Abstract
Summary
This meta-analysis pooled results from 23 qualifying individual cohort studies and found that depression was significantly associated with an increased risk of fractures and bone loss.
Introduction
The association between depression and risk of fracture remains controversial. We conducted a comprehensive meta-analysis to examine the effect of depression on the risk of osteoporotic fractures and bone loss.
Methods
We searched databases and reviewed citations in relevant articles for eligible cohort studies. Two investigators independently conducted study selection, appraisal, and data abstraction through the use of a standardized protocol. Random effect models were used for meta-analysis. Cochrane Q and I2 statistics were used to assess heterogeneity. Funnel plots and rank correlation tests were used to evaluate publication bias.
Results
Twenty-three studies were included for meta-analysis. In studies that reported hazard ratio (HR) as the outcome (nine studies [n = 309,862]), depression was associated with 26% increase in fracture risk (HR = 1.26, 95% CI, 1.10–1.43, p < 0.001). Studies that reported risk ratio (RR) as the outcome (seven studies [n = 64,975]) suggested that depression was associated with 39% increase in fracture risk (RR = 1.39, 95% CI, 1.19–1.62, p < 0.001). Among studies that reported hip bone mineral density (BMD) as an outcome (eight studies [n = 15,442]), depression was associated with a reduced mean annual bone loss rate of 0.35% (0.18–0.53%, p < 0.001). The increased risk of fracture and bone loss associated with depression was consistent in all meta-analysis having modified inclusion criteria and in different subgroup analyses as well. Significant heterogeneity was observed in the meta-analysis; however, no significant publication bias was detected.
Conclusion
Depression is associated with a significant increased risk in fracture and bone loss. Effective prevention may decrease such risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fracture has become a global public health issue. Across the world, osteoporosis has caused more than 8.9 million fractures per year [1]; among the population with age over 50, more than 30% of women will encounter osteoporotic fractures, while approximately 20% of men will incur the same issues [2, 3]. The progressive aging of the worldwide population leads to a dramatic increase in the incidence of osteoporotic fracture. For incidence in hip fracture alone, a threefold increase is anticipated by the year 2050 [4]. In consequence of frequent osteoporotic fracture, excess mortality and severe disability will cause an escalation of health and social burdens affiliated with osteoporotic fracture, especially for European countries [5].
Similar to osteoporosis, depression is a chronic, prevalent condition, which affects 18% of men and 26% of women in the USA [6]. Cross-national lifetime prevalence figures show that up to 21.4% of the population suffers from depressive symptoms that qualify for a clinical diagnosis [7]. Many studies have examined the association of depression and decreased BMD, bone loss, osteoporosis, and fracture. Several meta-analyses have reported that depression is a significant risk factor for low BMD [8,9,10,11]. However, results from these meta-analyses have not been consistent. These meta-analyses included either studies with cross-sectional or case-control design, a different set of available publications [9], and/or different outcomes related to osteoporosis [8, 9]. None of these meta-analyses assessed the association between depression and the risk of osteoporotic fracture in a prospective cohort design. Although one meta-analysis used fracture as outcome and included cohort studies [12] only, it was conducted 7 years ago; additionally, the number of included cohort studies was small and did not include numerous large eligible studies published recently [13,14,15,16,17,18,19,20,21,22]. As well, the association between depression and risk of bone loss was not fully assessed due to very few eligible studies available. Hence, none of the prior meta-analyses to date have offered a comprehensive review and analysis of all relevant prospective cohort data in investigating the association between depression and the risk of fracture and bone loss.
Our aim was to conduct an updated meta-analysis to examine all eligible prospective cohort studies that have assessed the effect of depression on the risk of fracture and bone loss, and to obtain more comprehensive, accurate, and precise results about this effect.
Materials and methods
This meta-analysis followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [23], with reference to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [24]. The objectives, literature search strategy, inclusion criteria, exclusion criteria, methods of study selection, data abstraction, and methods of statistical analysis were described in our protocol, which was preregistered in the International Prospective Register of Systematic Reviews. The protocol is available at the following URL: https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017060022.
Eligibility criteria
Due to the bidirectional relationship between depression and fracture, only original perspective cohort studies that evaluated the effects of depression on the risk of osteoporotic fractures and/or bone loss were included. Studies examining the effect of fracture on depression were not eligible. Case-control and cross-sectional studies were excluded. We only included studies using human subjects, without the restriction of age, sex, race, and geographic location.
In the present meta-analysis, the term “depression” refers to clinical depression, depressive disorder, or depressive mood. Eligible exposures were unipolar depression, depressive disorder that was clinically diagnosed, or depressive mood assessed by a standardized psychometric tool. Bipolar depressive disorder and bipolar depression were not eligible [25]. The primary outcome for the present meta-analysis is fracture, which includes osteoporotic fracture or fragility fracture. Osteoporosis without fracture is not an eligible primary outcome. Included studies for the primary outcome must have reported adjusted hazard ratio (HR) or relative risk (RR) of fracture. The secondary outcome is bone loss, which refers to decreased BMD associated with depression. The eligible cohort studies for the secondary outcome must have reported difference of BMD change over the follow-up period between depressed and non-depressed participants. Studies that only used depression as a continuous variable in analysis and did not report HR, RR, or BMD change between depressed and non-depressed participants were not eligible.
Literature search
A comprehensive literature search of MEDLINE from January 1966 through July 2017 was conducted with PubMed (Supplemental Table 1). The medical subject headings (MESH) used for this search were “depression,” “depressive disorder,” “bone loss,” “bone fracture,” “fracture,” “bone density,” and “osteoporosis.” We also use the key word “bone mineral density” for the literature search. The search was limited to human studies and English language only. We also searched in the Web of Science, Scopus, Embase, and PsyInfo databases, using a similar strategy. The literature search was conducted independently by two investigators (B.L. and S.T.). Librarians were consulted before and during the search to ensure comprehensiveness. We also searched unpublished data including conference materials, abstracts, thesis, and dissertations, using the Google search engine. The two investigators also independently reviewed each reference list of original studies [13,14,15,16,17,18,19,20,21,22, 26,27,28,29,30,31,32,33,34,35,36,37,38] and related review and prior meta-analysis articles.
Study selection
Reviewers B.L. and S.T. independently screened titles and abstracts of all references found in the literature search so as to further identify potential eligible studies. Only irrelevant references agreed by both reviewers were excluded at this stage and any uncertain references were included for full-text retrieval and examination. The two reviewers then independently reviewed the full contents of selected references to assess their eligibility for this meta-analysis. Cohen’s kappa statistic was used to assess the agreement between the two investigators. Disagreement and uncertainty regarding eligibility were discussed and adjudicated by a third reviewer (Q.W.).
Data abstraction
The following study characteristics were abstracted: author(s), study name, year of publication, study design, study population and country, distribution by age, sex, and race. Important factors such as outcome, duration of follow-up, cohort size, assessment of depression, depression criterion, outcomes (BMD, fractures), menopausal status (women only), estrogen supplement (women only) and other medications, confounders that were adjusted by multivariate analysis, hazard ratio (HR), and/or relative risk (RR) of fracture associated with depression and its standard errors were all abstracted for this meta-analysis. Lastly, the mean difference of BMD changes between depressed and non-depression group and the corresponding SD or SE was gathered. When original studies presented multiple adjusted estimates for the same outcome, the estimate that had been adjusted for the largest number of confounders was used. By using a standardized data extraction form, relevant data was abstracted from all eligible studies. The two reviewers independently conducted data abstraction and entry. Both checked the data at least twice for accuracy. Authors were contacted when additional information was needed.
Study appraisal
To examine risk of bias and to assess the quality of the methodology in the original studies, the Newcastle-Ottawa quality assessment scale for cohort studies was applied. A quality score was calculated for each study based on a pre-specified questionnaire [39]. Each study was evaluated by the following eight criteria for quality assessment: representativeness of the exposed cohort, selection of non-exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at the start of the study, comparability of cohorts on the basis of the design or analysis, assessment of outcome, follow-up duration long enough for outcomes to occur, and adequacy of follow-up of cohorts. The score ranged from 0 to 9, where a score of 9 indicates the strongest regarding methodology. The information about the Newcastle-Ottawa Scale for each study is summarized in Supplemental Table 2. Based on the suggestion by the MOOSE group [23], we did not use the quality scores as weights in conducting meta-analyses. The quality scores were used only in pre-specified sensitivity analysis.
Statistical analysis
The primary outcome measures were adjusted HR and RR for fracture. However, the cohort studies that reported odds ratio (OR) were also considered, since OR is approximately the same as the RR, if the absolute risk of incident fracture is low. For the concern of normalization and variance stabilization, natural logarithms of RR and HR were used in the analysis instead of using HR or RR directly. The variance of log-transformed RR and HR was calculated using CI or p value given in the original studies. Adjusted RR and HR reported in the original studies were analyzed separately. In order to calculate the overall effect size, the reciprocal of the variance for each study was used as the weight of the corresponding study. The amount of bone loss or the percentage of BMD loss during different lengths of follow-up was normalized as the annual percentage of bone loss for each study. For most of the studies involving bone loss, the variance of annual percentage of loss was computed using 95% CIs or p value. For specific studies that did not report CI or p value, variance was calculated by pooling the variance from different groups in the study [19].
We conducted several sensitivity analyses to assess the robustness of our major findings, and the effect of depression to fracture outcome was assessed with different inclusion criteria. Multiple pre-specified subgroup analyses were conducted to determine if fracture risk or difference of BMD change was influenced by sex, demographic region, duration of follow-up, and sample size of the study cohort, and if the study controlled for calcium intake and for antidepressant use.
We assessed the heterogeneity by Cochrane Q statistic and I2. The existence of heterogeneity was expected since the participants in the original cohort studies differed in sex, age, race, and ethnicity, and the fact that these included studies were conducted in different settings and regions. The Cochrane Q statistics suggested significant heterogeneity for HR (p < 0.01) and annualized bone loss (p < 0.01), but no significant heterogeneity was observed for RR (p = 0.18). To be conservative, we used a random effect model in all analyses, even if no significant heterogeneity was detected.
To examine for possible publication bias, funnel plots were constructed for visual inspection. We also employed a Begg’s rank correlation test as a quantitative method to assess publication bias. R statistical software was used to perform all data analyses.
Results
Study characteristics
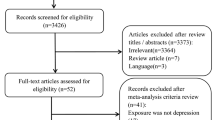
The study selection process is presented in Fig. 1. We identified 5547 potential references after removing duplicate records. The titles and the abstracts of all these references were screened, which resulted in 103 full-text articles assessed for eligibility, with modest agreement between the two investigators (k = 0.67). After full review of the 103 articles, 23 studies met the inclusion criteria and were included in our meta-analysis [13,14,15,16,17,18,19,20,21,22, 26,27,28,29,30,31,32,33,34,35,36,37,38], with high agreement between the two investigators (k = 0.90). All eligible articles were published in peer-reviewed journals. The characteristics of 23 articles are summarized in Supplemental Table 3. Of these included cohort studies, 15 reported fracture as an outcome [15,16,17,18, 20, 22, 26,27,28,29,30,31,32,33,34], 7 reported BMD as an outcome [13, 14, 19, 21, 36,37,38], and 1 reported both [35]. Among the studies that reported fracture as an outcome, 9 reported HR as an outcome [15, 16, 22, 26, 27, 32,33,34,35], while 7 of them reported RR or OR [17, 18, 20, 28,29,30,31]. The mean duration of follow-up varied from 1 [37] to 22 [27] years. The number of subjects ranged from 100 in the study by Whooley et al. [36] to 135,110 in the National Health Research Institute’s study conducted in Taiwan [15]. Among all included studies, 11 were conducted in the USA and 11 studies included women only. However, sex was controlled in all of the other 12 studies. Only 3 studies [33, 34, 37] did not control for age effect.
Meta-analysis
In 16 studies that reported fracture as the outcome, 9 studies reported HRs [n = 309,862] and 7 reported RRs [n = 64,975]. Figure 2a is the forest plot of all studies that reported HR (hazard ratio) as an outcome. In the forest plot, the HRs (95% CIs) of fracture by depression for each study and the overall HR are included. HR is greater than 1 in 9 of the 10 studies. Overall, a 26% increase in fracture risk (HR = 1.26, 95% CI, 1.10–1.43, p < 0.001) was associated with depression. Figure 2b is the forest plot of all studies that reported RR as an outcome. RR is greater than 1 in 6 of the 7 studies. Overall, depression was associated with 39% increase in fracture (RR = 1.39, 95% CI, 1.19–1.62, p < 0.001). Figure 2c is the forest plot of all 8 studies that reported BMD at the hip, indicating that depression was associated with annual bone loss of 0.35% (95% CI, 0.18–0.53%, p < 0.001). Higgins’s I2 and Cochrane’s Q suggest high heterogeneity among the studies that reported HR and among studies that reported BMD. However, for the studies that reported RR, no significant heterogeneity was observed.
Sensitivity analysis
Table 1 summarizes the results of sensitivity analysis. The estimated fracture risk associated with depression remained significant when studies were included with different criteria. For instance, the overall HR increased slightly to 1.34 when outcome measure was limited to only osteoporotic fracture. When analysis was confined to studies with high-quality score (≥ 7), the overall HR decreased slightly to 1.22. After excluding two studies with small sample size (< 1000), the overall HR varied little. Finally, when the analysis was confined to the 7 studies with following-up > 5 years, the overall HR decreased to 1.18. The estimated fracture risk associated with depression remained similar in sensitivity analysis with studies that reported RR as outcome. For example, when analysis was confined to studies with high-quality score (≥ 7) or to studies with following-up > 5 years, the overall RR decreased slightly to 1.34. When the meta-analysis was confined to the 6 studies with sample size > 1000, the overall RR increased slightly to 1.43. In addition, the overall RR increased slightly to 1.43 when outcome measure was limited to osteoporotic fracture only. Finally, when analysis was confined to 5 European studies, the overall RR increased slightly to 1.47. The estimated annual bone loss of hip associated with depression increased slightly when the meta-analysis was confined to 7 studies conducted in the USA only or 7 studies with duration of follow-up < 10 years. For studies that reported BMD at the spine, depression was associated with annual bone loss of 0.32% (95% CI, 0.13–0.77%, p < 0.001). For studies that reported BMD at the trochanter, depression was associated with annual bone loss of 0.57% (0.40–0.74%, p < 0.001).
Subgroup analysis
The subgroup analysis results for primary outcome measure using HR are summarized in Table 2. The HR for fractures was higher in the studies with longer duration of follow-up (≥ 10 years), conducted in the USA, with smaller population size (< 10,000). In addition, the studies that did not control for smoking (HR 1.38, 95% CI 1.07–1.79; [4 studies {n = 217,249}) had a higher overall HR compared to the studies that controlled for smoking (HR 1.19, 95% CI 0.98–1.46; [5 studies {n = 92,613}]). Likewise, the studies that did not control for antidepressant use (HR 1.43, 95% CI 0.94–2.18; [2 studies {n = 9245}]) had a higher overall HR compared to the studies that controlled for antidepressant use (HR 1.23, 95% CI 1.06–1.41; [7 studies {n = 300,617}]). For subgroup analysis of studies that reported RR (Table 3), the estimated risk was higher in the studies with sample size greater than 10,000, with adjustments for antidepressants or for smoking. Among the studies that reported BMD at the hip as an outcome, the studies with only female participants (effect size 0.33%, 95% CI 0.14–0.52%; [6 studies {n = 12,878}]) had lower mean annual bone loss rate compared to the studies with only male participants (effect size 0.49%, 95% CI 0.17–0.82; [2 studies {n = 2564}]). The studies with less than 5 years follow-up (effect size 0.48%, 95% CI 0.43–0.53%; [5 studies {n = 6943}]) had a higher mean annual bone loss rate compared to the studies with greater or equal to 5 years follow-up (effect size 0.19%, 95% CI, − 0.06 to 0.44%; [3 studies {n = 8499}]). However, the differences of all estimated risk associated with depression between subgroups were not significant in all subgroup analyses. Therefore, we did not conduct meta-regression analysis.
Publication bias
Publication bias was examined using funnel plots. For fracture outcomes, publication bias was suspected by observing the funnel plots (Supplemental Figure A and B). Hence, we performed the Begg rank correlation test and the results showed that publication bias was not significant for HR (p = 0.18) or for RR (p = 0.11). For BMD outcome, visual inspection of the funnel plot indicated that publication bias was also suspected (Supplemental Figure C). However, the rank correlation test showed that publication bias for BMD outcome was not significant (p = 0.46). We performed the trim-and-fill correction procedure and found no trim or fill data point was needed for all outcome measures.
Discussion
Our meta-analysis demonstrated that depression is prospectively associated with a significant increase in the risk of fracture and bone loss. The increased risk associated with depression remained consistent and statistically significant in all sensitivity analyses based on various inclusion criteria (Table 1) and in all subgroup analyses grouped by various participant and study characteristics (Tables 2, 3, and 4). Such consistent findings from all these analyses suggest our results are robust and consistent. Given that both depression and osteoporosis are prevalent worldwide, our meta-analysis findings will no doubt have important implications for public health globally.
The results of our meta-analysis are in accordance with findings from those former meta-analyses [8,9,10, 40] that assessed the association of depression and decreased BMD. These prior meta-analyses either included case-control and cross-sectional studies, and were thus not able to address whether depression is prospectively associated with increased risk of fracture and bone loss. One of our previous meta-analyses [12] included cohort studies only and assessed the effect of depression on risk of fracture; however, because it was published 7 years ago, it was not able to integrate findings from large studies published in recent years [13,14,15,16,17,18,19,20,21,22], thus rendering the results less robust. Due to small number of included studies, our prior meta-analysis lacked sufficient data to assess the effect of depression on bone loss. In this updated meta-analysis, we were able to include several new qualified studies with large sample size. The large sample size in the current meta-analysis equipped us to conduct comprehensive sensitivity analyses and subgroup analyses not only for fracture outcome, but also for bone loss outcome in the perspective study design. The significant, consistent association between depression and bone loss found in the meta-analysis, as well as in all sensitivity analyses and subgroup analyses, further indicated, as well as substantiated, that bone loss creates a pathway for depression, which then in turn increases fracture risk. As well, we observed some variations in the association of depression with risk of fracture and bone loss between subgroups. These variations may be caused by heterogeneity, as these original studies were conducted at various settings in different geographic regions. As well, participants varied in distribution of age, gender and race/ethnicity, and depression, and BMD was measured using various tools and instruments. However, the difference of effect size between subgroups was not significant in all subgroup analysis. As this updated meta-analysis includes all eligible cohort data, it is more robust in assessing the casual association between depression and risk of bone loss and fracture.
The underlying mechanism of how depression increases risk of fracture has not been fully elucidated. Depression may alter concentrations of many hormones that affect bone formation and/or bone resorption. Depression continually activates the hypothalamic-pituitary-adrenal (HPA) axis and causes an elevated cortisol level. Hypercortisolemia is considered an important causal factor in decreased bone formation associated with depression. Levels of inflammatory cytokines such as interleukin-1β, interleukin-2, and interleukin-6 are elevated in depression, and elevated levels of these pro-inflammatory markers are linked to decreased BMD [41]. Depression is associated with several other key regulators of bone formation [42], including gonadal hormones estrogen and testosterone. In addition, the processes for human reproduction and growth become inhibited in depressive states, which results in decreased levels of estrogen and growth hormone/insulin growth factors. These hormone changes can cause decreased bone formation and increased bone resorption, and can contribute to bone deficit in depressive states [43]. Other factors, including leptin, vitamin D, and parathyroid hormone, may also play roles in the causal link between depression and decrease bone mass. Many poor health behaviors associated with depression, such as smoking, increased alcohol drinking, and decreased physical activity, have been found to impact bone metabolism [44]. In this meta-analysis, we observed significant association between depression and bone loss at several skeletal sites. These findings indicated bone loss may be a pathway between depression and fracture. On the other hand, depressed people are more likely to have difficulty in attending to the environment and are less likely to take necessary safety precautions, thus increasing the probability of falling [45]. This illustrates another pathway for depression to increase the risk of fracture. Fractures occur as a result of both decreased BMD and a higher propensity to fall, and depression is likely to increase fracture risk through both pathways.
This meta-analysis has some limitations. First, language bias was expected since only English studies were included. Second, publication bias cannot be ruled out, even all tests for this bias were not significant. Third, most original reports employed self-report scales to determine if participants were depressed, which may be subjected to misclassification bias and thus underestimate the risk of fracture and bone loss associated with depression. In addition, we were not able to conduct subgroup analysis based on ethnicity because information about ethnicity was not provided in most original reports. Finally, some original reports included in this meta-analysis lacked data on medication use, including corticosteroid and glucocorticoid. The impact of medications on the observed association between depression and risk of fracture and bone loss needs further investigation. There are differences in the risk of bias across studies selected, which was detected by observing that the effect of size is associated with the quality score. Duration of follow-up, demographic region of the study, the gender of the participants, and population size may influence the overall effect size substantially. High heterogeneity among studies selected was observed when we used HR and BMD as our outcomes. We applied the random effect model and the standardized outcome to address the issue.
In conclusion, our updated meta-analysis of prospective cohort studies further confirmed that depression is significantly associated with an increased risk of osteoporotic fracture and bone loss. Due to the high prevalence of depression and osteoporosis worldwide, the observed link between depression and bone loss and fracture has important implications for public health globally, especially with increased aging of the population worldwide. Prevention and treatment of depression may substantially decrease the risk of osteoporosis and osteoporotic fracture. Further studies are warranted to investigate the underlying mechanisms for how depression causes an increased risk of bone loss and fracture.
Abbreviations
- BMD:
-
Bone mineral density
- HR:
-
Hazard ratio
- OR:
-
Odd ratio
- RR:
-
Risk ratio
- MDD:
-
Major depressive disorder
- CI:
-
Confidence interval
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Melton LJ, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL (1998) Bone density and fracture risk in men. J Bone Miner Res 13:1915–1923
Melton LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL (1992) Perspective. How many women have osteoporosis? J Bone Miner Res 7:1005–1010
Gullberg B, Johnell O, Kanis JA (1997) World-wide projections for hip fracture. Osteoporos Int 7:407–413
Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Shim RS, Baltrus P, Ye J, Rust G (2011) Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005-2008. J Am Board Fam Med 24:33–38
Kessler RC, Ustün TB (2004) The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 13:93–121
Schweiger JU, Schweiger U, Hüppe M, Kahl KG, Greggersen W, Fassbinder E (2016) Bone density and depressive disorder: a meta-analysis. Brain Behav 6:e00489
Stubbs B, Brefka S, Dallmeier D, Stubbs J, Vancampfort D, Denkinger MD (2016) Depression and reduced bone mineral density at the hip and lumbar spine: a comparative meta-analysis of studies in adults 60 years and older. Psychosom Med 78:492–500
Wu Q, Magnus JH, Liu J, Bencaz AF, Hentz JG (2009) Depression and low bone mineral density: a meta-analysis of epidemiologic studies. Osteoporos Int 20:1309–1320
Yirmiya R, Bab I (2009) Major depression is a risk factor for low bone mineral density: a meta-analysis. Biol Psychiatry 66:423–432
Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG (2010) Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporos Int 21:1627–1635
Diem SJ, Harrison SL, Haney E, Cauley JA, Stone KL, Orwoll E, Ensrud KE, Osteoporotic Fractures in Men Research, G (2013) Depressive symptoms and rates of bone loss at the hip in older men. Osteoporos Int 24:111–119
Diem SJ, Ruppert K, Cauley JA, Lian Y, Bromberger JT, Finkelstein JS, Greendale GA, Solomon DH (2013) Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab 98:4355–4363
Cheng B-H, Chen P-C, Yang Y-H, Lee C-P, Huang K-E, Chen VC (2016) Effects of depression and antidepressant medications on hip fracture: a population-based cohort study in Taiwan. Medicine (Baltimore) 95:e4655
Williams LJ, Pasco JA, Jackson H, Kiropoulos L, Stuart AL, Jacka FN, Berk M (2016) Depression as a risk factor for fracture in women: a 10 year longitudinal study. J Affect Disord 192:34–40
Williams LJ, Berk M, Henry MJ, et al (2014) Depression following fracture in women: a study of age-matched cohorts. BMJ Open 4. https://doi.org/10.1136/bmjopen-2013-004226
Gale CR, Dennison EM, Edwards M, Sayer AA, Cooper C (2012) Symptoms of anxiety or depression and risk of fracture in older people: the Hertfordshire cohort study. Arch Osteoporos 7:59–65
Cizza G, Mistry S, Nguyen VT, Eskandari F, Martinez P, Torvik S, Reynolds JC, Gold PW, Sinaii N, Sinai N, Csako G, Group, P. S (2012) Do premenopausal women with major depression have low bone mineral density? A 36-month prospective study. PLoS One 7:e40894
Jørgensen TSH, Hansen AH, Sahlberg M, Gislason GH, Torp-Pedersen C, Andersson C, Holm E (2015) Nationwide time trends and risk factors for in-hospital falls-related major injuries. Int J Clin Pract 69:703–709
Rauma PH, Honkanen RJ, Williams LJ, Tuppurainen MT, Kröger HP, Koivumaa-Honkanen H (2016) Effects of antidepressants on postmenopausal bone loss—a 5-year longitudinal study from the OSTPRE cohort. Bone 89:25–31
Bolton JM, Morin SN, Majumdar SR, Sareen J, Lix LM, Johansson H, Odén A, McCloskey EV, Kanis JA, Leslie WD (2017) Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry 74:641–648
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Moher D, Liberati A, Tetzlaff J, Altman DG, Group, P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264–269):W64
Davidson GC, Neale JM (2001) Abnormal psychology, 6th edn. John Wiley, New York
Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS (1999) Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med 159:484–490
Mussolino ME (2005) Depression and hip fracture risk: the NHANES I epidemiologic follow-up study. Public Health Rep 120:71–75
Forsén L, Meyer HE, Søgaard AJ, Naess S, Schei B, Edna TH (1999) Mental distress and risk of hip fracture. Do broken hearts lead to broken bones? J Epidemiol Community Health 53:343–347
Søgaard AJ, Joakimsen RM, Tverdal A, Fønnebø V, Magnus JH, Berntsen GKR (2005) Long-term mental distress, bone mineral density and non-vertebral fractures. The Tromsø study. Osteoporos Int 16:887–897
Piirtola M, Vahlberg T, Isoaho R, Aarnio P, Kivelä S-L (2008) Predictors of fractures among the aged: a population-based study with 12-year follow-up in a Finnish municipality. Aging Clin Exp Res 20:242–252
Tolea MI, Black SA, Carter-Pokras OD, Kling MA (2007) Depressive symptoms as a risk factor for osteoporosis and fractures in older Mexican American women. Osteoporos Int 18:315–322
Whitson HE, Sanders L, Pieper CF, Gold DT, Papaioannou A, Richards JB, Adachi JD, Lyles KW (2008) Depressive symptomatology and fracture risk in community-dwelling older men and women. Aging Clin Exp Res 20:585–592
Ojo F, Al Snih S, Ray LA, Raji MA, Markides KS (2007) History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans. J Natl Med Assoc 99:412–418
Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, Ensrud KE, Barrett-Connor E, Cummings SR, Orwoll E (2007) Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res 22:211–219
Spangler L, Scholes D, Brunner RL, Robbins J, Reed SD, Newton KM, Melville JL, Lacroix AZ (2008) Depressive symptoms, bone loss, and fractures in postmenopausal women. J Gen Intern Med 23:567–574
Whooley MA, Cauley JA, Zmuda JM, Haney EM, Glynn NW (2004) Depressive symptoms and bone mineral density in older men. J Geriatr Psychiatry Neurol 17:88–92
Milliken LA, Wilhelmy J, Martin CJ, Finkenthal N, Cussler E, Metcalfe L, Guido TA, Going SB, Lohman TG (2006) Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in postmenopausal women exercising for 1 year. J Gerontol A Biol Sci Med Sci 61:488–494
Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE (2007) Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med 167:1240–1245
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Liu Y, Wang Z, Xiao W (2017) Risk factors for mortality in elderly patients with hip fractures: a meta-analysis of 18 studies. Aging Clin Exp Res. https://doi.org/10.1007/s40520-017-0789-5
Ganesan K, Teklehaimanot S, Tran T-H, Asuncion M, Norris K (2005) Relationship of C-reactive protein and bone mineral density in community-dwelling elderly females. J Natl Med Assoc 97:329–333
Khosla S, Melton LJ, Atkinson EJ et al (1998) Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274. https://doi.org/10.1210/jcem.83.7.4924
Gkiatas I, Lykissas M, Kostas-Agnantis I, Korompilias A, Batistatou A, Beris A (2015) Factors affecting bone growth. Am J Orthop Belle Mead NJ 44:61–67
Alghadir AH, Gabr SA, Al-Eisa E (2015) Physical activity and lifestyle effects on bone mineral density among young adults: sociodemographic and biochemical analysis. J Phys Ther Sci 27:2261–2270. https://doi.org/10.1589/jpts.27.2261
Anstey KJ, Burns R, von Sanden C, Luszcz MA (2008) Psychological well-being is an independent predictor of falling in an 8-year follow-up of older adults. J Gerontol B Psychol Sci Soc Sci 63:P249–P257
Funding
The project was supported by the Fund of Knowledge from University of Nevada, Las Vegas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Wu, Q., Liu, B. & Tonmoy, S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int 29, 1303–1312 (2018). https://doi.org/10.1007/s00198-018-4420-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4420-1