Abstract
A thermostable DNA polymerase I from a mesophilic Bacillus sphaericus strain C3-41 was characterized in this study. The polI was cloned, sequenced and over-expressed in Escherichia coli. The expressed 110 kDa fusion protein of PolI was stable at 70°C for 1 h. Compared with DNA polymerase I of E. coli (TaKaRa), the relative polymerase activity of this PolI was 3.33 ± 0.1 RFU μl−1 at 37°C using fluorescent quantitative analysis. It showed higher polymerase activity than E. coli PolI at higher temperature, with a relative activity of 3.75 ± 0.1 RFU μl−1 at 70°C. The polI sequence analysis and the protein structure prediction indicated that this protein had a high similarly to other PolI from thermophilic micro-organisms. This information is of importance for future study for evolution of the house-keeping gene polI in entomopathogenic bacterium B. sphaericus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacillus sphaericus is an aerobic, mesophilic and spore-forming bacterium, with a terminal swelled sporangium and spherical spore. All the B. sphaericus strains have been grouped into 49 serotypes on the basis of the flagella agglutination. Most strains are non-entomopathogenic whereas only nine serotypes (H1, H2, H3, H5, H6, H9, H25, H26, and H48) exhibit toxicity against mosquito larvae. Due to the specific toxicity to mosquito larvae of binary toxin (Bin) and mosquitocidal toxins (Mtxs) produced during the sporulation and vegetative stages, respectively, these toxic strains were widely used as bio-pesticides in the field as part of vector control programs.
Bacillus sphaericus C3-41, a highly active strain isolated from a mosquito-breeding site in China in 1987, has different levels of toxicity against Culex sp., Anopheles sp. and Aedes sp. This strain belongs to the flagella serotype H5a5b, like strains 2362 and 1593 (Yuan et al. 2001) and it has been developed as commercial larvicide (JianBao®, JianBao Co., Foshan City, China) for mosquito larvae control in China.
The DNA polymerase is an enzyme involved in DNA replication and repair in most organisms (Chatelier et al. 2004). Extensive research has been conducted on isolation and characterization of DNA polymerases from various organisms, including bacteria, yeasts and human beings. Besides the basic polymerase activity, DNA polymerase may also contain 5′→3′ and 3′→5′ exonuclease activity in the N-terminal chain of the polypeptide (Harini et al. 2004). Until now, a number of thermostable DNA polymerases have been isolated from thermophilic eubacteria and thermophilic archaea, such as Vent® DNA polymerase from Thermococcus litoralis (Perler et al. 1996), Taq DNA polymerase from an extreme thermophile Thermus aquaticus (Chien et al. 1976) and Bst DNA polymerase from Bacillus stearothermophilus (Kong et al. 1998). These DNA polymerases are stable at high temperature and have been used to DNA amplification for specific scientific purposes. All the reported thermostable DNA polymerases have been detected only in thermophiles, but not in mesophilies. Thus, it is reasonable to screen new enzymes or enzymatic complexes from thermophilic organisms in the future (Pavlov et al. 2004). However, some archaic mesophilic bacteria, in particular those which are evolutionally conserved, might preserve their thermostable polymerase activity.
During research aimed at the screening of thermostable proteins in B. sphaericus strains C3-41, a heat-stable protein was detected during bacterial vegetative and sporulation stages, showing some DNA polymerase activity. In this paper, a DNA polymerase gene (polI) was cloned, sequenced and over-expressed in Escherichia coli. The purified polymerase was stable at 70°C and had polymerase activity and 5′→3′ and 3′→5′ exonuclease activity. This new heat stable polymerase might have applications in gap filling nick translation and strand displacement in laboratory manipulation. The study is also of importance for further interpretation of the evolution of the house-keeping gene polI in entomopathogenic bacterium B. sphaericus.
Materials and methods
Bacterial strains and culture conditions
Bacillus sphaericus C3-41 strain was isolated from a soil sample collected in South China in 1987 (Zhang et al. 1987) and was grown for 12 h at 30°C in Luria–Bertani (LB) broth. E. coli strains DH5α, BL21 and recombinant E-pBSP, E-pFUC were grown at 37°C in LB medium supplemented with 30 μg kanamycin ml−1 and 1 mM IPTG.
Cloning and sequencing
Plasmids were isolated from E. coli by standard alkaline lysine procedure. Cloning experiments and restriction endonuclease analysis were carried out as described (Sambrook and Russell 2001). Total genomic DNA was prepared from B. sphaericus C3-41 by the method of Bourgouin et al. (1990) and sequenced in the Beijing Genomics Institute, Chinese Academy of Science. BLAST and CLUSTAL W programs (Wingren et al. 2003) were used for nucleotide and protein homology searches and multiple sequence alignments, respectively, and the DNASTAR software package (DNASTAR, Madison, WI, USA) was used for DNA analysis.
The polI gene was amplified from C3-41 chromosomal DNA by PCR. The PCR primers were designed according to ORF prediction and gene annotation of the C3-41 genome sequence: polI-1 (5′-GG GAA TCC ATG ACA AAA GAA AAA TTA -3′) and polI-2 (5′-GG GTC GAC TTA TTT TGC TTC ATA CCA -3′) and contained appropriate restriction sites (EcoRI/SalI, underlined). PCR reaction mixture includes 0.5 μg chromosomal DNA, 100 pmol of each primer and 1.25 U pfu Taq polymerase (Fermentas, Hanover, MD, USA) in a total volume of 50 μl. The following PCR procedure was used: 94°C for 5 min; 30 cycles of 94°C for 1.5 min, 48°C for 1.5 min, 70°C for 2.5 min; followed by a final extension cycle at 72°C for 10 min. A ∼2.6 kb PCR fragment was obtained and subsequently cloned into the cloning vector pMD18-T (TaKaRa®, TaKaRa Bio Inc., China) in E. coli DH5α for DNA sequencing.
The amplified PCR fragment was digested by EcoRI and SalI, and then introduced into an EcoRI/SalI digested plasmid pET28a (Novagen, Madison, WI, USA), a His-tag expression vector, resulting in the recombinant plasmid pBSP, which contained the entire encoding region of polI.
Nucleotide sequence accession number
The nucleotide sequence data of the polI open reading frame were submitted to the EMBL, Genbank and DDBJ nucleotide sequence databases under accession number of DQ309765.
Expression of recombinant PolI
The recombinant plasmid pBSP was transformed into E. coli BL21 and a recombinant E. coli colony, designated as E-pBSP, was selected and confirmed for further investigation. The overnight culture of E-pBSP was transferred to 20 ml fresh LB medium containing kanamycin (30 μg ml−1) and then the culture was incubated for 3 h at 37°C under continuous shaking (200 g), and 1 mM IPTG was then added for inducing the expression of PolI. After 4 h incubation at 30°C under continuous shaking, the culture was collected for SDS-PAGE analysis (Bao and Cohen 2004).
For large-scale expression, overnight culture of E-pBSP was transferred to 500 ml fresh LB medium and the culture was shaken at 37°C for 3 h, then IPTG was added to a final concentration of 1 mM in culture and the culture was shaken continuously for another 4 h at 30°C. Cells were harvested by centrifugation (10,000 g, 4°C) and stored at −20°C.
Purification of recombinant PolI
Recombinant PolI protein was purified by a His-Bind Resin chromatography kit (Novagen) using a procedure modified from the original one supplied by the manufacturer. Cells were lysed by sonification at 4°C and the cell debris was removed by centrifugation (14,000 g, 20 min, and 4°C). The supernatant was filtrated by a 0.45 μm membrane and the filtered solution was added to the equilibrated Ni-NTA resin column (Novagen). The eluted protein solution was dialyzed in buffer A (20 mM Tris–HCl pH 8.0, 40 mM KCl, 0.1 mM EDTA) and then stored in buffer B (20 mM Tris–HCl pH 8.0, 40 mM KCl, 0.1 mM EDTA, 10 mM ME, 50% (v/v) glycerol) at −70°C.
Polymerase activity assay of PolI
The comparative DNA polymerase activity of B. sphaericus DNA polymerase I was assayed by fluorescent quantitative analysis (Villbrandt et al. 2000; Bruck et al. 2002). In the PCR system, excessive template DNA and primers were added. In 50 μl total reaction mixture, 0.5 μg of a recombinant plasmid pFUC (a derivative of pUC18 with a DNA insert) was used as DNA template and 100 pM of DNA fragments matching the sequence of the insert of pFUC were used as primers. Then 0.1 mM dATP, dGTP, dCTP and fluorescein-12-dUTP were added with 5 μl E. coli DNA pol I buffer (pH 8.0). The reaction system incubated at 94°C for 5 min to allow denaturing of the double strands DNA and then at 46°C for 1 min for annealing. Afterwards, 1 μl E. coli DNA polymerase I was added as positive control, 1 μl sterilized H2O as negative control and 1 μl of purified B. sphaericus DNA polymerase for testing polymerase activity, respectively. Sets of these samples were placed at different temperature (30, 37, 40, 50, 60, 70, and 80°C) for 2 h and then incubated on ice for 10 min. The fragment DNA was precipitated by adding 100 μl cold ethanol, then centrifuged at 12,000 g and 4°C for 15 min. The un-incorporated fluorescent activity in supernatant was assayed at 528 nm using a Synergy™ HT Multi-Detection Microplate Reader (BIO-TEK®, Bio-Tek Instruments Inc., Winooski, VT, USA), and the incorporated fluorescent activities in amplified DNA fragment were calculated by subtracting the un-incorporated activity from the total activity.
Fluorescent activity assays were done in duplicate and performed at least three times. The fluorescent quantity catalyzed by 1 μl E. coli DNA PolI at 37°C for 2 h was defined as 5 RFU. The comparative polymerase activity of B. sphaericus DNA polymerase I was calculated and expressed according to the units of E. coli DNA PolI.
Exonuclease activity of PolI
For qualitative assay of 5′→3′ exonuclease activity, 50 μg of HindIII digested pUC18 was incubated with 4 μl dNTPs (2.5 mM each), 10 μl E. coli DNA pol I buffer (pH 8.0) (TaKaRa) and add sterile Milli-Q water to 95 μl. This reaction mixture was heated at 75°C for 5 min, then 5 μl of the purified B. sphaericus DNA polymerase I was added. E. coli polymerase I (TaKaRa) was used as a control (Aliotta et al. 1996). The following steps were performed as part of the TaKaRa protocol of E. coli DNA PolI. The 5′-blunted pUC18 DNA was recycled, ligated by T4-ligase (TaKaRa) and transformed to E.coli DH5α. The ligated plasmids were purified from transformed E.coli DH5α and then digested by HindIII to test the existence of HindIII site. The absence of HindIII site in ligated plasmid indicated the 5′→3′ exonuclease activity of PolI.
The activity of 3′→5′ exonuclease was assayed as the same method whereas the plasmid pUC18 was digested by PstI.
Thermostability of polymerase I
Purified DNA polymerase was incubated at temperature from 40 to 100°C for 30 min and then placed on ice for 10 min. The heat-treated solution was centrifuged at 12,000 g for 20 min at 4°C. The comparative DNA polymerase activity of supernatant was tested by the method described above (Tsujimoto et al. 2003).
Analysis of the polI sequence
The DNA homology of polI from B. sphaericus and those from other 15 bacteria was analyzed by BLAST T and CLUSTAL W, and the evolution distance and relationships were displayed by the NJ arithmetic method. The comparative analysis of B. sphaericus C3-41 polI gene was been handled in EMBL (http://www.ebi.ac.uk/embl) and SWISS-PROT (http://www.expasy.ch/sprot).
Based on the sequence of PolI from B. sphaericus C3-41 and the known protein crystal structure of PolI in PDB (http://www.rcsb.org/pdb), a 3D structure homology model of the PolI protein from B. sphaericus was predicted up using CPHmodels 2.0 (http://www.cbs.dtu.dk/services/CPHmodels).
Results
Cloning and sequencing analysis of Bacillus sphaericus C3-41 polI gene
The preliminary bioinformatics analysis of the C3-41 genome sequence revealed that an ORF had high similarity to polI sequences from other thermophile bacteria, indicating this ORF might have polymerase activity. Using a set of primers designed according to the sequence of this ORF, a ∼2.7 kb DNA fragment was amplified from the chromosomal DNA of C3-41 by PCR. Sequence analysis showed that the amplified DNA fragment was composed of 2,628 bp, encoding 875 aa with a predicated molecular weight of 98.66 KDa. Further analysis indicated that a promoter sequence (5′→3′) and a ribosome-binding site (5′→3′) was located upstream of this fragment, corresponding to the expected −35 and −10 sequences, and that an expected transcription termination structure was found downstream of the TAA termination codon.
Purification and characterizing of recombinant Polymerase I in Escherichia coli
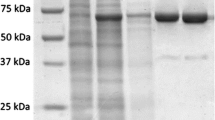
For expression of this protein, the recombinant plasmid pBSP, which contained the entire encoding region of polI under the control of an IPTG-inducible promoter, was introduced into E. coli BL21, giving a recombinant strain E-pBSP. SDS-PAGE analysis showed that this recombinant strain could express the fusion protein after 4 h inducement of IPTG, producing a protein with a molecular weight of about 100 kDa (data not shown). Using a Ni2+ affinity chromatography system, this fusion protein could be purified, giving a single band with a molecular weight of 105 kDa on SDS-PAGE (Fig. 1).
The relative polymerase activity of this protein was assayed by fluorescent quantitative analysis. The results indicated that both PolI from E. coli and B. sphaericus have peak polymerase activity at different temperatures. The highest polymerase activities of PolI from E. coli were observed at temperatures between 37 and 50°C, with a comparative unit in the range of 4.17 ± 0.1–5.00 ± 0.1 RFU, whereas the PolI from B. sphaericus had an activity of 3.33 ± 0.1 RFU. With the increase of temperature, the polymerase activity of both PolI decreased. However, the PolI from E. coli had lower activity than that from B. sphaericus at higher temperature. At 70°C, the former had a relative activity of 2.92 ± 0.2 RFU, while the latter had an activity of 3.75 ± 0.1 RFU (Table 1, Fig. 2).
Thermostability studies revealed that PolI from B. sphaericus was stable at high temperature, with a half-life of 1 h at 80°C, indicating this PolI was a thermostable PolI (Fig. 3).
In addition to possessing a polymerase activity resembling PolI from other thermostable Bacillus species, PolI from B. sphaericus could cut the cohesive end of HindIII and PstI digested plasmid pUC18 both from 5′→3′ and 3′→5′, resulting the deletion of HindIII and PstI sites in the ligated plasmid, respectively, indicating this PolI possessed 5′→3′ exonuclease activity and 3′→5′exonuclease activity. No obvious difference of exonuclease activity between the DNA PolI from B. sphaericus and E. coli was observed (data not shown).
Phylogenetic analysis
Amino acid sequence analysis of PolI from B. sphaericus revealed that this protein contained 202 aliphatic AA (23.086%), 85 aromatic AA (9.714%), 448 non-polar AA (51.2%), 427 polar AA (48.8%), and 254 charged AA (29.2%). The molecular weight of the PolI is 98.6 KDa, and its isoelectric point is 4.79.
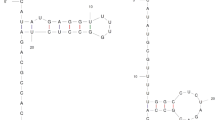
Based on polI DNA sequences (Fig. 4a) and their encoding amino acid sequences (Fig. 4b) of 16 bacterial strains,two phylogenetic tree were constructed. Genetically, B. sphaericus is close to thermophilic bacteria such as Geobacillus sterarothermophilus and Streptococcus thermophilus, with 76 and 72% at the amino acid sequence level, respectively. Furthermore, alignment results revealed that the aliphatic amino acids, such as leucine (L), glycine (G), alanine (A), proline (P), etc. of PolI from 16 strains were highly conserved at the same locus and that the sequence similarity in the 5′ terminal was higher than in other regions. One 3D model of PolI from B. sphaericus C3-41 was predicted based on the established crystal structure of PolI from other thermophile bacteria, such as T. aquaticus (PDBcode: 1cmw_A), B. stearothermophilus (PDBcode: 2bdp_A) and DNA Polymerase I Klenow Fragment from E. coli (PDBcode: 1d8y_A). Like other PolI, the PolI from B. sphaericus, contains a proofreading exonuclease domain and a polymerase domain consisting of a right hand with finger, palm and thumb subdomains. The finger and thumb subdomains provide a region that could bind target DNA during replication (Pavlov et al. 2004) and a nearly identical catalytic center could be found in the palm subdomain. In general, all highly conserved regions and catalytically essential amino acid sequences for the exonuclease and polymerase domains (Joyce and Steitz 1994) are very similar in all polymerase structure of T. aquaticus, B. stearothermophilus, B. sphaericus and DNA polymerase I Klenow fragment from E. coli.
Discussion
A thermostable PolI from a mesophilic B. sphaericus strain C3-41 was cloned and characterized. In the qualitative analysis of this polymerase I, the comparative polymerase activity was calculated by comparison with the observed activity of commercial E. coli PolI (TaKaRa). In the standard reaction environment (37°C), the comparative activity of PolI from B. sphaericus was 3.33 ± 0.1 RFU μl−1, while E. coli PolI had an activity of 5.00 ± 0.1 RFU. With increasing temperature, the relative activity of E. coli PolI dropped to 2.92 ± 0.2 RFU, while for B. sphaericus PolI activity increased to 3.75 ± 0.1 RFU at 70°C (Fig. 2). In addition, the B. sphaericus PolI was stable at high temperature and was resistant to heat treatment at 80°C for 1 h, indicating it was a thermostable PolI.
The thermostablity of a protein is based on its amino acid composition. Deckert et al. (1998) reported the charged and non-charged amino acids in thermostable protein accounted for 29.84 and 26.79%, respectively, of the total composition. A similar percentage of charged and non-charged amino acid was observed in B. sphaericus PolI (29.29 and 23.06%, respectively). It has been demonstrated that charged residues and non-charged amino acids, especially proline and arginine residues, were important for stabilizing the protein structure high temperatures (Vogt et al. 1997). It was noted that there are 28 proline residues mostly located in the turning in the predicted model of B. sphaericus PolI. Also, 34 Arg (3.9%), an abundant residue in most thermostable proteins, were found in B. sphaericus PolI (Krell et al. 2004). All this observations are consistent with the observed thermostability of B. sphaericus PolI.
To date, several thermostable PolI proteins have been detected or characterized in Bacillus species, but all of them are from thermophiles, such as B. stearothermophilis, B. tusciae, B. thermocloacace, and B. caldotenax (Sellmann et al. 1992; Vellore et al. 2004), with an optimal growth temperature over 50°C. B. sphaericus is a mesophilic entomopathogenic bacterium and has optimal growth temperature from 30 to 37°C. and so thermostable proteins are not necessary for their survival and evolution. B. sphaericus is an archaic organism and its spore has even been found in 25–40-million-year-old amber (Cano et al. 1995). However, some identified genes (such as bin and mtx gene) among different serotypes and isolates show extremely high levels of similarity (Yuan et al. 1999, 2001). The genetic stability of this organism relies on the conservative replication of its genes. Probably this archaic evolutionally conserved bacterium has maintained its genome relatively consistently from its beginning era and this may be explained why it has a pol1 sequence of polI which is highly homologous to that of thermophiles. However, the evolution of this entomopathogenic bacterium is still to be investigated.
The DNA and amino acid sequence alignment of 16sRNA and PolI indicated that B. sphaericus was close to the Staphylococcus species, and the sequence of 3′→5′ exonuclease activity region had considerable similarity among S. aureus, S. thermophilus and B. sphaericus C3-41. Furthermore, analytical comparison of the 16S rRNA gene sequences revealed that all Bacillus strains had same physiological and ecological characters (data not shown). Recent studies suggested that Bacillus pasteuni, Bacillus psychrophilus and Bacillus globiporus were classified into genus Sporosacrine (Yoon et al. 2001). The physiological properties of these species are very similar to B. sphaericus and different to other bacillus (Nakamura 2000). The other neighbors of the B. sphaericus were assigned to one group through 16s-23s ITS alignment (Xu and Cote 2003). All these analyses reveal that B. sphaericus may be a special microbe and is worthy of more detailed investigation.
References
Aliotta JM, Pelletier JJ, Ware JL, Moran LS, Benner JS, Kong HM (1996) Thermostable Bst DNA polymerase I lakes a 3′→5′ proofreading exonuclease activity. Genet Anal 12:185–195
Bao K, Cohen SN (2004) Reverse transcriptase activity innate to DNA polymerase I and DNA topoisomerase I proteins of streptomyces telomere complex. Proc Natl Acad Sci 101:14361–14366
Bourgouin C, Delecluse A, Torre F, Szulmajster J (1990) Transfer of the toxin protein genes of Bacillus sphaericus into Bacillus thuringiensis subsp. israelensis and their expression. Appl Environ Microbiol 56:340–344
Bruck I, Yuzhakov A, Yurieva O, Jeruzalmi D, Skangalis M, Kuriyan J, O’Donnell M (2002) Analysis of a multicomponent thermostable DNA polymerase III replicase from an extreme thermophile. J Biol Chem 19:17334–17348
Cano RJ, Monica K, Borucki (1995) Revival and identification of bacterial spores in 20-to 40-million-year-old Dominican amber. Science 268:1060–1064
Chatelier EL, Bécherel OJ, Alencon E, Canceill D, Ehrlich SD, Fuchs RP, Janniére L (2004) Involvement of DnaE, the second replicative DNA polymerase from Bacillus subtilis, in DNA mutagenesis. J Biol Chem 16:1757–1767
Chien A, Edgar DB, Trela JM (1976) Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol 127:1550–1557
Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL,Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olsen GJ, Swanson RV (1998) The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 392:353–358
Harini S, Griffiths K, Flynn EK, Astatke M, Shih PJ, Lee JE, Gerard GF, Gibbs MD, Bergquist PL (2004) Thermophilic bacterial DNA polymerases with reverse-transcriptase activity. Extremophiles 8:243–251
Joyce CM, Steitz TA (1994) Function and structure relationships in DNA polymerases. Annu Rev Biochem 63:777–822
Kong HM, Pelletier JJ, Aliotta JM (1998) Over-expression and purification of truncated thermostable DNA polymerase by protein fusion. US Patent 5814506
Krell T, Greco1 F, Engel1 O, Dubayle J, Dubayle J, Kennel A, Charloteaux B, Brasseur R, Chevalier M, Sodoyer R, Habit RE (2004) HIV-1 gp41 and gp160 are hyperthermostable proteins in a mesophilic environment characterization of gp41 mutants. Eur J Biochem 271:1566–1579
Nakamura LK (2000) Phylogeny of Bacillus sphaericus-like organisms. Int J Syst Evol Microbiol 50:1715–1722
Pavlov AR, Pavlova N, Kozyavkin SA, Slesarev AI (2004) Recent developments in the optimization of thermostable DNA polymerases for efficient applications. Trends Biotechnol 22:253–260
Perler F, Kumar S, Kong H (1996) Thermostable DNA polymerase. Adv Protein Chem 48:377–435
Sambrook J, Russell J (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sellmann E, Schroder KL, Knoblich IM, Westermann P (1992) Purification and characterization of DNA polymerases from Bacillus species. J Bacteriol 174:4350–4355
Tsujimoto Y, Watanabe A, Nakano K, Watanabe K, Matsui H, Tsuji K, Tsukihara T, Suzuki Y (2003) Gene cloning, expression, and crystallization of a thermostable exo-inulinase from Geobacillus stearothermophilus KP1289. Appl Microbiol Biotechnol 62:180–185
Vellore J, Moretz SE, Lampson BC (2004) A group II intron-type open reading frame from the thermophile Bacillus (Geobacillus) stearothermophilus encodes a heat-stable reverse transcriptase. Appl Environ Microbiol 70:7140–7147
Villbrandt B, Sobek H, Frey B, Schomburg D (2000) Domain exchange: chimerase of Thermus aquaticus DNA polymerase, Escherichia coli DNA polymerase I and Thermotoga neapolitana DNA polymerase. Protein Eng 13:645–654
Vogt G, Woell S, Argos P (1997) Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol 269:631–643
Wingren C, Edmundson AB, Borrebaeck CAK (2003) Designing proteins to crystallize through-strand pairing. Protein Eng 16:255–264
Xu D, Cote JC (2003) Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 39 end 16S rDNA and 59 end 16S–23S ITS nucleotide sequences. Int J Syst Evol Microbiol 53:695–704
Yoon JH, Lee KC, Weiss N, Kho YH, Kang KH, Park YH (2001) Sporosarcina aquimarina sp. nov., a bacterium isolated from seawater in Korea, and transferof Bacillus globisporus (Larkin and Stokes 1967), Bacillus psychrophilus (Nakamura 1984) and Bacillus pasteurii (Chester 1898) to the genus Sporosarcina as Sporosarcina globispora comb. nov., Sporosarcina psychrophila comb. nov. and Sporosarcina pasteurii comb. nov., and emended description of the genus Sporosarcina. Int J Syst Evol Microbiol 51:1079–1086
Yuan ZM, Neilsen-LeRoux C, Pasteur N, Delecluse A, Charles JF, Frutos R (1999) Cloning and expression of the bin genes of Bacillus sphaericus C3-41 in a crystal minus B. thuringiensis subsp. Israelensis. Wei Sheng Wu Xue Bao 39:29–35
Yuan ZM, Rang C, Maroun RC, Victor JP, Frutos R, Pasteur N, Vendrely C, Jean-Francois C, Nielsen-LeRoux C (2001) Identification and molecular structural prediction analysis of a toxicity determinant in the Bacillus sphaericus crystal larvicidal toxin. Eur J Biochem 268:2751–2760
Zhang YM, Liu EY, Cai QX, Chen ZS (1987) Isolation of two high toxin bacillus sphaericus strains. Inseticidal Microorg 1:98–99
Acknowledgments
We thank Dr. Simon Rayner for useful suggestion and critical reading of the manuscript, and Mr. Cai Quanxin for his technical assistance. The project was supported by grants (KSCX2-SW-301-10, KSCX2-SW-315) from the Chinese Academy of Sciences, a 973 project (2003CB114201) and a grant (30470037) from NFSC, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bei, H., Haizhou, L., Xiaomin, H. et al. Preliminary characterization of a thermostable DNA polymerase I from a mesophilic Bacillus sphaericus strain C3-41. Arch Microbiol 186, 203–209 (2006). https://doi.org/10.1007/s00203-006-0135-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0135-3