Abstract
The objective of this study was to assess the effects of some culture conditions [temperature (20, 30, 37 °C), incubation time (48, 72, 120 h), pH (5.0, 6.0, 7.0), NaCl concentration (0, 3, 6%), carbon (glucose, fructose, lactose), nitrogen (sodium nitrate, ammonium sulfate, bacto-peptone), and mineral sources (calcium carbonate, ferric chloride)] on the exopolysaccharide (EPS) production by lactic acid bacteria (LAB) strains (belonging to Lactobacillus (L.) plantarum, L. namurensis, and Pediococcus (P.) ethanolidurans species) isolated from naturally fermented pickles. The maximum EPS production was determined at 30 °C and pH 6.0. The highest amount of EPS was obtained after 120 h of incubation, with glucose as carbon source, bacto-peptone as nitrogen source and calcium carbonate as mineral source for most of the tested strains. The EPS formation was not stimulated by NaCl, indicating that EPS formation of the tested strains was not a stress response. L. plantarum MF460 produced the highest amount of EPS at 30 °C after 48 h of incubation, which was 515.48 mg/L. One of the most pronounced results of this study was that the EPS production of L. plantarum MF556 strain was increased up to 512.81 mg/L with the addition of calcium carbonate to MRS medium. The effect of different culture conditions, particularly of incubation time, carbon, nitrogen, and mineral sources, on the EPS production often vary depending on the strain. Therefore, these apparent strain specific results demonstrated that the optimum culture conditions for the enhanced EPS production should be specifically determined for each LAB strain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exopolysaccharides (EPS) are high-molecular-weight, carbohydrate biopolymers produced extracellularly by a great variety of microorganisms including some bacteria, microalgae, yeasts and fungi (De Vuyst and Degeest 1999). Certain lactic acid bacteria (LAB) species are capable of forming EPS that are either covalently linked to the cell surface, forming capsules, or secreted into the environment, forming slimes. These two types of EPS are called as capsular polysaccharides (CPS) or slime EPS (further referred to as EPS), respectively (Degeest et al. 2001b).
EPS produced by some strains of LAB have recently aroused considerable interest due to their industrially important physicochemical properties as well as their potential beneficial effects on human health. In terms of potential technological applications, EPS-producing LAB can be widely used as viscosifying, stabilizing, emulsifying, or gelling agents in the dairy food industry due to their positive effect on the rheological and textural properties of fermented milk products such as yoghurt and cheese (Bengoa et al. 2018; Caggianiello et al. 2016). In addition to their role in the development of technological properties of fermented food products, EPS from LAB also possess health-promoting effects because of their antitumor, immunostimulatory, antiulcer or cholesterol-lowering activities. Moreover, EPS are thought to play a role in the protection of the bacterial cells against harsh environmental conditions, such as desiccation, osmotic stress, pH and antimicrobial factors, such as bacteriophages, toxic metal ions, sulfur dioxide, ethanol and antibiotics. EPS may also be involved in biofilm formation, adhesion of cells onto solid surfaces and cellular recognition (De Vuyst and Degeest 1999).
Although EPS-forming LAB, with its advantage as being generally regarded as safe (GRAS) status, have significant potential for the development of functional foods either as starter and coadjunct cultures or by in situ production of bioactive polymers with both technological and health benefits (Torino et al. 2015), the relatively low production of EPS (50–400 mg/L) by LAB, compared with the industrial microbial exopolysaccharides, such as xanthan, is the limiting factor hindering their industrial exploitation (Caggianiello et al. 2016; Cirrincione et al. 2018). In addition, the degradation of EPS upon extended incubation of the EPS-producing LAB strain is one of the main obstacle hindering the industrial production of these polymers. The EPS production levels reported for LAB strains varies between 45 and 350 mg/L under non-optimized culture conditions, and varies between 150 and 600 mg/L under optimal culture conditions (De Vuyst et al. 2001; Degeest et al. 2001b). The EPS yield should be at least 10–15 g/L in order for it to be applicable in food industry. The highest EPS production level reported to date for lactobacilli was 2.775 g/L which was obtained using L. rhamnosus RW-9595 M (Macedo et al. 2002). The second highest recorded yield was 2.5 g/L for L. kefiranofaciens WT-2B (Maeda et al. 2004). However, these values are still not comparable with the considerably high yields (30–50 g/L xanthan gum) obtained with the commercial EPS-producer, Xanthomonas campestris (Caggianiello et al. 2016). Aside from dextran produced by Leuconostoc mesenteroides, EPS originated from LAB have not been industrially used as food additive yet (Torino et al. 2015). Nevertheless, in spite of the lower yields, even the low amounts of EPS produced by LAB could be adequate for their exploitation for in situ applications (Cirrincione et al. 2018). Furthermore, it is possible to enhance EPS production by manipulating the culture conditions since the EPS yield produced by LAB largely depends on the microbial growth conditions (temperature, pH, oxygen tension, incubation time, etc.) and the composition of the medium (carbon and nitrogen sources, growth factors, etc.) (Bengoa et al. 2018; Degeest et al. 2001b). In addition, the EPS production by LAB has been observed to be strain-dependent. Therefore, several attemps have been made to obtain higher yields of EPS by selecting new bacterial strains or by optimization of culture conditions (Looijesteijn et al. 2000). Although there have been a considerable amount of studies on EPS production by LAB strains isolated from fermented dairy products (Aslim et al. 2005; Bengoa et al. 2018) and sourdough (Abedfar et al. 2018; Dertli et al. 2016; Liu et al. 2018), to the best of our knowledge, research on EPS-producing LAB strains originated from fermented vegetables (Grosu-Tudor and Zamfir 2013; Kim et al. 2008) has rarely been reported. In addition, to date, the main LAB species that have been reported to produce EPS include L. rhamnosus (Macedo et al. 2002), L. plantarum (Imran et al. 2016; Zhang et al. 2013), L. helveticus (Li et al. 2014), L. delbrueckii subsp. bulgaricus (Aslim et al. 2005; Grobben et al. 1998), Streptococcus thermophilus (De Vuyst et al. 1998; Degeest et al. 2002), Lactococcus lactis subsp. lactis (Pan and Mei 2010) and P. pentosaceus (Shin et al. 2016). However, there are only few reports (Llamas-Arriba et al. 2018) on the EPS production of L. namurensis and P. ethanolidurans strains which were involved in our tested strains. Therefore, the objective of this study was to assess the effects of some culture conditions (incubation time, temperature, medium pH, NaCl concentration and different carbon, nitrogen, and mineral sources) on the EPS production by LAB strains isolated from traditional pickles.
Materials and methods
LAB strains and growth conditions
Ten indigenous LAB strains isolated from spontaneously fermented pickles and maintained in the Culture Collection of the Ankara University, Department of Food Engineering were used in this study. The test strains were preselected among 152 LAB strains based on their ropiness character. For qualitative evaluation of EPS, the cultures were inoculated onto de Man Rogosa and Sharpe (MRS) agar plates and the strains were considered as potential EPS-producer if mucoid or ropy colonies were observed. Eight L. plantarum, one L. namurensis and one P. ethanolidurans strains which were previously identified molecularly by 16S rRNA gene sequencing were used in this study and the GenBank accession numbers of these strains are listed in Table 1 (Elmaci et al. 2015). The LAB strains were cryopreserved in 30% (v/v) glycerol solution at − 65 °C until use. Before each experiment, the stock cultures were activated in MRS broth at 30 °C for 48 h.
Isolation and quantification of EPS
The isolation of EPS was performed according to the method previously described by Aslim et al. (2006) with certain modifications. Briefly, the LAB cultures, which were grown in 10 mL MRS broth, were treated with 1 mL of 80% (v/v) trichloroacetic acid solution and then the cell suspension was stirred by turning upside down at room temperature. The stirred cultures were maintained at − 18 °C for 10 min. Removal of cell pellets and protein were performed by centrifugation at 5000 rpm for 20 min. For EPS precipitation, 5 mL of the collected supernatant was mixed with 10 mL of cold ethanol (95%, v/v) and then the mixture was stirred by turning upside down and maintained at − 18 °C for 30 min. Recovery of EPS was then performed by centrifugation at 5000 rpm for 15 min. The pellets obtained after centrifugation were washed with 5 mL of ethanol by centrifugation. The remaining pellets after the evaporation of ethanol were kept at − 18 °C until analysis.
The content of total sugar in the precipitated and isolated EPS was determined spectrophotometrically as glucose equivalent according to a modified Miller method using DNS (3,5-dinitrosalicylic acid) reagent (Forouhi and Gunn 1983). Briefly, for the hydrolysis reaction 2 mL of 2 N HCl was added to 1 mL of sample. It was held in boiling water bath for 2 h. After cooling the hydrolyzed samples, 2 mL of 2 N NaOH was added for neutralization. After the hydrolysis step, the samples were appropriately diluted and 1 mL of the diluted samples was mixed with 2 mL of DNS reagent (containing 1% dinitrosalicylic acid, 1% NaOH, 0.16% phenol). The samples were then held in boiling water bath for 15 min. After cooling the test tubes to room temperature, 1 mL of Rochelle salt solution (40% sodium–potassium tartrate) was added to stabilize the red-brown color. The absorbance of the samples was measured at 575 nm by a SHIMADZU UV-1280 spectrophotometer. The EPS concentration (expressed as mg/L) was determined using glucose standard solutions (0–640 mg/L).
Factors affecting the EPS production by LAB strains
Effect of temperature on EPS production
Effect of temperature on EPS production was performed by inoculating 10 mL MRS broth with freshly grown LAB cultures. In all experiments, 1% (v/v) inoculum was used. The inoculated cultures were incubated at 20, 30, and 37 °C for 120 h and then the EPS concentration was determined by the aforementioned method.
Effect of incubation time on EPS production
To evaluate the effect of incubation time on EPS production, three different incubation times were tested: 48, 72, and 120 h. For this purpose, the freshly grown cultures were inoculated into 10 mL MRS broth and incubated at 30 °C. The EPS concentration produced by the LAB strains was determined by the aforementioned method after each incubation period.
Effect of medium pH on EPS production
To study the effect of pH on EPS production, the initial pH of MRS medium was adjusted to three pH values of 5.0, 6.0 and 7.0 with 2 N NaOH or 2 N HCI. The 48-h cultures of LAB strains, activated in MRS broth at 30 °C, were then inoculated into pH-adjusted MRS broth. The EPS concentration was determined after 120 h of incubation at 30 °C.
Effect of NaCl concentration on EPS production
The 48-h cultures of LAB strains, activated in MRS broth at 30 °C, were inoculated into MRS broth containing 0%, 3%, and 6% NaCl. After 120 h of incubation at 30 °C, the EPS concentration was determined.
Effect of different carbon, nitrogen and mineral sources supplementation on EPS production
Different carbon sources at 2% concentration (glucose, lactose and fructose) or nitrogen sources at 1% concentration (sodium nitrate, ammonium sulfate and bacto-peptone) or mineral sources (ferric chloride (FeCl3) at 0.5% or calcium carbonate (CaCO3) at 2%) were added individually to MRS broth, and the standard MRS broth without any supplement were used as control. The 48-h cultures of LAB strains, activated in MRS broth at 30 °C, were then inoculated into MRS broth containing different carbon, nitrogen or mineral sources. After incubation at 30 °C for 120 h, the EPS concentration was determined.
Statistical analysis
All experiments were performed in two independent replicates. The experimental data were statistically analyzed by one-way ANOVA using the Minitab Software Version 14 (Minitab Inc., State College, PA, USA) followed by the Duncan’s multiple range test at the 5% level.
Results
Effect of temperature on EPS production
The EPS production amounts of the tested LAB strains at varied temperatures are given in Table 2. There was significant interaction effect between temperature and LAB strains (P < 0.05). The EPS production ability of all tested LAB strains was the highest at 30 °C which is the optimum growth temperature of the strains. Therefore, 30 °C was selected as the incubation temperature for further experiments. EPS production of all LAB strains dramatically decreased as the culture temperature was increased from 30 to 37 °C. Although to a lesser extent, a similar decreasing trend was also observed for 20 °C, the lowest culture temperature tested in this study. Particularly, the amount of EPS produced by L. plantarum MF460 at 20 °C (418.09 mg/L) was similar to that at 30 °C (433.61 mg/L). Among the ten tested LAB strains, maximum amount of EPS was produced by L. plantarum MF460 (433.61 mg/L) followed by L. plantarum MF303 (408.53 mg/L) and L. plantarum MF556 (401.29 mg/L) at 30 °C. On the other hand, the lowest EPS concentration was found for L. plantarum MF176 (56.15 mg/L), followed by L. namurensis MF210 (68.56 mg/L) and P. ethanolidurans MF194 (72.18 mg/L) at 37 °C. The most dramatical decrease in EPS production was observed in strain MF176 when the temperature increased from 30 to 37 °C, as the EPS concentration was decreased by about 85% (from 377.50 to 56.15 mg/L).
Effect of incubation time on EPS production
As seen in Table 3, EPS production was significantly influenced by the interaction effect of incubation time and LAB strains (P < 0.05). For 6 of the 10 tested strains, maximum EPS production was achieved after 120 h of incubation. Therefore, 120 h was selected as the incubation time for further experiments. The EPS production of L. plantarum MF232 was not affected by the incubation time since the difference between the EPS yields with respect to the three different incubation periods was statistically insignificant. The results showed that 48 h was the optimum incubation time for strains MF303 and MF460. L. plantarum MF460 produced the highest amount of EPS after 48 h of incubation, which was 515.48 ± 16.97 mg/L. There is a tendency for the EPS production of these two strains to decrease as the incubation time increases. In L. plantarum MF169, maximum EPS production was obtained at 72 h which was 313.60 mg/L. L. plantarum strains MF460, MF556, MF176, and MF303 exhibited significantly higher production of EPS as compared to the other strains tested at their optimum incubation time.
Effect of medium pH on EPS production
As shown in Table 4, there was a statistically significant interaction effect of pH and LAB strains for EPS concentration (P < 0.05). All strains exhibited maximum EPS production at pH 6.0 which is similar with the pH of MRS broth (pH 5.8). pH 5.0 and 7.0 resulted in a decrease in EPS yield, especially at pH 7.0. The highest amount of EPS was obtained by L. plantarum MF460 (470.47 mg/L) followed by L. plantarum MF303 (419.02 mg/L) and L. plantarum MF176 (396.27 mg/L) under pH 6.0 condition. At pH 7.0, the lowest EPS values were recorded for P. ethanolidurans MF194 strain with the concentration of 85.52 mg/L.
Effect of NaCl concentration on EPS production
In all tested strains, the addition of NaCl caused a reduction in EPS concentration (P < 0.05) (Table 5). The LAB strains examined in this study produced the highest quantity of EPS in medium containing no NaCl whereas the EPS yield was significantly lower in medium containing 3% and 6% NaCl. Similar with the previous results regarding the effects of temperature, incubation time and pH in this study, the strain which produced the greatest amount of EPS (488.57 mg/L) was L. plantarum MF460. The EPS concentration decreased by ranging from 65 to 87% depending on the strains tested as the NaCl concentration was increased from 0 to 6%. However, this reduction effect was less pronounced in high EPS-producing strains in this study, including L. plantarum MF303 and L. plantarum MF460.
Effect of different carbon source supplementation on EPS production
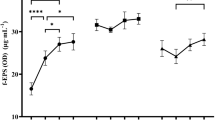
According to the variance analysis results, the interaction effects between different carbon sources and LAB strains on the EPS concentration were found to be statistically significant (P < 0.05). Figure 1 shows that supplementation of additional carbon source including glucose, fructose, and lactose in MRS broth did not substantially enhance the EPS production of the tested LAB strains, except for the strains MF210, MF232, MF303 in which the addition of glucose led to a slight increase in EPS production. Addition of 2% (w/v) glucose to MRS broth resulted in no change (L. plantarum MF169, L. plantarum MF176, P. ethanolidurans MF194, L. plantarum MF400, L. plantarum MF460, L. plantarum MF556) or slight increase (L. namurensis MF210, L. plantarum MF232, L. plantarum MF303) in the EPS production. Contrary to expectations, fructose even leads to decrease in EPS production of all tested LAB strains in comparison with the control MRS broth. In addition, supplementation of MRS broth with lactose also decreased EPS production, but to a lesser extent than fructose. However, the yields of EPS produced by L. plantarum MF169, L. plantarum MF460, and L. plantarum MF556 were not influenced by the addition of lactose. Although the control MRS medium containing 20 g/L glucose in its composition was the best carbon source, the supplement sugars can be arranged as glucose, lactose, and fructose in decreasing order of the EPS yield.
Effect of different nitrogen source supplementation on EPS production
According to the variance analysis results, the interaction effects between different nitrogen sources and LAB strains on the EPS concentration were found to be statistically important (P < 0.05). As seen in Fig. 2, the addition of sodium nitrate affected the EPS concentrations in different ways since it caused slight reduction in 6 strains, slight increase in 3 strains, and no change in 1 strain. Similarly, the effect of supplementation with bacto-peptone on EPS production varied depending on the strain. For instance, addition of bacto-peptone resulted in higher yields of EPS in 6 of the tested strains whereas caused reduction or no change in the rest of the tested strains. Amongst the nitrogen sources tested, the highest amount of EPS was obtained with L. plantarum MF460 in MRS broth containing bacto-peptone and sodium nitrate which was 447.58 mg/L and 444.28 mg/L, respectively. For all tested LAB strains in this study, the lowest EPS yields were obtained with the addition of ammonium sulfate. When comparing the nitrogen supplements tested in this study in terms of their effectiveness on the EPS production, they could be arranged in descending order as bacto-peptone, sodium nitrate, and ammonium sulfate.
Effect of supplementation with different nitrogen sources of sodium nitrate, ammonium sulfate, and bacto-peptone when compared with the control without supplementation on the EPS yield by LAB strains in MRS broth at 30 °C for 120 h. *Results are presented as means ± SD for duplicate. Bars indicate standard deviations
Effect of different mineral source supplementation on EPS production
The effect of different mineral sources as compared with control medium on EPS production by LAB strains was evaluated. The two-way ANOVA revealed that the EPS concentration was affected by the interaction of different mineral sources and LAB strains (P < 0.05). As can be seen from Fig. 3, the addition of calcium carbonate to MRS broth promoted the EPS production by most of the tested strains, especially by L. plantarum MF556. On the other hand, the supplementation with either ferric chloride or calcium carbonate resulted in lower yields of EPS in 3 of the tested strains (L. plantarum MF169, L. plantarum MF176, L. namurensis MF210) since higher yields were obtained in the control group. The effect of ferric chloride addition on EPS production seems to be strain-dependent since there is no consistency in the extent of EPS production in response to the supplementation. The EPS concentration was decreased in 5 strains, increased in 4 strains, and was remained unchanged with the addition of ferric chloride. Despite the fact that L. plantarum MF460 was considered to be the best EPS-producer in the other tests of this study, L. plantarum MF556 produced the maxiumum amount of EPS (512.81 mg/L) as a result of calcium carbonate addition.
Effect of supplementation with different mineral sources of calcium carbonate (CaCO3) and ferric chloride (FeCl3) when compared with the control without supplementation on the EPS yield by LAB strains in MRS broth at 30 °C for 120 h. *Results are presented as means ± SD for duplicate. Bars indicate standard deviations
Discussion
It is well known that one of the most important parameters that influence the EPS biosynthesis is temperature (Li et al. 2014). In this study, any increase or decrease in the culture temperature from optimum growth temperature (30 °C) caused a decrease in EPS production. The highest tested temperature (37 °C) resulted in the lowest EPS production in all tested LAB strains in this study. For mesophilic LAB strains, the EPS production seems to be stimulated under sub-optimal conditions for growth, such as low temperatures, whereas it has generally been reported to be growth-associated for thermophilic LAB strains (De Vuyst et al. 1998; Degeest et al. 2001b). In contrast with this phenomenon for mesophilic LAB strains, EPS production of the tested strains in our study seems to occur at condition favourable for bacterial growth. Similarly, Zhang et al. (2011) reported that L. fermentum F6 produced higher amount of EPS at optimum growth temperature (37 °C). Polak-Berecka et al. (2014) also found that EPS production by L. rhamnosus was greater at 37 °C than at 30 °C. In general, the optimum temperature for EPS production was reported to be approximately 25 and 40 °C for mesophilic and thermophilic LAB, respectively (Harutoshi 2013). Several studies showed that low temperatures markedly enhanced polymer production levels of mesophilic LAB. For instance, in a study conducted by Tsuda and Miyamoto (2010), the highest EPS yield was obtained at 25 °C, which was below the optimum growth temperature (30 °C) of the tested L. plantarum 301102S strain. On the other hand, L. plantarum T10 produced higher quantity of EPS (410.44 mg/L) at 35 °C than at 30, 40, and 45 °C (Khanh and Thao 2016). For L. sakei, Degeest et al. (2001a) also observed higher EPS yields at temperatures lower than the optimum growth temperature of the test strain (30 °C). Similarly, Bengoa et al. (2018) reported that L. paracasei strains produced higher amount of EPS after incubation at 20 °C than at 30 or 37 °C. The stimulatory effect of low temperature on EPS production by mesophilic LAB has been explained by the fact that under sub-optimal growth temperatures much slower cell wall polymers biosynthesis is exhibited by slowly growing cells. Therefore, more isoprenoid lipid carrier precursors (undecaprenol (C55) lipid carrier) will be used for the EPS biosynthesis rather than for the production of cell wall material, resulting in increased EPS production. Because undecaprenol plays an essential role both in the biosynthesis of EPS and the cell wall components such as peptidoglycan, lipopolysaccharide, and teichoic acid (Bengoa et al. 2018; Degeest et al. 2001b; Vandenberg et al. 1995). But there is also another possibility that at optimum growth temperatures, microorganism may use excess sugar for cell wall biosynthesis, which could be the possible explanation for the coupled effect between growth and EPS production of our tested LAB strains with respect to temperature.
Although the optimum pH for EPS formation has been found to vary depending on the LAB strain and the experimental conditions, it is generally around pH 6.0 (Aslim et al. 2005; Degeest et al. 2001b), which is in accordance with our results. Khanh and Thao (2016) reported that L. plantarum T10 strain produced the highest EPS amount (397.72 mg/L) at pH 5.5, which was almost similar with our results. Similar results were also reported for other lactobacilli. Haj-Mustafa et al. (2015) who worked on L. rhamnosus 519 strain reported that the maximum EPS amount was obtained at pH 5.7 and above that pH, EPS amount was reduced. For L. delbrueckii subsp. bulgaricus B3 and G12 strains, the greatest amount of EPS was obtained at pH 6.2, as compared with the other pH values tested (pH 4.0, 4.5, 5.0, 5.5, 6.0, and 7.0) (Aslim et al. 2005). Contrary to our results in which the neutral pH led to a remarkable reduction in the amount of EPS, Imran et al. (2016) indicated that the neutral pH promoted the EPS production in L. plantarum NTMI05 and NTMI20 strains.
For most of the tested LAB strains in this study, the maximum EPS production was attained after 120 h of incubation period. The lack of EPS breakdown during the extended incubation period up to 120 h could be attributed by the fact that the EPS production level reached to the maximum at end of fermentation, as in the case of Streptococcus thermophilus SST111 (Vaningelgem et al. 2004). For the strains MF303 and MF460 as well as MF169 in which the maximum amount of EPS was produced at 48 h and 72 h, respectively, decrease in EPS production was determined after their peak EPS value in terms of incubation time. The EPS degradation upon extended incubation of the EPS-producing LAB strains has been reported, probably due to their glycohydrolase activity (Degeest et al. 2001b). In a study by Imran et al. (2016), the higher amount of EPS by L. plantarum NTMI05 and NTMI20 was obtained after 72 h of incubation in comparison with 24, 48, 96, and 120 h. Khanh and Thao (2016) found that the highest EPS amount for L. plantarum T10 strain was produced at 48 h, followed by a decrease in EPS production after 48 h of incubation. Haj-Mustafa et al. (2015) also reported that the highest amount of EPS was produced by the strain L. rhamnosus 519 at 49 h and the amount of EPS diminished after 49 h of fermentation. Zhang et al. (2013) indicated that the amount of EPS produced by L. plantarum C88 strain reached to a peak value of 69 mg/L in the late stationary growth phase (32 h) and then started to decrease in the early decline growth phase. For the tested LAB strains in this study, a direct relationship was found between optimum temperature and pH conditions for growth and EPS yields, which is an indication for possible coupling of growth and EPS production. However, because of the lack of results with regard to the kinetics of growth, which is one of the deficiencies of our study, we can not conclude whether EPS production is synchronized with growth or not. In the growth-associated type of EPS production, the onset of EPS production is generally synchronized with growth, followed by showing a maximum rate in the logarithmic growth phase and reaching a maximum towards the end of the logarithmic growth. This kind of growth-associated EPS production indicates primary metabolite kinetics. On the other hand, the continued EPS production was also observed beyond or only in the stationary phase, and thereby followed secondary metabolite kinetics (Degeest et al. 2001b). As stated by Pham et al. (2000), in the case of glucose-grown cells, L. rhamnosus R strain continued to produce EPS beyond the declining phase. However, EPS was not further produced after growth has finished in the lactose added medium. Therefore, Pham et al. (2000) concluded that EPS from LAB should not be considered as secondary metabolites, but rather as minor products diverted away from glycolysis. Although the growth kinetics were not studied, our results regarding the effect of incubation time seem to be accordance with the findings proposed by Pham et al. (2000) since 120 h is a long time period for EPS production, implying the decline growth phase.
It was examined whether the EPS yields of LAB strains could be enhanced under NaCl stress condition, since EPS are thought to be produced as a defense to protect the bacterial cells in response to stress factors (Caggianiello et al. 2016). However, the results of this study indicated that EPS formation was not stimulated by NaCl since the lower yields of EPS were obtained with increasing NaCl concentrations. Similar results with regard to the negative impact of high NaCl stress condition on EPS production have also reported for L. helveticus ATCC 15807 (Torino et al. 2005) and P. parvulus 2.6 (Velasco et al. 2006). From the technological point of view, the decreased ability of the tested LAB strains to produce EPS in the presence of NaCl limits their potential in situ application for the manufacture of fermented dairy products, i.e., cheese.
Based on the effectiveness of the carbon sources on the EPS production, the tested sugars could be ranked as follows: glucose > lactose > fructose, when comparing them between each other. In agreement with our results, Zivkovic et al. (2015) reported that L. paraplantarum BGCG11 colonies lost their ropiness character in the growth medium supplemented with fructose as the sole carbon source. Similarly, it was noted that the EPS production by L. delbrueckii subsp. bulgaricus NCFB 2772 was 30 mg/L and 95 mg/L, with fructose and glucose, respectively (Grobben et al. 1997). Several studies have shown that glucose was an effective carbon source for EPS production by several LAB strains. Imran et al. (2016) indicated that L. plantarum NTMI05 and NTMI20 strains exhibited higher EPS production in the presence of glucose, as compared with the other carbon sources tested (galactose, lactose, and sucrose). Similarly, Polak-Berecka et al. (2014) reported that glucose, followed by galactose and lactose exhibited maximum EPS production by L. rhamnosus E/N. L. rhamnosus E/N was found to produce threefold higher EPS with glucose, galactose or lactose than with fructose, maltose or saccharose as a carbon source. Zhang et al. (2011) reported that the amount of EPS produced by L. fermentum F6 increased to varying extents with the addition of different sugars to the skim milk, with glucose giving the maximum amount of EPS. According to their study, the effectiveness of the sugars was in the order of glucose, fructose, lactose and galactose, with EPS concentrations of 33.05, 16.40, 13.94, 12.50 mg/L, respectively. According to a study conducted by Cerning et al. (1994) showed that glucose was the most suitable carbon source for the production of EPS by L. casei CG1, whereas lactose was not. They also stated in their study that the concentration of sugar had a remarkable effect on EPS yield and the presence of 20 g/L glucose in the medium resulted in maximum amount of EPS production. In light of that finding, a possible explanation for the results of our study would be that further increasing the glucose content did not result in further enhancement in the EPS production since the glucose content of MRS broth (20 g/L) was more than enough for the maximum EPS production.
Yeast extract and peptone are needed for either cell growth or EPS production since they are complex nutrients that include vitamins, minerals, purins and pyrimidins playing significant role in these processes (Degeest et al. 2002). The carbon source is mainly utilized for the production of energy required for cell growth and EPS production, as well as for the biosynthesis of biomass and EPS precursors. Although amino acids do not directly play role in EPS production, they may provide key carbon and nitrogen sources for the synthesis of essential cell components. Since the EPS production is growth-associated in some LAB and is an energy demanding process, the EPS production might be favoured by a high C:N ratio and adequate amounts of carbon and nitrogen sources (Degeest et al. 2001b, 2002; Harutoshi 2013).
Similar to the effect of various carbon sources, the supplementation with different nitrogen sources (sodium nitrate, ammonium sulfate, and bacto-peptone) also did not stimulate the EPS production of the tested LAB strains. The EPS production by LAB may be either stimulated or inhibited by the addition of different nitrogen sources, indicating this effect appears as strain-dependent (Khanh and Thao 2016). Hence, in this study, EPS production responses to sodium nitrate and bacto-peptone addition varied significantly among the tested strains. Ammonium sulfate as an inorganic nitrogen source led to decrease in EPS synthesis of all LAB strains. Similar to ammonium sulfate, sodium nitrate, which was another inorganic nitrogen source tested, negatively affected the EPS production of most of the tested strains. Similarly, several researchers have inferred that inorganic nitrogen sources leaded to relatively lower amount of EPS, as compared to organic nitrogen sources (Ergene and Avci 2018; Razack et al. 2013). It was proposed that some essential amino acids could not be derived from inorganic sources of nitrogen (Razack et al. 2013). In contrast to this phenomenon, a study reported that the EPS production of a probiotic L. plantarum strain was increased from 0.14 to 1.08 g/L with the addition of ammonium sulphate as an inorganic nitrogen source (Ismail and Nampoothiri 2010). Although the effect of bacto-peptone addition on EPS production varied depending on the strain, bacto-peptone as an organic nitrogen source gave higher EPS yields in comparison with the other nitrogen sources tested. In accordance to our result, in a study by Li et al. (2014) soybean peptone was reported to be the best nitrogen source for EPS production of L. helveticus MB2-1. Khanh and Thao (2016) reported that yeast extract was the most suitable nitrogen source for EPS production of L. plantarum T10.
In studying the effect of two different mineral salt sources, calcium carbonate was considered as a good accelerator for EPS production. The most pronounced effect of calcium carbonate addition was observed in strain MF556 that the highest amount of EPS was found to be 512.81 mg/L which was also the second highest EPS amount reached throughout the study. As is the case for the effect of carbon and nitrogen sources, the effect of mineral sources on EPS production varied depending on the strain. In contrast with calcium carbonate, ferric chloride addition resulted in decrease in EPS amount of half of the tested strains, as compared with the control MRS broth. Mineral salts such as metal cations (Mg2+ and Ca2+) may have a catalytic role as coenzyme or components of coenzyme on cellular metabolism (Li et al. 2014). Li et al. (2014) reported that for the strain L. helveticus MB2-1, maximum EPS production was found to be 479 mg/L as a result of 3 g/L MgSO4 addition to basal whey medium, followed by CaCl2 with 405 g/L of EPS production. With regard to the iron requirements of LAB, Grobben et al. (2000) found that the omission of Fe2+ ion did not affect either the growth or the EPS production of L. delbrueckii subsp. bulgaricus NCFB 2772.
In conclusion, our results revealed that the microbial growth conditions and the medium composition have a considerable effect on EPS production by L. plantarum, L. namurensis and P. ethanolidurans strains. The EPS production was enhanced by up to 515.48 mg/L for L. plantarum MF460 and 512.81 mg/L for L. plantarum MF556 by altering the culture conditions. These values are relatively high values in comparision with the reported EPS levels for LAB strains ranging from 150 to 600 mg/L (De Vuyst et al. 2001; Degeest et al. 2001b). However, the highest yields obtained in this study are still not comparable with the EPS level produced by L. rhamnosus RW-9595 M (2.775 g/L) which was described as the highest EPS-producer strain within LAB (Macedo et al. 2002). From the findings of our study, the effects of different culture conditions, particularly of incubation time, carbon, nitrogen, and mineral sources, on the EPS production often vary depending on the strain. Therefore, these apparent strain specific results demonstrated that the optimum culture conditions for the enhanced EPS production should be specifically determined for each LAB strain. It has already been known that there is not any single set of cultural conditions in which the maximum EPS yields are ensured for all EPS-producing LAB. It is also important to point out that the single factor method, which was used in the present study, is time consuming and may overlook the interactions between the different variables. Therefore, further studies using response surface methodology should be conducted for the optimization of culture conditions and fermentation media to enhance the yield of the highest EPS-producer in this study such as L. plantarum MF460 and L. plantarum MF556.
References
Abedfar A, Hosseininezhad M, Sadeghi A, Raeisi M, Feizy J (2018) Investigation on "spontaneous fermentation" and the productivity of microbial exopolysaccharides by Lactobacillus plantarum and Pediococcus pentosaceus isolated from wheat bran sourdough. Lwt-Food Sci Technol 96:686–693. https://doi.org/10.1016/j.lwt.2018.05.071
Aslim B, Yuksekdag ZN, Beyatli Y, Mercan N (2005) Exopolysaccharide production by Lactobacillus delbruckii subsp bulgaricus and Streptococcus thermophilus strains under different growth conditions. World J Microb Biot 21:673–677. https://doi.org/10.1007/sl1274-004-3613-2
Aslim B, Beyatli Y, Yuksekdag ZN (2006) Productions and monomer compositions of exopolysaccharides by Lactobacillus delbrueckii subsp bulgaricus and Streptococcus thermophilus strains isolated from traditional home-made yoghurts and raw milk. Int J Food Sci Technol 41:973–979. https://doi.org/10.1111/j.1365-2621.2005.01155.x
Bengoa AA, Llamas MG, Iraporda C, Duenas MT, Abraham AG, Garrote GL (2018) Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol 69:212–218. https://doi.org/10.1016/j.fm.2017.08.012
Caggianiello G, Kleerebezem M, Spano G (2016) Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biot 100:3877–3886. https://doi.org/10.1007/s00253-016-7471-2
Cerning J, Renard CMGC, Thibault JF, Bouillanne C, Landon M, Desmazeaud M, Topisirovic L (1994) Carbon source requirements for exopolysaccharide production by Lactobacillus-Casei Cg11 and partial structure-analysis of the polymer. Appl Environ Microb 60:3914–3919
Cirrincione S, Breuer Y, Mangiapane E, Mazzoli R, Pessione E (2018) 'Ropy' phenotype, exopolysaccharides and metabolism: study on food isolated potential probiotics. LAB Microbiol Res 214:137–145. https://doi.org/10.1016/j.micres.2018.07.004
De Vuyst L, Degeest B (1999) Heteropolysaccharides from lactic acid bacteria. Fems Microbiol Rev 23:153–177. https://doi.org/10.1016/S0168-6445(98)00042-4
De Vuyst L, Vanderveken F, Van de Ven S, Degeest B (1998) Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth-associated biosynthesis. J Appl Microbiol 84:1059–1068
De Vuyst L, De Vin F, Vaningelgem F, Degeest B (2001) Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int Dairy J 11:687–707. https://doi.org/10.1016/S0958-6946(01)00114-5
Degeest B, Janssens B, De Vuyst L (2001a) Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0–1: production kinetics, enzyme activities and EPS yields. J Appl Microbiol 91:470–477. https://doi.org/10.1046/j.1365-2672.2001.01404.x
Degeest B, Vaningelgem F, De Vuyst L (2001b) Microbial physiology, fermentation kinetics, and process engineering of heteropolysaccharide production by lactic acid bacteria. Int Dairy J 11:747–757. https://doi.org/10.1016/S0958-6946(01)00118-2
Degeest B, Mozzi F, De Vuyst L (2002) Effect of medium composition and temperature and pH changes on exopolysaccharide yields and stability during Streptococcus thermophilus LY03 fermentations. Int J Food Microbiol 79:161–174. https://doi.org/10.1016/S0168-1605(02)00116-2
Dertli E, Mercan E, Arici M, Yilmaz MT, Sagdic O (2016) Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. Lwt-Food Sci Technol 71:116–124. https://doi.org/10.1016/j.lwt.2016.03.030
Elmaci SB, Tokatli M, Dursun D, Ozcelik F, Sanlibaba P (2015) Phenotypic and genotypic identification of lactic acid bacteria isolated from traditional pickles of the Cubuk region in Turkey. Folia Microbiol 60:241–251. https://doi.org/10.1007/s12223-014-0363-x
Ergene E, Avci A (2018) Effects of cultural conditions on exopolysaccharide production by Bacillus sp ZBP4. Tarim Bilim Derg 24:386–393
Forouhi E, Gunn DJ (1983) Some effects of metal-ions on the estimation of reducing sugars in biological media. Biotechnol Bioeng 25:1905–1911. https://doi.org/10.1002/bit.260250717
Grobben GJ et al (1997) Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose. Appl Microbiol Biot 48:516–521. https://doi.org/10.1007/s002530051089
Grobben GJ et al (1998) Enhancement of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 with a simplified defined medium. Appl Environ Microb 64:1333–1337
Grobben GJ, Boels IC, Sikkema J, Smith MR, De Bont JAM (2000) Influence of ions on growth and production of exopolysaccharides by Lactobacillus delbrueckii subsp bulgaricus NCFB 2772. J Dairy Res 67:131–135. https://doi.org/10.1017/S002202999900391x
Grosu-Tudor SS, Zamfir M (2013) Functional properties of lactic acid bacteria isolated from Romanian fermented vegetables. Food Biotechnol 27:235–248. https://doi.org/10.1080/08905436.2013.811082
Haj-Mustafa M, Abdi R, Sheikh-Zeinoddin M, Soleimanian-Zad S (2015) Statistical study on fermentation conditions in the optimization of exopolysaccharide production by Lactobacillus rhamnosus 519 in skimmed milk base media. Biocatal Agric Biote 4:521–527. https://doi.org/10.1016/j.bcab.2015.08.013
Harutoshi T (2013) Exopolysaccharides of lactic acid bacteria for food and colon health applications. In: Kongo M (ed) Lactic acid bacteria—R & D for food, health and livestock purposes. IntechOpen, New York, pp. 515–538. https://doi.org/10.5772/50839. https://www.intechopen.com/books/lactic-acid-bacteria-r-d-for-food-health-and-livestock-purposes/exopolysaccharides-of-lactic-acid-bacteria-for-food-and-colon-health-applications
Imran MYM et al (2016) Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int J Biol Macromol 93:731–745. https://doi.org/10.1016/j.ijbiomac.2016.09.007
Ismail B, Nampoothiri KM (2010) Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 192:1049–1057. https://doi.org/10.1007/s00203-010-0636-y
Khanh TBTD, Thao DTT (2016) Optimal conditions for exopolysaccharide production by Lactobacillus plantarum T10. J Sci Technol 54:40–47
Kim MJ, Seo HN, Hwang TS, Lee SH, Park DH (2008) Characterization of exopolysaccharide (EPS) produced by Weissella hellenica SKkimchi3 isolated from Kimchi. J Microbiol 46:535–541. https://doi.org/10.1007/s12275-008-0134-y
Li W et al (2014) Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. Lwt-Food Sci Technol 59:732–739. https://doi.org/10.1016/j.lwt.2014.06.063
Liu AP et al (2018) Diversity of isolated lactic acid bacteria in Ya'an sourdoughs and evaluation of their exopolysaccharide production characteristics. Lwt-Food Sci Technol 95:17–22. https://doi.org/10.1016/j.lwt.2018.04.061
Llamas-Arriba MG, Perez-Ramos A, Puertas AI, Lopez P, Duenas MT, Prieto A (2018) Characterization of Pediococcus ethanolidurans CUPV141: a beta-d-glucan- and heteropolysaccharide-producing bacterium. Front Microbiol. https://doi.org/10.3389/fmicb.2018.02041
Looijesteijn PJ, van Casteren WHM, Tuinier R, Doeswijk-Voragen CHL, Hugenholtz J (2000) Influence of different substrate limitations on the yield, composition and molecular mass of exopolysaccharides produced by Lactococcus lactis subsp cremoris in continuous cultures. J Appl Microbiol 89:116–122. https://doi.org/10.1046/j.1365-2672.2000.01082.x
Macedo MG, Lacroix C, Gardner NJ, Champagne CP (2002) Effect of medium supplementation on exopolysaccharide production by Lactobacillus rhamnosus RW-9595M in whey permeate. Int Dairy J 12:419–426. https://doi.org/10.1016/S0958-6946(01)00173-X
Maeda H, Zhu X, Suzuki S, Suzuki K, Kitamura S (2004) Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2B. J Agr Food Chem 52:5533–5538. https://doi.org/10.1021/jf049617g
Pan DD, Mei XM (2010) Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp lactis 12. Carbohyd Polym 80:908–914. https://doi.org/10.1016/j.carbpol.2010.01.005
Pham PL, Dupont I, Roy D, Lapointe G, Cerning J (2000) Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl Environ Microb 66:2302–2310. https://doi.org/10.1128/Aem.66.6.2302-2310.2000
Polak-Berecka M, Wasko A, Kubik-Komar A (2014) Optimization of culture conditions for exopolysaccharide production by a probiotic strain of Lactobacillus rhamnosus E/N. Pol J Microbiol 63:253–257
Razack SA, Velayutham V, Thangavelu V (2013) Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes. Turk J Biol 37:280–288. https://doi.org/10.3906/biy-1206-50
Shin JS et al (2016) Exopolysaccharide fraction from Pediococcus pentosaceus KFT18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J Appl Microbiol 120:1390–1402. https://doi.org/10.1111/jam.13099
Torino MI, Hebert EM, Mozzi F, de Valdez GF (2005) Growth and exopolysaccharide production by Lactobacillus helveticus ATCC 15807 in an adenine-supplemented chemically defined medium. J Appl Microbiol 99:1123–1129. https://doi.org/10.1111/j.1365-2672.2005.02701.x
Torino MI, de Valdez GF, Mozzi F (2015) Biopolymers from lactic acid bacteria. Novel applications in foods and beverages Front Microbiol. https://doi.org/10.3389/fmicb.2015.00834
Tsuda H, Miyamoto T (2010) Production of exopolysaccharide by Lactobacillus plantarum and the prebiotic activity of the exopolysaccharide. Food Sci Technol Res 16:87–92. https://doi.org/10.3136/fstr.16.87
Vandenberg DJC et al (1995) Production of a novel extracellular polysaccharide by Lactobacillus-Sake 0–1 and characterization of the polysaccharide. Appl Environ Microb 61:2840–2844
Vaningelgem F, Zamfir M, Adriany T, De Vuyst L (2004) Fermentation conditions affecting the bacterial growth and exopolysaccharide production by Streptococcus thermophilus ST 111 in milk-based medium. J Appl Microbiol 97:1257–1273. https://doi.org/10.1111/j.1365-2672.2004.02418.x
Velasco S, Arskold E, Paese M, Grage H, Irastorza A, Radstrom P, van Niel EWJ (2006) Environmental factors influencing growth of and exopolysaccharide formation by Pediococcus parvulus 2.6. Int J Food Microbiol 111:252–258. https://doi.org/10.1016/j.ijfoodmicro.2006.06.008
Zhang YC, Li SY, Zhang CH, Luo YK, Zhang HP, Yang ZA (2011) Growth and exopolysaccharide production by Lactobacillus fermentum F6 in skim milk. Afr J Biotechnol 10:2080–2091
Zhang L et al (2013) Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol 54:270–275. https://doi.org/10.1016/j.ijbiomac.2012.12.037
Zivkovic M, Miljkovic M, Ruas-Madiedo P, Strahinic I, Tolinacki M, Golic N, Kojic M (2015) Exopolysaccharide production and ropy phenotype are determined by two gene clusters in putative probiotic strain Lactobacillus paraplantarum BGCG11. Appl Environ Microb 81:1387–1396. https://doi.org/10.1128/Aem.03028-14
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mıdık, F., Tokatlı, M., Bağder Elmacı, S. et al. Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles. Arch Microbiol 202, 875–885 (2020). https://doi.org/10.1007/s00203-019-01799-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01799-6