Abstract
The purpose of this study was to screen for high-yield exopolysaccharide (EPS) producing lactic acid bacteria (LAB) from sugar beet molasses and to evaluate the production capacity of EPS using sugar beet molasses. A highly EPS-producing LAB strain LSBM1 isolated from sugar beet molasses was identified, and the fermentation conditions for EPS using sugar beet molasses as the substrate were optimized by the single-factor experiment and response surface methodology. Strain LSBM1 was identified as Leuconostoc suionicum based on morphological characteristics, and 16S rRNA gene sequence analysis. The optimal culture medium for EPS production was composed of 300 g/L beet molasses, 20.0 g/L yeast extract, 20.0 g/L K2HPO4·3H2O, 0.01 g/L MgSO4·7H2O, 0.01 g/L NaCl, 0.01 g/L CaCl2, 0.01 g/L MnSO4·H2O, 0.01 g/L FeSO4·7H2O, 0.01 g/L. The maximum EPS yield (31.78 g/L) was achieved under the optimized conditions of loading volume 200 mL/250 mL, inoculum size 2%, initial pH value 8.1, culture temperature of 37.59 °C, and culturing period 38.5 h. The in-vitro antioxidant activity showed that LSBM1 EPS had good scavenging activity against DPPH and ABTS radicals. Together, these results provide a LAB strain and the production conditions for obtaining high EPS yields using sugar beet molasses as substrate, and suggest that the harvested LAB-EPS might have potential applications as functional ingredients in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar beet molasses is the principal by-product of the sugar beet industry. It is characterized by its viscous, dark brown, semi-fluid state with a sucrose content ranging from 40 to 56%. This by-product is rich in sucrose, glucose, fructose, and other carbohydrates, as well as vitamins, inorganic salts, and a small amount of crude protein. Due to its nutritional properties, beet molasses provides sufficient nutrition for microorganism fermentation. As such, it represents an economical and readily available material for industrial fermentation (Manfredini et al. 1980). In order to increase economic value of the sugar beet molasses, much efforts have been devoted by researchers worldwide for its bio-conversion and valorization, including the production of animal feed (Manfredini et al. 1980), bio-digestants, bio-ethanol (El-Sayed et al. 2016), bacterial cellulose (Öz et al. 2022), and the development of high value-added chemical materials (Ertan et al. 2021).
Lactic acid bacteria-derived exopolysaccharides (LAB-EPS) are biological polymers that are secreted by lactic acid bacteria (Mironescu et al. 2011), including α-d-glucan, β-d-glucan, fructans, galactans, etc. (Yoon et al. 2004). LAB-EPS has great potential for application in various fields such as food and medicine. For instance, LAB-EPS can be used as non-caloric sweeteners, thickeners, stabilizers, and other functional ingredients for food industry. They can enhance the gel properties of food products, improve processing characteristics, and enhance the overall taste, texture, and flavor of food items (Korcz et al. 2021). Furthermore, LAB-EPS displays various biological activities including anti-tumor effects (Wu et al. 2021), anti-biofilm (Liu et al. 2017), immunomodulation (Zhu et al. 2019), probiotic effects in the intestinal tract (Kim et al. 2018), and promotion of mineral absorption (Lynch et al. 2018). However, low yields with high costs of LAB-EPS production is a major barrier for their industrial-scale production in food and biomedical industry. As a result, there has been growing interest in seeking high-yielding LAB-EPS strains and exploring inexpensive and efficient culture substrates to enhance EPS production. These research efforts aim to overcome the limitations and promote the practical application of LAB-EPS in various fields.
Sugar beet molasses can indeed serve as a cost-effective and abundant carbon source for LAB-EPS production. EPS production by GRAS (generally recognized as safe) bacteria using beet molasses not only provides a more economical fermentable carbon sources, but also represents a promising bio-conversion avenue for value-added valorization of molasses (Sharma et al. 2016). However, EPS production by LAB strains using beet molasses has not been reported. In this study, a LAB strain LSBM1 isolated from sugar beet molasses was characterized as a high-yielding LAB-EPS producer. Thus, this work aimed to identify the LSBM1 strain based on 16S rRNA gene sequence analysis, optimize its fermentation conditions of EPS production using sugar beet molasses, and further evaluate the antioxidant activity of EPS. The results of this work could provide valuable insight into the value-added reuse of sugar beet molasses for LAB-EPS production.
Materials and Methods
Materials and Culture Medium
All beet molasses used in this study were provided by COFCO Sugar Holding Co. Ltd (China). MRS medium (g/L): peptone 10.0, beef extract 5.0, yeast extract 4.0, glucose 20.0, K2HPO4 ·3H2O2.0, ammonium citrate 2.0, sodium acetate 5.0, MgSO4·7H2O 0.2, MnSO4·H2O 0.05, Tween-80 1.0 mL, agar 20.0, 0.5% CaCO3. EPS-producing medium (Shukla et al. 2011) (g/L): sucrose 20.0, yeast extract 20.0, K2HPO43H2O 20.0, MgSO4·7H2O 0.2, MnSO4·H2O 0.01, FeSO4·7H2O 0.01, CaC12·2H2O 0.01, NaC1 0.01.
Isolation and Screening of EPS-Producing LAB Strains
Isolation of LAB strains refers to the method of Zhou et al. (2018) with some modifications. Briefly, 10 g of beet molasses was mixed with 45 mL of sterile physiological saline in a triangular flask. The mixture was fully shaken to ensure thorough mixing. Afterward, the mixture was diluted, and 200 μL of the dilution was spread onto the MRS agar plates. The plates were then incubated at 37 °C for 48 h. Colonies exhibiting calcium dissolution zone were selected, then repeated purified by scribing method to obtain pure strain isolate, and then stored at 4 °C for further use. The obtained strains were inoculated into the EPS-producing medium and cultured at 37 °C for 48 h. The colonies were picked and pulled with a sterile inoculation ring to observe their viscous state. The single colonies displaying long and viscous pulling characteristics were selected as the target EPS-producing lactic acid bacteria.
Identification of Strain LSBM1
Isolated strain LSBM1 was inoculated onto MRS plate and cultured at 37 °C for 48 h. The morphological characteristics of the colony were observed. To further characterize the bacterial cells, Gram staining was performed and staining cells were examined using the optical microscope (Nikon E100, Nanjing Yongxin Optics Co., Ltd, China). Additionally, the bacteria cells were fixed, dehydrated, and sprayed with gold, and the cell morphology was observed by scanning electron microscope (Zeiss EVO 18, Carl Zeiss, Germany). Referring to the method of Wang et al. (2022), 2 mL of strain MRS cultures was taken and centrifuged at 12,000 r/min for 1 min to obtain the bacterial cells precipitate. The genetic DNA of the strain was extracted using a bacterial genome extraction kit using universal bacterial primers, 27F (5′-GAGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGCTACCTTGTTACGAC-3′). PCR amplification was performed according to the methods by Aita et al. (2022). The PCR products were verified by 1% agarose gel electrophoresis and then sent to Chengdu Prime Biotech Co. Ltd. for DNA sequencing. The sequence results were submitted to the GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for homologous Blast comparison with existing nucleotide sequences in database. The nucleotide sequences of the type strains with high similarity to 16S rRNA sequence of strain LSBM1 were selected and then downloaded, and the Neighbor-Joining phylogenetic tree was constructed using MEGA 5.0 software.
Fermentation and Quantification of EPS
Crude EPS was extracted and determined using the method of Du et al. (2017) with some modifications. Briefly, the strains were inoculated into the EPS-producing medium with 2% inoculum size and left to incubate at 37 °C for 48 h. After inactivation at 80 °C for 30 min, the fermentation broth was centrifuged at 10,000 r/min for 10 min to remove the cells. Then, an equivalent volume of 10% (v/v) trichloroacetic acid (TCA) was added to the cell-free supernatant. After incubation for 4 h, proteins were removed by centrifugation at 4 °C and 4000×g for 30 min. The resulting supernatant was carefully collected, and then three volumes of cold 95% ethanol were slowly added to the supernatant. The mixture was left to settle overnight at 4 °C, after which the supernatant was removed by centrifugation at 4000 r/min for 30 min, and the precipitation of EPS was harvested. The obtained precipitate was dissolved in deionized water and subjected to dialysis using a membrane with a cutoff of 14,000 Da. Finally, the separated EPS was concentrated and freeze-dried for further analysis. Total EPS yield was determined by the phenol-sulfuric acid method (Zeng et al. 2022) using the UV Spectrophotometer (UV-759 s, Lengguang Technology, Shanghai, China).
One-way Experimental Design for EPS Fermentation
To evaluate the influence of various factors on the yield of EPS produced by strain LSBM1, several experiments were conducted, including fermentation time (18 h, 24 h, 36 h, 42 h, 48 h), experiments including fermentation temperature (25 °C, 30 °C, 35 °C, 40 °C, 45 °C), initial pH (5, 6, 7, 8, 9), molasses addition (2%, 4%, 10%, 20%, 30%), loading volume (50 mL/250 mL, 75 mL/250 mL, 100 mL/250 mL, 150 mL/250 mL, 200 mL/250 mL), and inoculum size (1%, 2%, 3%, 4%, 5%).
Response Surface Optimization of EPS Fermentation
Based on the single factor test results, fermentation time, temperature, and initial pH were selected as independent variables, and EPS yield as response values. Box–Behnken design containing 17 runs were performed to enhance the progression variables, and the trials were planned using Design Expert 13.0 software (State-Ease Inc., Minneapolis, USA). Analysis of variance (ANOVA), response surface plots, and the coefficient of determination (R2) were used to assess the rate of EPS production that followed (Table 1).
Evaluation of In-Vitro Antioxidant Activities of EPS
The scavenging capacity of crude EPS against DPPH radicals, hydroxyl radicals (–OH), and superoxide anion radicals (O2−) was determined by the method of Wang et al. (2017). The total reducing capacity of EPS was determined by the method of Lee et al. (2006).
Results and Discussion
Morphologic and Genotypic Characteristics of Strain LSBM1
A total of 11 strains of LAB bacteria were isolated from beet molasses, among which strain LSBM1 had the strongest ability to form mucilage on the EPS-producing medium plate. Morphological observation revealed that the colonies of LSBM1 on MRS medium appeared milky white, opaque, with flattened surfaces and neat edges (Fig. 1A), and cells were rounded in shape, displayed a positive gram staining result, and showed no budding (Fig. 1B). SEM observation further revealed that the bacterial cells were rounded to oval, with a size of approximately 0.5 μm (Fig. 1C).
Sequencing results showed that the 16S rRNA sequence of strain LSBM1 had an effective fragment length of 1420 bp (GenBank accession number: OR886617). Blast comparison analysis indicated that the 16S rRNA sequence of LSBM1 exhibited higher similarity to the sequences of Leuconostoc mesenteroides and Leu. suionicum in the NCBI database. To further specify its taxonomic position, sequences of various types strains sharing high similarity were selected to construct a phylogenetic tree. Figure 2 displayed the clustering of strain LSBM1 with Leu. suionicum LMG 8159 (NR109003), with a remarkable sequence similarity of 99.79%. Hence, based on these results, strain LSBM1 was identified as Leu. suionicum. To the best of our knowledge, this study reported for the first time a isolated strain of Leu. suionicum with high EPS production from sugar beet molasses.
One-Factor Experiment Analysis
As presented in Fig. 3, fermentation time, fermentation temperature, pH, beet molasses addition, and loading volume significantly (P < 0.05) influenced the EPS yield of strain LSBM1. As shown in (Fig. 3A), the highest EPS yield of 3.43 ± 0.14 g/L was obtained at the point of 42 h of fermentation time. As the fermentation continued, the EPS yield decreased. The EPS yield increased with the fermentation temperature, specifically, the highest EPS yield reached 5.69 ± 0.12 g/L at a temperature of 40 °C. However, the significant decrease in EPS yield at 45 °C, which can be attributed to the slowing down of bacterial multiplication and a decrease in the number of cells in the bacterial culture. When controlling the initial pH of the fermentation system, the highest EPS yield of 5.52 ± 0.30 g/L was obtained at pH 8 (Fig. 3C). Increasing the addition of beet molasses resulted in a clear upward trend in EPS yield (Fig. 3D). However, when the additional amount of molasses surpassed 30%, the rheological properties of the fermentation broth became a high viscosity state and unfavorable for fermentation. Therefore, the optimal beet molasses addition was determined to be 30%. The EPS yield also showed dependence on the loading volume. Increasing the loading volume led to an increase in EPS yield, reaching its maximum at 200 mL/250 mL with a yield of 3.77 ± 0.01 g/L (Fig. 3E). Conversely, the influence of inoculum size on EPS yield was not significant (Fig. 3F), and a selected inoculum size of 2% are presented.
Effect of culture conditions on the EPS yields by strain LSBM1. A: Fermentation time; B: fermentation temperature; C initial pH; D: beet molasses addition; E: loading volume; F: inoculum size; error bars are represented the standard errors (se) of the mean value (n = 3). Different lower-case letters indicate significant differences (P < 0.05)
Response Surface Experiment Analysis
Based on the results of the one-way experiment, the initial pH (A), fermentation temperature (B), and fermentation time (C) were selected as the test factors, and Box–Behnken response surface design was employed to optimize these conditions, and the corresponding results were presented in Table 2. Following regression analysis, a complete Quadratic polynomial model was obtained to describe the relationship between the EPS yield (Y) and initial pH, fermentation temperature, and fermentation time: Y = 29.85 + 1.41 × A − 3.66 × B − 1.88 × C + 1.06 × A × B − 0.68 × A × C + 1.22 × B × C − 4.03 × A2 − 4.34 × B2 − 2.28 × C2.
Table 3 displays the analysis of variance for the regression model. The F-value of the Quadratic polynomial model was 17.45 (P > 0.05), suggesting the absence of significant differences in the out-of-fit term (P = 2.34 > 0.05). These findings indicated that there were no outliers, and the model is suitably fitted. The coefficient of determination R2 0.9573 indicated that the sample variation of 95.7% for EPS produced by LSBM1 was attributed to the independent variables and 4.3% of the total variation cannot be explained by the model. As shown in Table 3, the fermentation temperature (B) and fermentation time (C) had significant effects on EPS yield (P < 0.05), while the initial pH value (A) did not present a significant effect on EPS yield. The interaction among the three factors in the model was not significant (P > 0.05). The quadratic terms A2, B2, and C2 of the factors in the model had significant (P < 0.05) effects on EPS yields.
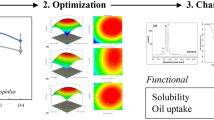
The response surfaces and contours of the two-way interaction of initial pH value (A), fermentation temperature (B) and fermentation time (C) plotted according to the multiple regression equations are shown in Fig. 4. The response surface curves of each factor were all in the shape of a parabola with a downward sloping opening, which indicated the existence of a maximum response value. As can be seen from the contour lines, the contour lines are more dense in the direction of fermentation temperature (B) compared to initial pH value (A) and fermentation time (C), indicating that fermentation temperature (B) has a greater effect on the response peak. This is consistent with the significant level of ANOVA results.
3D Response surface plots and corresponding contour plots of the three variables on EPS production of Leu. suionicum LSBM1. A Interaction between initial pH and fermentation time; B interaction between initial pH and fermentation temperature; C interaction between fermentation temperature and fermentation time
Verification of EPS Maximum Yield
The optimal fermentation parameters for EPS production by strain LSBM1 were predicted by the regression model as follows: initial pH 8.16, fermentation temperature 37.59 °C, fermentation time 38.62 h, and the predicted maximum EPS yield was up to 31.37 g/L, which is a significant improvement of 13.24-fold compared to the yield before optimization. To validate the reliability of the response surface experiments, the optimal solution determined by the experiments was rounded to the nearest integer (pH 8.1, temperature 37 °C, and time 38.5 h) to carry out fermentation and validation experiments. The results showed that the EPS yield under the optimal conditions was 31.78 ± 0.14 g/L, this actual yield closely aligned with the predicted value, indicating that the experimental results obtained from the response surface optimization were reliable.
The yield of LAB-EPS depends on the genetic properties of different LAB species and different strains of the same species. Wang et al. (2017) optimized the fermentation conditions for the synthesis of EPS by Lactobacillus plantarum KX041 by response surface methodology, the maximum EPS yield reached 599.52 mg /L. Tho. et al. (2021) optimized EPS yield up to 17.32 g/L using Lactobacillus plantarum VAL6 with heat and CO2 treatment. Ale et al. (2020) reported that the medium optimization with Lactobacillus fermentum Lf2 resulted in the EPS yield of up to about 1 g/L, whereas Han et al. (2014) optimized the medium with Leu. mesenteroides BD1710 to ferment tomato juice and its optimized EPS yield was about 32 g/L, Xing et al. (2018) isolated Leu. mesenteroides DRP105 from homemade sauerkraut in Northeastern China, and its optimized EPS yield was as high as 53.79 g/L. Ge et al. (2023) isolated Leu. citreum BH10 from birch sap and optimized the fermentation conditions, and the final EPS yield up to 55.96 g/L.
Overall, Leuconotoc spp. synthesized EPS with higher yields than LAB species of other well-known genus, which has significant cost advantages in large-scale industrial production applications. In our study, the maximum yield of 31.78 ± 0.14 g/L EPS produced by LSBM1 was obtained, which is comparable to the EPS yield reported for Leuconotoc spp. in previous literature.
In Vitro Antioxidant Activity of EPS
DPPH is a stable free radical compound with a maximum absorption peak at 517 nm, and it is widely used in assays to evaluate the scavenging capacity of antioxidants. The scavenging activity of different concentrations of EPS and ascorbic acid for DPPH free radicals is shown in Fig. 5A. As can be seen from the figure, the scavenging ability of both DPPH radicals increased with increasing concentration in a concentration-dependent manner. When the concentration of EPS from LBSM1 reached 8 mg/mL, its maximum scavenging rate reached 67.41 ± 0.33%, which was higher than the scavenging activity of Leuconostoc mesenteroides LM187 EPS at the same concentration of DPPH free radicals (50.2%) (Zhang et al. 2021), suggesting that it has a strong DPPH scavenging activity. In contrast, in the report by Tang et al. (2017), the DPPH radical scavenging rate of EPS at 4 mg/L was 53.31%. This difference may be related to the hydrogen ion-giving capacity of polysaccharides from different sources.
Antioxidant activities of EPS produced by Leu. suionicum LSBM1. A: The radical scavenging activities of EPS to DPPH free radicals; B: the radical scavenging ability of EPS to OH free radicals; C: the radical scavenging ability of EPS to ABTS; D: the radical scavenging activity of reducing the power of EPS
OH− is an important reactive oxygen species with strong electron-gaining ability, which is a dangerous and active free radical that can be generated by immunity in organisms and freely enter cell membranes, causing serious damage, especially to proteins (Lin et al. 2017), so the scavenging capacity against hydroxyl radicals is also a major important index for evaluating antioxidant activity. The OH− radicals scavenging capacity of LSBM1-EPS reached 23.94 ± 0.32% when the concentration of LSBM1 EPS was 8 mg/mL. The hydroxyl radical scavenging capacity reached 23.94 ± 0.32% at a concentration of 8 mg/mL, indicating that LSBM1-EPS has a certain capacity to scavenge OH− radicals by giving electrons or hydrogen ions, but the free radical scavenging ability is relatively weak in a certain concentration range (Fig. 5B).
The ABTS scavenging assay is widely used to study the antioxidant capacity of antioxidants, and the method is characterized by simple operation, high specificity, and wide applicability. The higher ABTS scavenging activity is attributed to the ability of EPS to convert reactive radicals to a stable state and to terminate free radical chain reactions by providing electrons to the radicals (Liu et al. 2016). The scavenging activity of strain EPS on ABTS is shown in Fig. 5C. The scavenging activity on ABTS was enhanced with the increase of mass concentration at EPS mass concentration from 0 to 8 mg/mL, and the scavenging rate was as high as 78.58 ± 1.30% when the EPS concentration reached 8 mg/mL, which was similar to the report by Yang et al. (2021), using LAB to induce the soy protein. The scavenging rate of the obtained EPS was comparable to the ABTS scavenging rate of 58.70 ± 1.28% at 5 mg/mL concentration (Yang et al. 2021). However, EPS from LSBM1 exceeded the ABTS scavenging rate of Pantoea alhagi NX-11 APES1, approximately 20% at 5 mg/mL (Sun et al. 2021).
There is a direct correlation between the antioxidant capacity of antioxidants and the reducing capacity, so the reducing capacity determination of EPS is an important index to reflect the antioxidant capacity. Figure 5D shows the total reducing capacity graph of different concentrations of EPS. The absorbance was 0.3315 ± 0.001 at the concentration of 8 mg/mL. The lower reducing capacity may be due to the fact that EPS is a macromolecular substance with a less reducing end. Therefore, the test showed that EPS has a low reducing capacity, which is consistent with previous studies (Dou et al. 2015).
The antioxidant activity of LAB-EPS is affected by a variety of factors, including the proportion of monosaccharide composition, molecular mass, configuration and proportion of glycosidic bonds, and functional group composition. Some studies have shown that the atoms of EPS with more glyoxylates are negatively charged, have stronger spatial site resistance when scavenging free radicals, and have stronger antioxidant and radical scavenging capacity. The reducing ability of EPS was significantly lower than ascorbic acid because ascorbic acid, as a recognized strong reductant, has a strong dehydrogenation ability. So the overall scavenging activity was lower than that of the control.
Conclusion
In this study, an EPS-producing strain of Leu. suionicum LSBM1 was isolated from sugar beet molasses, and the fermentation conditions of EPS production were optimized by one-way and response surface methodology. The maximum EPS yield reached 31.78 ± 0.14 g/L, an increase by 13.24-fold compared to the pre-optimized yield. Additionally, a preliminary evaluation of the in-vitro antioxidant activity of the LSBM1-EPS was performed. The results displayed that the crude EPS exhibited substantial scavenging activity against DPPH and ABTS radicals, with scavenging rates of 67.41 ± 0.33% and 78.58 ± 1.30% respectively, at a concentration of 8 mg/mL. These findings indicate the potential application and development of LSBM1-EPS as a natural food additive or antioxidant. In summary, this study not only provides insights into the value-added conversion and comprehensive utilization of beet molasses, but also supports the further development and industrial application of functional LAB-EPS. The upcoming experimental studies will contribute to a deeper understanding of the EPS produced by strain LSBM1, such as structural characterization, biosynthesis mechanisms, and health benefits evaluation.
References
Aita, G.M., and H.W. Young. 2022. EPS-producing microorganisms from Louisiana’s crusher juice and the effect of processing conditions on EPS production. Sugar Tech 25 (2): 482–490. https://doi.org/10.1007/S12355-022-01235-Y.
Ale, E.C., V.A. Batistela, G.C. Olivar, J.B. Ferrado, and A.G. Binetti. 2020. Statistical optimisation of the exopolysaccharide production by Lactobacillus fermentum Lf2 and analysis of its chemical composition. International Journal of Dairy Technology 73 (1): 76–87. https://doi.org/10.1111/1471-0307.12639.
Dou, J., Y. Meng, L. Liu, J. Li, D. Ren, and Y. Guo. 2015. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. International Journal of Biological Macromolecules 72: 31–40. https://doi.org/10.1016/j.ijbiomac.2014.07.053.
Du, R., H. Xing, Y. Yang, H. Jiang, Z. Zhou, and Y. Han. 2017. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydrate Polymers 174: 409–416. https://doi.org/10.1016/j.carbpol.2017.06.084.
El-Sayed, H.M. 2016. Isolation of fermentative microbial isolates from sugar cane and beet molasses and evaluation for enhanced production of bioethanol. Energy Sources, Part a. Recovery, Utilization, and Environmental Effects 38 (15): 2117–2180. https://doi.org/10.1080/15567036.2015.1030050.
Ertan, F., B. Keskinler, and A. Tanriseven. 2021. Exploration of Cupriavidus necator ATCC 25207 for the production of poly (3-hydroxybutyrate) using acid treated beet molasses. Journal of Polymers and the Environment 29 (7): 2111–2125. https://doi.org/10.1007/S10924-020-02020-2.
Ge, Z., F. Azi, X. Bao, X. Yin, X. Feng, and M. Zhang. 2023. Optimization and characterization of exopolysaccharides from Leuconostoc citreum BH10 and its functional properties in vitro. Food Production, Processing and Nutrition 5: 23. https://doi.org/10.1186/S43014-023-00134-3.
Han, J., F. Hang, B. Guo, Z. Liu, C. You, and Z. Wu. 2014. Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydrate Polymers 112: 556–562. https://doi.org/10.1016/j.carbpol.2014.06.035.
Kim, K., G. Lee, H.D. Thanh, J.H. Kim, M. Konkit, and S. Yoon. 2018. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. Journal of Dairy Science 101 (7): 5702–5712. https://doi.org/10.3168/jds.2017-14151.
Korcz, E., and V. László. 2021. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends in Food Science and Technology 110: 375–384. https://doi.org/10.1016/J.TIFS.2021.02.014.
Lee, S.C., S.M. Jeong, S.Y. Kim, H.R. Park, K.C. Nam, and D.U. Ahn. 2006. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chemistry 94 (4): 489–493. https://doi.org/10.1016/j.foodchem.2004.12.001.
Lin, L., J. Xie, S. Liu, M. Shen, W. Tang, and M. Xie. 2017. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. International Journal of Biological Macromolecules 99: 665–673. https://doi.org/10.1016/j.ijbiomac.2017.03.040.
Liu, Y., B. Zhang, S.A. Ibrahim, S.S. Gao, H. Yang, and W. Huang. 2016. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydrate Polymers 145: 71–77. https://doi.org/10.1016/j.carbpol.2016.03.020.
Liu, Z., Z. Zhang, L. Qiu, F. Zhang, X. Xu, and H. Wei. 2017. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. Journal of Dairy Science 100 (9): 6895. https://doi.org/10.3168/jds.2016-11944.
Lynch, K.M., E. Zannini, A. Coffey, and E.K. Arendt. 2018. Lactic acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization, and health benefits. Annual Review of Food Science and Technology 9 (1): 155–176. https://doi.org/10.1146/annurev-food-030117-012537.
Manfredini, M., and C. Cavani. 1980. Distillery effluents as animal feed: The use of condensed beet molasses stillage (CBMS) in broiler feeding. Animal Feed Science and Technology 5 (3): 233–239. https://doi.org/10.1016/0377-8401(80)90033-4.
Mironescu, M., and I.D. Mironescu. 2011. Rheological behavior of a novel microbial polysaccharide. Romanian Biotechnological Letters 16 (2): 6105–6114.
Öz, Y.E., and M. Kalender. 2022. A novel static cultivation of bacterial cellulose production from sugar beet molasses: Series static culture (SSC) system. International Journal of Biological Macromolecules 225: 1306–1314. https://doi.org/10.1016/j.ijbiomac.2022.11.190.
Sharma, S.N., K. Patel, U. Lata, M. Singh, R.S. Krishania, and S.P. Singh. Sangwan. 2016. A novel approach of integrated bioprocessing of cane molasses for production of prebiotic and functional bioproducts. Bioresource Technology 219: 311–318. https://doi.org/10.1016/j.biortech.2016.07.131.
Shukla, S., and A. Goyal. 2011. Optimization of fermentation medium for enhanced glucan sucrase and glucan production from Weissella confusa. Brazilian Archives of Biology and Technology 54: 1117–1124. https://doi.org/10.1590/S1516-89132011000600006.
Sun, L., Y. Yang, P. Lei, S. Li, H. Xu, R. Wang, Y. Qiu, and Z. Wen. 2021. Structure characterization, antioxidant and emulsifying capacities of exopolysaccharide derived from Pantoea alhagi NX-11. Carbohydrate Polymers 261: 117872. https://doi.org/10.1016/j.carbpol.2021.117872.
Tang, W.D., M. Wang, W. Han, X. Chen, X. Jiang, M. Zhang, Q. Wu, and J. Wei. 2017. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydrate Polymers 173: 654–664. https://doi.org/10.1016/j.carbpol.2017.06.039.
Tho, N.P., L.T. Son, N.T. Tho, B.D. Cuong, H.P. Toan, and H. Khanh. 2021. Enhancing the production and monosaccharide composition of exopolysaccharides of Lactobacillus plantarum VAL6 by applying thermal stress and increased carbon dioxide concentration. Microbiology 90 (4): 527–537. https://doi.org/10.1134/S0026261721040147.
Wang, X., C. Shao, L. Liu, X. Guo, Y. Xu, and L. Xin. 2017. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. International Journal of Biological Macromolecules 103: 1173–1184. https://doi.org/10.1016/j.ijbiomac.2017.05.118.
Wang, X., M. Xu, D. Xu, K. Ma, C. Zhang, G. Wang, and W. Li. 2022. Structural and prebiotic activity analysis of the polysaccharide produced by Lactobacillus helveticus SNA12. Carbohydrate Polymers 296: 119971. https://doi.org/10.1016/j.carbpol.2022.119971.
Wu, J.Y., Y.H. Zhang, L. Ye, and C.L. Wang. 2021. The anti-cancer effects and mechanisms of LAB exopolysaccharides in vitro: A review. Carbohydrate Polymers 253 (5): 117308. https://doi.org/10.1016/j.carbpol.2020.117308.
Xing, H., R. Du, F. Zhao, and Z. Zhou. 2018. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. International Journal of Biological Macromolecules 112: 1208–1216. https://doi.org/10.1016/j.ijbiomac.2018.02.068.
Yang, X., Y. Ren, and L. Zhang. 2021. Structural characteristics and antioxidant properties of exopolysaccharides isolated from soybean protein gel induced by lactic acid bacteria. LWT-Food Science and Technology 150: 111811. https://doi.org/10.1016/j.lwt.2021.111811.
Yoon, E.J., S.H. Yoo, J. Cha, and H.G. Lee. 2004. Effect of levan’s branching structure on antitumor activity. International Journal of Biological Macromolecules 34 (3): 191–194. https://doi.org/10.1016/j.ijbiomac.2004.04.001.
Zeng, C., G. Ye, G. Li, H. Cao, Z. Wang, and S. Ji. 2022. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydrate Polymers 278 (2): 118928. https://doi.org/10.1016/j.carbpol.2021.118928.
Zhang, Q., J. Wang, Q. Sun, S.M. Zhang, X.Y. Sun, C.Y. Li, and J. Tang. 2021. Characterization and antioxidant activity of released exopolysaccharide from a potential probiotic Leuconostoc mesenteroides LM187. Journal of Microbiology and Biotechnology 31 (8): 1144–1153. https://doi.org/10.4014/JMB.2103.03055.
Zhou, Q., F. Feng, Y. Yang, and F. Yan. 2018. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. International Journal of Biological Macromolecules 107: 2234–2241. https://doi.org/10.1016/j.ijbiomac.2017.10.098.
Zhu, Y., X. Wang, W. Pan, X. Shen, Y. He, and H. Yin. 2019. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. International Journal of Biological Macromolecules 121: 342–349. https://doi.org/10.1016/j.ijbiomac.2018.09.201.
Acknowledgements
The authors would like to thank the Students’ Research Interesting Training Support (2023243) by Sichuan Agricultural University, China.
Funding
This research was supported by the Students’ Research Interesting Training Support (2023243) by Sichuan Agricultural University, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Long, X., Hou, X., Li, S. et al. Fermentation Optimization and In-Vitro Antioxidant Activity of Exopolysaccharides Produced by Leuconostoc suionicum LSBM1 Using Sugar Beet Molasses. Sugar Tech (2024). https://doi.org/10.1007/s12355-024-01396-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12355-024-01396-y