore et al. 2008). Additionally, popular commensal vaginal microbes, such as Lactobacillus crispatus and Lactobacillus gasseri, can generate H2O2 and have been associated with some benefits for women’s health, such as lower rates of bacterial vaginosis and HIV acquisition (Martin and Suarez 2010; Mitchell et al. 2015). They can not only inhibit the growth of pathogens, but also mediate pathogen competition, exclusion, and displacement in biofilm formation (Woo and Ahn 2013; Shokri et al. 2017; Wasfi et al. 2018). For example, L. fermentum has an inhibitory effect on the growth of P. aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) (Chen et al. 2013; Shokri et al. 2017). L. plantarum can form a strong biofilm, which inhibits the growth of pathogens (Jalilsood et al. 2015). L. acidophilus, L. rhamnosus, L. animalis, and L. gasseri all have antibacterial activity against clinical isolates of E. coli and Klebsiella pneumoniae (Halder and Mandal, 2016).
Lactic acid bacteria (LAB) are a well-established group of microorganisms, widely used in the food fermentation industry, and are generally recognized as safe (GRAS) microorganisms (Wu et al. 2017; Ghosh et al. 2019). In addition to being used in biological therapeutics, the most studied probiotic LAB, belonging to the Lactobacillus and Bifidobacterium genera, have been frequently applied in health promotion in both humans and animals (Gioia and Biavati 2018; Ghosh et al. 2019; Yadav and Jha 2019). For example, L. acidophilus, L. brevis, L. casei, L. fermentum, L. gasseri, L. johnsonii, L. paracasei, L. plantarum, L. rhamnosus, L. reuteri, and L. salivarius, B. adolescentis, B. animalis, B. bifidum, B. breve, and B. longum have all been extensively studied (Linares et al. 2016, 2017; Ghosh et al. 2019). Additionally, species of the Weissella genus that have high probiotic potential for inhibiting pathogen growth (Elavarasi et al. 2014), and can produce high amounts of exopolysaccharides and some species, especially W. cibaria, have been confirmed to produce large amounts of H2O2 (max. 3.2 mmol/L) (Endo et al. 2009; Fusco et al. 2015), have received much attention (Fusco et al. 2015).
In this study, we screened LAB isolated from the intestinal tracts of pigs and feces of dogs for anti-S. aureus activity. We found that two isolates, L. animalis 30a-2 and W. cibaria C34, which were presumed to produce H2O2 efficiently against S. aureus, have good antimicrobial activity against clinical isolates of MRSA and some other common pathogens. Both strains also showed well-characterized probiotic properties, such as high acid/bile salt tolerance. Our results suggest that these bacterial strains have potential as probiotics and should be studied for further application, like improve animal health by preventing serious infection.
Materials and methods
Isolation of lactic acid bacteria
LAB strains were isolated from the gastrointestinal tract mucosa of 30 pigs (in a slaughterhouse) and the feces of 30 dogs (in an animal shelter) in Taichung, Taiwan. The isolation method was based on a previous protocol but with slight modifications (Silva et al. 2013). The ileal mucosa (1 cm in size) of pigs was sampled, washed three times with sterilized phosphate-buffered saline (PBS), pH 7.4, and homogenized in 10 mL PBS. Additionally, 1 g of feces from dogs was sampled and homogenized in 10 mL PBS. The homogenized samples were then allowed to stand at 4 °C for 30 min. The supernatants were serially diluted with sterile 0.9% NaCl solution and plated onto Rogosa Selective Lactobacillus (SL) agar (Difco Laboratories, Detroit, USA) with 1% CaCO3 (Merck, Darmstadt, Germany) for the isolation of putative LAB strains. After 48 h of incubation at 37 °C in anaerobic jars, single colonies were picked up and anaerobically sub-cultured in deMan-Rogosa Sharpe broth (Difco Laboratories, Detroit, USA) at 37 °C for 48 h. All isolates were first screened for anti-S. aureus ATCC 27735 activity using an agarose/agar assay procedure (Somkuti and Steinberg 2002). Five of the best anti-S. aureus ATCC 27735 strains were further morphologically identified via Gram staining, KOH reaction, catalase activity, and fermentation of 49 carbohydrates (API 50 CHL, Bio-merieux, Marcy I’Etoile, France).
Characterization of antimicrobial substances
Five lactobacilli strains with good anti-S. aureus activity were further characterized for the production of antimicrobial substances, such as organic acid, hydrogen peroxide, and bacteriocin. For the reduction of organic acid production, the lactobacilli strains were cultured on MRS agar with a reduced concentration of glucose (0.2%, w/v) under anaerobic conditions for 24 h. The resulting colonies were collected and laid on a 1.5% BHI agar (BHI, Oxoid, Basingstoke, England) plate after spreading 20 μL of S. aureus ATCC 27735 (1.5 × 108 CFU/mL) (Somkuti and Steinberg 2002). The assembled assay plates were kept at 4 °C for 30 min before being transferred to a 37 °C incubator for up to 16 h under aerobic conditions. The plates were scored for the presence of inhibition zones resulting from the diffusion of antimicrobial substances produced by Lactobacillus colonies on the top layer. Nisin-producing L. lactis ATCC 11454 (positive control) (Millette et al. 2004) and L. acidophilus ATCC 4356 (negative control), purchased from the Food Industry Research and Development Institute (FIRDI), Taiwan, were used as reference strains in all tests.
For the bacteriocin and H2O2 assays, we repeated the above-mentioned procedure but added protease (0.5 mg/mL) (Protease EC 3.4.24.31 (5.2 U/mg) Type XIV from Streptomyces griseus, Sigma, USA) or catalase (0.4 mg/mL) (Catalase EC 1.11.1.6 (2,860 U/mg) from bovine liver, Sigma, USA) into 1.5% BHI agar before spreading the S. aureus ATCC 27735 (1.5 × 108 CFU/mL) and laying lactobacilli colonies onto the agar plate. H2O2 production by lactobacilli was detected using a TMB-Plus plate, following a previous protocol (Rabe and Hillier 2003).
Molecular identification of 5 good anti-S. aureus activity lactobacilli
Bacterial DNA was isolated using the Genomic DNA Purification Kit (Biokit Biotechnology Inc., Taiwan) according to the manufacturer’s instructions. PCR was used to amplify the 16S rDNA of lactobacilli using the universal forward primer 27F (5´-AGAGTTTGATCCTGGCTCAG-3´) and reverse primer 1492R (5´-TACGGYTACCTTGTTACGACTT-3´) (de Lillo et al. 2006). In addition, the 16S-23S rDNA intergenic spacer region (ISR) was amplified using the forward primer p2 (5´-CTTGTACACACCGCCCGTC-3´) and the reverse primer p7 (5´-GGTACTTAGATGTTTCAGTTC-3´) (Rachman et al. 2003; Ben Belgacem et al. 2009). PCR was performed using the following thermal profile: 94 °C for 5 min; 35 cycles of 94 °C for 20 s, 54 °C for 30 s, 72 °C for 1/1.5 min; and finally 72 °C for 5 min. Each PCR reaction contained 10 × PCR buffer, 0.1 μM of each primer pair, 10 mM dNTPs, 25 ng of DNA, and 0.25 μL of Taq polymerase (Takara Bio Inc., Japan), made up to a final volume of 25 μL. All of the amplified 16S rDNA fragments and 16S-23S ISR were cloned into the pGEM-T vector (Promega, USA) and sequenced from both directions. DNA sequence analysis, including blast and alignment, was performed using Vector NTI® software V10 (Invitrogen, USA). The nucleotide sequences of the five LAB isolates determined in this study were deposited in the GenBank (NCBI) database.
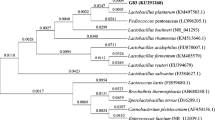
The sequences of 16S rDNA and 16S-23S rDNA ISR were performed BLAST analysis in GenBank database and to construct phylogenetic tree based on relevant 16S rDNA and 16S-23S rDNA ISR sequences. Regarding 16S rDNA analysis, it was involved 14 nucleotide sequences, including the five LAB sequences obtained in this study and nine sequences belonging to Lactobacillus species that were received from the blasting results of GenBank database. In addition, the sequence of E. coli (X80725), which was used as an outgroup, was included. The species identity of LAB 30a-2 and C34 was further determined using 16S-23S rDNA ISR sequences. 16S-23S rDNA ISR nucleotide sequences of Lactobacillus spp. and Weissella spp., obtained from GenBank, were used for the classification of 30a-2 and C34 strains with the outgroup of S. aureus (SAU11789). Multiple sequence alignment was conducted using the CLUSTAL W program, and phylogenetic trees were constructed using MEGA X software with the neighbor-joining method (Kumar et al. 2018). Bootstrapping was performed for 1000 replicates, and the evolutionary distances were computed using the Kimura two-parameter method.
Commercial and clinical isolates of pathogenic bacterial strains
The pathogenic bacteria used as indicator strains of antibacterial activity were purchased from FIRDI, Hsinchu, Taiwan, and included S. aureus ATCC 27735, Bacillus cereus ATCC 11778, Listeria monocytogens ATCC 19111, Acinetobacter baumannii ATCC 15151, E. coli K12, Pseudomonas aeruginosa ATCC 27853, Salmonella choleraesuis ATCC 13312, Salmonella enteritidis ATCC 13076, Salmonella typhimurium ATCC 13311, Shigella flexneri ATCC 29903, Shigella sonnei ATCC 25931, and Yersinia enterocolitica BCRC 12986. Furthermore, 16 clinical isolates of MRSA with multidrug resistance were obtained from the National Taichung, Fong-Yuan, Nantou, and Chang-Hua Hospitals in central Taiwan. Gram-negative bacteria included three clinical strains of multidrug-resistant A. baumannii (MDRAB); extended-spectrum β-lactamase (ESBL) E. coli and P. aeruginosa were obtained from the National Fong-Yuan Hospital. The antibiotic susceptibility tests for these clinical isolates were determined using the disc diffusion method, according to the Clinical and Laboratory Standard Institute (CLSI) (Weinstein 2018). All of the pathogenic strains were grown in BHI broth (BHI, Oxoid, Basingstoke, England) at 37 °C under aerobic conditions.
Antimicrobial activity of isolated lactobacilli against pathogenic bacteria
Potential lactobacilli with anti-S. aureus activity were further evaluated for their antimicrobial spectrum against the pathogenic bacteria from FIRDI and clinical isolates mentioned above, including multidrug-resistant Gram-positive and Gram-negative pathogenic bacteria. The diameters of the inhibition zones on the 1.5% TSA plates were measured. The antimicrobial activity of the lactobacilli against pathogenic bacteria was classified according to the diameter of inhibition zone as follows: < 10 mm, none (−); > 10 mm, weak (+); > 15 mm, middle (+ +); > 20 mm, strong (+ + +); or > 25 mm, very strong (+ + + +) inhibition. Each assay was performed in triplicate.
Probiotic characteristics of the identified lactobacilli
The identified strains were used to assess their tolerance of low pH and bile salt and antibiotic susceptibility profile. The results were used for the evaluation of the strains as potential probiotics according to the previous protocol but with some modifications (Lim et al. 2004; Ruiz et al. 2013). The isolates were incubated in MRS broth at 37 °C for 15 h under anaerobic conditions. To assess the tolerance to low pH, the overnight cultures were diluted 100-fold into 3 mL of different pH values (i.e., 2, 3, and 6.5) of MRS broth in 15 mL culture tubes and were then anaerobically incubated at 37 °C for 3 h. Additionally, to assess the tolerance to bile salt, the overnight cultures of isolates were diluted 100-fold into 3 mL of MRS broth supplemented with 0.3% oxgall (w/v) in 15 mL culture tubes and were then anaerobically incubated at 37 °C for 24 h. Bacteria were spread onto MRS agar plates and anaerobically incubated at 37 °C for 48 h, and then, bacterial viability counts were performed.
The antibiotic susceptibility profile was assessed using the antimicrobial disc diffusion test (Zhou et al. 2005; Weinstein 2018) with some modifications as follows: 1 mL of fresh culture (1.5 × 108 CFU/mL) was added to a bottle containing 100 mL 1% MRS agar (cooled to 45 °C), mixed gently, and poured equally into 9 cm Petri dishes. Then, the antimicrobial disks were placed onto the agar surface using an antimicrobial disc dispenser and then incubated for 24 h. The diameters of the resulting inhibition zones were then measured.
Statistical analysis
The data are presented as the mean values and standard deviations (mean ± SD), calculated from triplicate trials. Statistical significance of the results was evaluated using one-way ANOVA and Duncan’s multiple range tests (P < 0.05) using SPSS 10.0.7 software.
Results and discussion
Isolation of lactic acid bacteria with antagonistic activity against S. aureus ATCC 27735
The important feature of probiotic lactobacilli is its antagonistic activity against bacterial pathogens. Here, we isolated a total of 1167 putative LAB strains from the intestinal tracts of pigs and feces of dogs, of which 45 strains showed antagonistic activity against S. aureus ATCC 27735. The probability of obtaining LAB against S. aureus ATCC 2735 from pigs was 3.7% (23/625) and from dogs was 4.1% (22/542), but only five strains generated substantial levels of anti-S. aureus ATCC 27735 activity, determined by inhibition zones that were clearly larger than 10 mm. These were designated as follows: 5-12H (13 mm), 6-3H (12 mm), 4-12E (11 mm), C34 (15 mm), and 30a-2 (21 mm) (Fig. 1). C34 strain was isolated from the feces of dogs, whereas the other four strains were isolated from the intestinal tracts of pigs. It seems that LAB with good anti-S. aureus ATCC 27735 activity can be easily isolated from pigs. This phenomenon was probably because of the food sources; that is, the diet of pigs is more diverse than that of dogs. Furthermore, this information could be a good clue for future isolation of good LAB antagonistic activity against S. aureus.
Anti-S. aureus activity of the five LABs. Five of best anti-S. aureus ATCC 27735 isolated strains were identified from 45 isolated strains with anti-S. aureus activity. Label 1: L. lactis ATCC11454, a nisin-producing strain was used as a positive control and Label 7: L. acidophilus ATCC 4356 was a negative control in this assay; Label 2: the isolated strain 5-12H; Label 3: the isolated strain 6-3H; Label 4: the isolated strain 4-12E; Label 5: the isolated strain C34; Label 6: the isolated strain 30a-2
The five isolates were subjected to Gram staining and KOH reaction, and were identified as Gram-positive bacteria and KOH negative (Powers 1995). Additionally, based on their morphology, 30a-2, C34, and 4-12E were identified as Gram-positive bacilli, whereas 6-3H and 5-12H were Gram-positive cocci. All five isolates were catalase-negative and calcium carbonate-positive. Following the characterization of sugar fermentation profiles using an API 50 CHL kit, the 5-12H and 6-3H strains yielded the same fermentation profile and were considered to be the same strain, which was subsequently identified as L. lactis. Additionally, the 4-12E, C34, and 30a-2 strains were identified as Lactobacillus reuteri, Weissella confusa, and Lactobacillus animalis, respectively (Supplementary Table 1).
Molecular identification using gene sequences of 16S rDNA and 16S-23S rDNA intergenic spacer region (ISR)
The five LAB strains with good anti-S. aureus activity (i.e., 5-12H, 6-3H, 4-12E, C34, and 30a-2) were further classified according to their 16S rDNA. The 16S rDNA sequences of the five LAB strains were deposited in the GenBank under the following accession numbers: 5-12H, MN044988; 6-3H, MN044989; 4-12E, MN044985; C34, KJ880099; and 30a-2, KJ880097. The results of the comparative 16S rDNA sequence analysis are presented in Table 1, and show that 5-12H and 6-3H strains were identified as L. lactis, with approximately 98.0% identity. Of the other three strains, C34 was identified as Weissella cibaria/Weissella confusa/Weissella kimchi with 99.8% identity, whereas 4-12E and 30a-2 were identified as Lactobacillus reuteri and Lactobacillus animalis/ Lactobacillus murinus, with 99.0% and 96.3% identity, respectively. The phylogenetic analysis based on 16S rDNA sequencing was performed with the E. coli (X80725) outgroup and showed that strains 5-12H and 6-3H were clustered together to form a monophyletic group with L. lactis GQ337885 (Fig. 2A). The 4-12E strain was grouped with L. reuteri CP014786 to form a monophyletic branch. The 30a-2 strain formed a monophyletic branch with Lactobacillus spp. (i.e., L. animalis AB911476, L. crispatus AB911456, and L. murinus KU196090), whereas the C34 strain was grouped with Weissella spp. (i.e., W. cibaria CP035267, W. confusa AB680186, and W. kimchi AF312874).
Phylogenetic tree of the 5 isolated LABs constructed using 16S rDNA sequences (a) and the isolated strains 30a-2 (b) and C34 (c) constructed using 16S-23S rDNA ISR sequences. a Sequences for 16S rDNA phylogenetic analysis were obtained from the GenBank database for the following strains: Lactobacillus animalis, Lactobacillus crispatus, Lactobacillus murinus, Lactobacillus reuteri, Weissella kimchi, Weissella cibaria, Weissella confusa, and Lactobacillus lactis. E. coli was used as an outgroup organism. The isolated strains are labeled in boxes. b The strain 30a-2 is labeled in the box. The ISR sequences for phylogenetic analysis were obtained from the GenBank database for the following strains: Lactobacillus animalis, Lactobacillus murinus, Lactobacillus agilis, Lactobacillus hordei, Lactobacillus salivarius, and Lactobacillus ruminis. c The isolated strain C34 is labeled in the box. The ISR sequences for phylogenetic analysis were obtained from the GenBank database for the following strains: Weissella cibaria, Weissella confusa, Weissella ceti, Weissella hellenica, Weissella jogaejeotgali, and Weissella paramesenteroides. Staphylococcus aureus was used as an outgroup organism for the analysis of ISR sequences. The GenBank accession number is included in brackets after the bacterial scientific name. The tree was constructed using the neighbor-joining method and tested by bootstrapping with 1000 replicates of data. Percentages are reported at the nodes, and the scale bar represents 0.05% sequence divergence
Although the C34 and 30a-2 strains have ambiguous results for the comparative 16S rDNA analysis, the comparative 16S-23S rDNA ISR analysis further confirmed that these two strains were close to the species of W. cibaria and L. animalis, with identities of 98.4% and 99.0%, respectively (Table 1). This was supported by performing the 16S-23S rDNA ISR sequence phylogenetic analysis with S. aureus (U11789) as the outgroup. The 30a-2 strain formed a monophyletic clade with L. animalis (CP039849) with a bootstrap value of 91%, whereas C34 was monophyletic with W. cibaria (CP022606) with a bootstrap value of 92% (Fig. 2b, c).
Weissella confusa and W. cibaria are two species with very close taxonomical relationship and physiological characteristics (Fusco et al. 2015). Here, we found that the C34 strain was identified as W. confusa using sugar fermentation characterization, whereas it was identified as W. cibaria using 16S-23S rDNA ISR. However, based on the molecular identification result, we classified C34 as a strain of W. cibaria. Taking the sugar fermentation characterization and molecular identification profiles of the five LAB strains together, the five isolates were confirmed as follows: L. lactis 5-12H; L. lactis 6-3H; L. reuteri 4-12E; L. animalis 30a-2; and W. cibaria C34.
Characterization of antimicrobial substances against S. aureus ATCC 27735
Substances produced by the five LAB strains against S. aureus ATCC 27735 were further characterized for the presence of antimicrobial substances such as organic acid, H2O2, and bacteriocin using an agarose/agar assay containing protease and catalase. Protease in agar plates can degrade peptides excreted from bacterial colonies; the result showed that the addition of protease caused the strains L. lactis 5-12H and L. lactis 6-3H and the positive control L. lactis ATCC11454 to lose their original S. aureus ATCC 27735 inhibition zones (Fig. 3a). It was therefore proposed that the antimicrobial substances produced by L. lactis 5-12H and L. lactis 6-3H are peptide-related bacteriocins, and probably nisins. This was supported by the identification of the nisin A gene in their genome (Supplementary Fig. 1). L. lactis is always recognized to transiently colonize in the gastrointestinal tract of animals. However, Mercier-Bonin and Chapot-Chartier studied the cell wall of L. lactis and determined that it also contains mucus/mucin binding proteins, especially of the LPxTG-protein family, supporting that L. lactis can be isolated even after washing the pig intestinal tracts three times (Mercier-Bonin and Chapot-Chartier 2017). However, L. reuteri 4-12E, W. cibaria C34, and L. animalis 30a-2 did not lose any inhibition zone when the disc plate containing protease was used; in fact, W. cibaria C34 and L. animalis 30a-2 had increased antimicrobial activity. We then added catalase to the agar plate and found that L. reuteri 4-12E, W. cibaria C34, and L. animalis 30a-2 lost their inhibition zone of S. aureus ATCC 27735, whereas L. lactis 5-12H, L. lactis 6-3H and the positive control L. lactis ATCC11454 maintained their clear inhibition zones (Fig. 3b). Therefore, we propose that the antimicrobial substances produced by W. cibaria C34 and L. animalis 30a-2 might be H2O2. This was supported by the TMB-Plus plate assay, which showed colonies as a blue color when the bacteria produced H2O2 (Rabe and Hillier 2003) (Supplementary Fig. 2).
Characterization of antimicrobial substances produced by the five LABs. Anti-S. aureus assay of the five LABs. Agar plates containing protease (a) or catalase (b) for characterizing antimicrobial substances (i.e., bacteriocin and H2O2) from isolated strains. Five of the best anti-S. aureus ATCC 27735 isolated strains were assessed. Label 1: L. lactis ATCC11454, a nisin-producing strain was used as a positive control and Label 7: L. acidophilus ATCC 4356 was a negative control in this assay; Label 2: the isolated strain 5-12H; Label 3: the isolated strain 6-3H; Label 4: the isolated strain 4-12E; Label 5: the isolated strain C34; Label 6: the isolated strain 30a-2
Generally, S. aureus can produce catalase to destroy H2O2 being produced by microorganisms (Mustafa 2014). Addition of protease to the agar plates increases the inhibition zone produced by W. cibaria C34 and L. animalis 30a-2, probably because the protease degrades external proteins produced by S. aureus for acquiring resistance to Lactobacillus sp., making it susceptible to H2O2 attack from W. cibaria C34 and L. animalis 30a-2. We also found that the inhibition zone produced by bacteriocin forms a clear regular circle, whereas the inhibition zone produced by H2O2 forms an irregular circle (Fig. 1a). This phenomenon could be a way to initially discriminate between bacteriocin and hydrogen peroxide produced by LAB.
Regarding L. reuteri, it is a well-studied probiotic bacterium and has been confirmed to produce organic acid, ethanol, and reuterin as antimicrobial substances (Mu et al. 2018). The reuterin produced from L. reuteri can establish a hostile environment for pathogens and can be resistant to proteolytic enzymes (Kang et al. 2011; Ghosh et al. 2019). In this study, we excluded reuterin biosynthesis from the isolate L. reuteri 4-12E, because the medium did not contain the starting material, glycerol, for reuterin biosynthesis (Vollenweider et al. 2010). L. reuteri 4-12E has anti-S. aureus activity in the presence of protease and is proposed that the anti-S. aureus activity is contributed by ethanol. The ethanol produced by L. reuteri 4-12E is oxidized when the agar plate contains catalase, resulting in the loss of the S. aureus inhibition zone (Fig. 3a, b).
Since L. lactis and L. reuteri are well-studied probiotics and both W. cibaria C34 and L. animalis 30a-2 among the 5 LAB isolates were found to have the best anti-S. aureus activity, especially, L. animalis 30a-2 was found to produce H2O2 for the first time; therefore, we further investigated the anti-MRSA activity, antimicrobial spectra, and potential probiotic characteristics of L. animalis 30a-2 and W. cibaria C34.
Antimicrobial activity of L. animalis 30a-2 and W. cibaria C34
Lactobacillus animalis 30a-2 and W. cibaria C34 were evaluated for their anti-MRSA activity toward 16 clinical strains of MRSA, which were isolated from patients in different hospitals. The MRSA strains are resistant to at least 14 antibiotics and only susceptible to the antibiotics linezolid and vancomycin (Supplementary Table 2). As shown in Table 2, L. animalis 30a-2 has a higher growth inhibitory effect on the 16 MRSA clinical isolates compared with W. cibaria C34; that is, L. animalis 30a-2 produced inhibition zones larger than 25 mm for seven MRSA isolates and between 10 and 25 mm for nine of the MRSA isolates; W. cibaria C34 only produced inhibition zones of between 10 and 25 mm for all 16 isolates.
Lactobacillus animalis 30a-2 and W. cibaria C34 were also evaluated for their antimicrobial spectra against eight commercial bacterial pathogens: Bacillus cereus, Listeria monocytogens, A. baumannii, E. coli, P. aeruginosa, Salmonella choleraesuis, Shigella flexneri, and Yersinia enterocolitica, and three clinically isolated pathogens: MDRAB, P. aeruginosa, and ESBL E. coli, which have different levels of resistance toward 15 antibiotics. For example, MDRAB strains can be resistant toward 14 or 15 antibiotics, P. aeruginosa strains can be resistant toward 8–12 antibiotics, and ESBL E. coli strains can be resistant toward 7–10 antibiotics (Supplementary Table 3). We found that compared with W. cibaria C34, L. animalis 30a-2 had a broader spectrum of inhibition versus commercial pathogens and clinically isolated bacteria (Table 3). Among 20 pathogenic strains tested, L. animalis 30a-2 can inhibit L. monocytogens ATCC19111, A. baumannii ATCC15151, E. coli K12, and MDRAB FY_A397, with an inhibition zone larger than 15 mm. It can inhibit 14 of the 20 pathogenic strains with an inhibition zone between 10 and 15 mm, and produced inhibition zones of less than 10 mm in only two strains. W. cibaria C34 produced inhibition zones greater than 15 mm in only two pathogenic strains, whereas the inhibition zones of between 10 and 15 mm were produced for six of the strains. W. cibaria C34 produced smaller inhibition zones (< 10 mm) for the other 12 pathogenic strains. Generally, L. animalis 30a-2 has better inhibitory effects against clinical isolates of MRSA and broader anti-pathogen spectra than that of W. cibaria C34.
Acid and bile acid tolerance of L. animalis 30a-2 and W. cibaria C34
The application potential of probiotics should be considered based on the viability of the isolated strains after being subjected to conditions of the gastrointestinal (GI) tract; for example, the acidic environment and bile secretions (Sahadeva et al. 2011). As shown in Table 4, the cell viability of L. animalis 30a-2 and W. cibaria C34 after a 3-h incubation in pH 6.5 MRS broth increased by 1.67 and 1.62 log units, respectively. However, when the pH of the MRS broth was reduced to 3.0, the cell viability only increased by 0.66 and 0.1 log units, respectively. At pH 2.0, the cell viability of both strains actually decreased by 1.2 and 1.4 log units, respectively. These results show that L. animalis 30a-2 has a higher tolerance for acid conditions compared with W. cibaria C34.
The results of bile tolerance for both L. animalis 30a-2 and W. cibaria C34 are shown in Table 5. Both strains have good tolerance to 0.3% bile acid (oxgall). After a 24-h incubation in MRS-oxgall broth, the cell viability of L. animalis 30a-2 and W. cibaria C34 increased by 1.91 and 2.59 log units, respectively, which was similar to that of both strains incubated in MRS broth alone. This suggests that both strains are resistant to an environment of high in bile acids.
References
Belgacem ZB, Dousset X, Prevost H, Manai M (2009) Polyphasic taxonomic studies of lactic acid bacteria associated with Tunisian fermented meat based on the heterogeneity of the 16S–23S rRNA gene intergenic spacer region. Arch Microbiol 191:711–720
Brunel AS, Guery B (2017) Multidrug resistant (or antimicrobial-resistant) pathogens—alternatives to new antibiotics? Swiss Med Wkly 147:w14553
Cerceo E, Deitelzweig SB, Sherman BM, Amin AN (2016) Multidrug-resistant Gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist 22:412–431
Chen PW, Jheng TT, Shyu CL, Mao FC (2013) Synergistic antibacterial efficacies of the combination of bovine lactoferrin or its hydrolysate with probiotic secretion in curbing the growth of meticillin-resistant Staphylococcus aureus. J Med Microbiol 62:1845–1851
Crespo-Piazuelo D, Estelle J, Revilla M, Criado-Mesas L, Ramayo-Caldas Y, Ovilo C, Fernandez AI, Ballester M, Folch JM (2018) Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci Rep 8:12727–12738
de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, Wade WG (2006) Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol Immunol 21:61–68
Di Gioia D, Biavati B (2018) Probiotics and prebiotics in animal health and food safety. Springer, Berlin
Elavarasi V, Pugazhendhi A, Priyadharsani TKP, Thamaraiselvi K (2014) Screening and characterization of Weissella cibaria isolated from food source for probiotic properties. Int J Comp Appl 1:29–32
Endo A, Futagawa-Endo Y, Kawasaki S, Dicks LM, Niimura Y, Okada S (2009) Sodium acetate enhances hydrogen peroxide production in Weissella cibaria. Lett Appl Microbiol 49:136–141
Espeche MC, Otero MC, Sesma F, Nader-Macias ME (2009) Screening of surface properties and antagonistic substances production by lactic acid bacteria isolated from the mammary gland of healthy and mastitic cows. Vet Microbiol 135:346–357
Fayol-Messaoudi D, Berger CN, Coconnier-Polter MH, Moal VL-L, Servin AL (2005) pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella entericaSerovar typhimurium. Appl Environ Microbiol 71:6008–6013
Fernandez L, Hancock RE (2012) Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev 25:661–681
Fleming D, Rumbaugh KP (2017) Approaches to dispersing medical biofilms. Microorganisms 5:1–16
Fusco V, Quero GM, Cho GS, Kabisch J, Meske D, Neve H, Bockelmann W, Franz CM (2015) The genus Weissella: taxonomy, ecology and biotechnological potential. Front Microbiol 6:155–176
Geisinger E, Isberg RR (2017) Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. J Infect Dis 215:S9–S17
Ghosh T, Beniwal A, Semwal A, Navani NK (2019) Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front Microbiol 10:502–520
Gresse R, Chaucheyras Durand F, Duniere L, Blanquet-Diot S, Forano E (2019) Microbiota composition and functional profiling throughout the gastrointestinal tract of commercial weaning piglets. Microorganisms 7:343–365
Halder D, Mandal S (2016) Antibacterial potentiality of commercially available probiotic Lactobacilli and curd Lactobacilli strains, alone and in combination, against human pathogenic bacteria. Transl Biomed 7:1–7
Jalilsood T, Baradaran A, Song AA, Foo HL, Mustafa S, Saad WZ, Yusoff K, Rahim RA (2015) Inhibition of pathogenic and spoilage bacteria by a novel biofilm-forming Lactobacillus isolate: a potential host for the expression of heterologous proteins. Microb Cell Fact 14:96–109
Kang MS, Oh JS, Lee HC, Lim HS, Lee SW, Yang KH, Choi NK, Kim SM (2011) Inhibitory effect of Lactobacillus reuteri on periodontopathic and cariogenic bacteria. J Microbiol 49:193–199
Kim J, An JU, Kim W, Lee S, Cho S (2017) Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the Illumina MiSeq platform. Gut Pathog 9:68–79
Kumar M, Dhaka P, Vijay D, Vergis J, Mohan V, Kumar A, Kurkure NV, Barbuddhe SB, Malik SV, Rawool DB (2016) Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int J Antimicrob Agents 48:265–270
Kumar A, Alam A, Rani M, Ehtesham NZ, Hasnain SE (2017) Biofilms: survival and defense strategy for pathogens. Int J Med Microbiol 307:481–489
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lim HJ, Kim SY, Lee WK (2004) Isolation of cholesterol-lowering lactic acid bacteria from human intestine for probiotic use. J Vet Sci 5:391–395
Linares DM, Ross P, Stanton C (2016) Beneficial microbes: the pharmacy in the gut. Bioengineered 7:11–20
Linares DM, Gomez C, Renes E, Fresno JM, Tornadijo ME, Ross RP, Stanton C (2017) Lactic acid bacteria and Bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front Microbiol 8:846–856
Maraolo AE, Cascella M, Corcione S, Cuomo A, Nappa S, Borgia G, De Rosa FG, Gentile I (2017) Management of multidrug-resistant Pseudomonas aeruginosa in the intensive care unit: state of the art. Expert Rev Anti Infect Ther 15:861–871
Martin R, Suarez JE (2010) Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl Environ Microbiol 76:400–405
Mercier-Bonin M, Chapot-Chartier MP (2017) Surface proteins of Lactococcus lactis: bacterial resources for muco-adhesion in the gastrointestinal tract. Front Microbiol 8:2247–2253
Millette M, Smoragiewicz W, Lacroix M (2004) Antimicrobial potential of immobilized Lactococcus lactis subsp. lactis ATCC 11454 against selected bacteria. J Food Prot 67:1184–1189
Mitchell C, Fredricks D, Agnew K, Hitti J (2015) Hydrogen peroxide-producing Lactobacilli are associated with lower levels of vaginal interleukin-1beta, independent of bacterial vaginosis. Sex Transm Dis 42:358–363
Mu Q, Tavella VJ, Luo XM (2018) Role of Lactobacillus reuteri in human health and diseases. Front Microbiol 9:757–773
Mustafa HSI (2014) Staphylococcus aureus can produce catalase enzyme when adding to human WBCs as a source of H2O2 productions in human plasma or serum in the laboratory. J Med Microbiol 4:249–251
Otero MC, Nader-Macias ME (2006) Inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus gasseri isolated from the vaginal tract of cattle. Anim Reprod Sci 96:35–46
Powers EM (1995) Efficacy of the Ryu nonstaining KOH technique for rapidly determining gram reactions of food-borne and waterborne bacteria and yeasts. Appl Environ Microbiol 61:3756–3758
Pridmore RD, Pittet AC, Praplan F, Cavadini C (2008) Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol Lett 283:210–215
Rabe LK, Hillier SL (2003) Optimization of media for detection of hydrogen peroxide production by Lactobacillus species. J Clin Microbiol 41:3260–3264
Rachman CN, Kabadjova P, Prevost H, Dousset X (2003) Identification of Lactobacillus alimentarius and Lactobacillus farciminis with 16S–23S rDNA intergenic spacer region polymorphism and PCR amplification using species-specific oligonucleotide. J Appl Microbiol 95:1207–1216
Rice LB (2006) Antimicrobial resistance in Gram-positive bacteria. Am J Med 119:S11–19
Ruiz L, Margolles A, Sanchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4:396
Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan HY, Tong EV, Wong SYW, Chan HK (2011) Survival of commercial probiotic strains to pH and bile. Int Food Res J 18:1515–1522
Satlin MJ, Walsh TJ (2017) Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus: three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis 19:e12762–e12769
Schmidt M, Unterer S, Suchodolski JS, Honneffer JB, Guard BC, Lidbury JA, Steiner JM, Fritz J, Kolle P (2018) The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 13:e0201279–e0201299
Sgouras DN, Panayotopoulou EG, Martinez-Gonzalez B, Petraki K, Michopoulos S, Mentis A (2005) Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin Diagn Lab Immunol 12:1378–1386
Shokri D, Khorasgani MR, Mohkam M, Fatemi SM, Ghasemi Y, Taheri-Kafrani A (2017) The inhibition effect of Lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob Proteins 10:34–42
Silva BC, Jung LR, Sandes SH, Alvim LB, Bomfim MR, Nicoli JR, Neumann E, Nunes AC (2013) In vitro assessment of functional properties of lactic acid bacteria isolated from faecal microbiota of healthy dogs for potential use as probiotics. Benef Microbes 4:267–275
Somkuti GA, Steinberg DH (2002) Agarose/agar assay system for the selection of bacteriocin-producing lactic fermentation bacteria. Biotechnol Lett 24:303–308
van Duin D, Paterson DL (2016) Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin N Am 30:377–390
Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol 10:57–73
Vollenweider S, Evers S, Zurbriggen K, Lacroix C (2010) Unraveling the hydroxypropionaldehyde (HPA) system: an active antimicrobial agent against human pathogens. J Agric Food Chem 58:10315–10322
Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM (2018) Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J Cell Mol Med 22:1972–1983
Weinstein MP (2018) M02. Performance standards for antimicrobial disk susceptibility tests, 13th edn. Clinical and Laboratory Standards Institute, Wayne
Woo J, Ahn J (2013) Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. Lett Appl Microbiol 56:307–313
Wu C, Huang J, Zhou R (2017) Genomics of lactic acid bacteria: current status and potential applications. Crit Rev Microbiol 43:393–404
Yadav S, Jha R (2019) Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J Anim Sci Biotechnol 10:2–12
Zhou JS, Pillidge CJ, Gopal PK, Gill HS (2005) Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int J Food Microbiol 98:211–217
Acknowledgements
The authors wish to acknowledge the help of Sam-Mi Chang from the Department of Laboratory Medicine, Fong-Yuan Hospital, Ministry of Health and Welfare, Taichung, Taiwan for providing the clinical strains of MRSA, MDRAB, P. aeruginosa, and ESBL E. coli. This work was supported by the Fong-Yuan Hospital, Ministry of Health and Welfare, grant no. 99022 and Central Taiwan University of Science and Technology, grant no. FYH1000305-100.05. We would like to thank two anonymous (unknown) reviewers and the editor for their comments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, CF., Lin, MY., Lin, CN. et al. Potential probiotic of Lactobacillus strains isolated from the intestinal tracts of pigs and feces of dogs with antibacterial activity against multidrug-resistant pathogenic bacteria. Arch Microbiol 202, 1849–1860 (2020). https://doi.org/10.1007/s00203-020-01908-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01908-w