Abstract
Summary
In this prospective cohort study, depressive symptoms were associated with higher rates of bone loss in older men. Poorer performance on physical function tests partly explained the association between depressive symptoms and bone loss, suggesting that efforts to increase exercise and improve physical performance in depressed men may be beneficial.
Introduction
The aim of this study was to ascertain whether depressive symptoms are associated with increased rates of bone loss at the hip in older men.
Methods
A population-based prospective cohort study of 2,464 community-dwelling men, aged 68 and older, enrolled in the Osteoporosis in Men Sleep Ancillary Study had depressive symptoms assessed by the Geriatric Depression Scale (GDS). Subjects were categorized as depressed if GDS ≥6 at the initial examination. Bone mineral density (BMD) at the hip was measured using dual-energy X-ray absorptiometry at the initial and follow-up examination (average 3.4 years between exams). Use of antidepressant medications was assessed by interview and verified from medication containers at the two examinations. A computerized dictionary was used to categorize type of medication.
Results
In a base model adjusted for age, race/ethnicity, and clinic site, the mean total hip BMD decreased 0.70 %/year in 136 men with a GDS score of ≥6 compared to 0.39 %/year in 2,328 men with a GDS score of <6 (p = 0.001). Walking speed and timed chair stand partly explained the association between depressive symptoms and rates of bone loss.
Conclusion
Depression, as defined by a score of 6 or greater on the Geriatric Depression Scale, is associated with an increased rate of bone loss at the hip in this cohort of older men. Adjustment for walking speed and timed chair stand attenuated the strength of the association, suggesting that differences in physical functioning do partially explain the observed association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although much research has examined the risk factors for osteoporosis in women, less is known about the risk factors for osteoporosis in men. In women, depression has been implicated as a risk factor for fractures [1–3], bone loss [4], and osteoporosis, although results have not always been consistent [5–8]. Little data regarding the relationships between depression, fractures, and bone loss in men have been available, although one recent cohort study [9] has reported an association between depressed mood and higher risk of fracture in men. The mechanisms underlying an association between depression and bone outcomes are not well understood but may include direct effects of depression on bone metabolism; indirect effects due to decreased physical activity, weight changes, and comorbid conditions associated with depression; and effects of medications used to treat depressive symptoms.
To determine whether depressive symptoms among older men are associated with increased rates of bone loss, we assessed depressive symptoms using the Geriatric Depression Scale and performed hip bone mineral density (BMD) measurements at two examinations (average 3.4 years between examinations) in a cohort of 2,464 men aged 68 years and older enrolled in the Osteoporosis in Men Sleep Study.
Methods
Participants
From March 2000 through April 2002, 5,994 men who were at least 65 years of age were recruited for participation in the baseline examination of the prospective Osteoporotic Fractures in Men (MrOS) study. Men were recruited from population-based listings in six regions of the USA: Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA. Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded [10, 11].
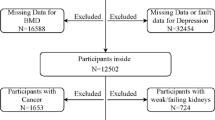
From December 2003 through March 2005, MrOS participants were invited to participate in an ancillary study to identify outcomes of sleep disorders in older men (MrOS sleep study). Of the 5,994 men enrolled in the overall study, 3,135 (52 %, >100 % of goal) completed the MrOS sleep examination. Of these, 2,464 (78.6 %) men had measurements of BMD at the sleep exam, completed the Geriatric Depression Scale (GDS) at the sleep exam, and had a follow-up BMD measurement at a follow-up exam (average 3.4 years later); these 2,464 men comprised the cohort for this analysis. Because depressive symptoms were assessed with a single question on the 12-item short form survey at the baseline MrOS visit, the original MrOS cohort was not utilized for this analysis. The institutional review board at each center approved the study protocol and written informed consent was obtained from all subjects.
Depressive symptoms
Depressive symptoms were evaluated at the sleep examination using the 15-item Geriatric Depression Scale short form (GDS) [12], a self-report scale consisting of 15 yes/no questions regarding symptoms of depression experienced in the previous week. A standard cutoff of ≥6 symptoms was used to define evidence of depression; the cutoff point of 6 or more symptoms has a sensitivity of 88 % and a specificity of 62 % compared with a structured clinical interview for depression [13].
Measurement of BMD
BMD at the total hip and two subregions (femoral neck and trochanter) was measured at the sleep and follow-up examinations (mean [SD], 3.4 [0.5] years between examinations) using dual-energy X-ray absorptiometry (DXA) with Hologic QDR-4500 W scanners (Hologic, Inc., Bedford, MA). Repeat measurements were performed on the same instruments used for the initial measurements. A central quality control laboratory, certification of DXA technicians, and standardized procedures for scanning were implemented to insure reproducibility of DXA measurements. At baseline, a hip phantom was circulated and scanned at the six clinical centers. Cross-calibration studies indicated no linear differences across scanners and the inter-scanner CV was 0.9 %. Each clinic scanned a hip phantom throughout the study to monitor longitudinal changes, and correction factors were applied to participant data as appropriate. In addition, multivariable models included an indicator variable for the individual center to adjust for interclinic differences. The rate of change in hip BMD was expressed as an annualized percentage of the initial value as percentage change in BMD per year.
Antidepressant medication use
Participants attending the clinic examinations were asked to bring all current (any use within the last 2 weeks) prescription and nonprescription medications with them to clinic. Interviewers completed a medication history for each participant, including name of medication and frequency of use. All prescription medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service drug vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) [14]. Subsequently, a physician (SJD) blinded to the outcome status reviewed the computerized drug data for antidepressant medication use and verified classification of medications as antidepressant medications.
Other measurements
The participants completed a questionnaire and were interviewed at each examination by trained staff, who asked about race/ethnicity, health status, smoking status, and alcohol use. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) [15]. To assess function, men were asked whether they had difficulty performing any of five independent activities of daily living [16–18]. A composite functional impairment score expressed the total number of activities ranging from 0 to 5 that a participant reported difficulty performing. Cognitive function was assessed with the Teng Modified Mini-Mental State Examination (maximum score 100) [19]. Body weight was measured by using a balance beam scale at all sites, except at one site (Portland) which used a digital scale. Height was measured by using a wall-mounted Harpenden stadiometer (Holtain, UK) [20]. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Change in weight over the study period was calculated by subtracting the weight at the sleep visit from the weight at the follow-up visit. Physical performance measures included chair stand time and walk speed. For the chair stand, the participants were asked to stand from a chair without using their arms; the time required to complete five chair stands without using arms was recorded [21]. For the narrow walk speed, the time (in seconds) to complete a 6-m course was recorded. Current use of bisphosphonates, thiazides, oral corticosteroids, vitamin D supplements, and calcium supplements was determined using the method described for ascertainment of antidepressant use. The number of selected comorbid conditions reported by the participants (stroke, diabetes mellitus, Parkinson’s disease, chronic obstructive pulmonary disease, myocardial infarction, angina, and congestive heart failure) was calculated for each participant.
Statistical analysis
X 2 tests for categorical variables, Fisher’s exact tests for categorical data with low expected cell counts, t tests for normally distributed continuous data, and Wilcoxon rank sum tests for skewed continuous data were used to compare characteristics at the sleep examination by category of GDS score (GDS <6 vs. GDS ≥6). For the primary analysis examining the association between category of GDS score and rate of change in BMD at the hip, the annualized mean percentage change in BMD and its 95 % confidence interval were calculated for the two categories of GDS (<6 vs. ≥6) score using the least-square-means procedure. To investigate mechanisms by which depressive symptoms might be associated with bone loss, we constructed a multivariable base model that included age, race, and clinic site, and then added potential confounders one at a time to determine their impact on the associations between depressive symptoms and bone loss: We hypothesized that the association between depressive symptoms and bone loss would be reduced after adjusting for these factors if they were in the causal pathway. A reduction of 10 % in the mean annualized percent change in BMD was considered as support for the hypothesis of mediation. Candidate covariates included known risk factors for bone loss in our cohort and characteristics related to category of GDS score at p < 0.1 independent of age.
In addition, we used a backward stepwise elimination procedure, with p ≤ 0.1 as a cutoff level for retention of covariates in the adjusted model. Candidate covariates were the same as above and included self-reported health status, number of instrumental activities of daily living (IADL) impairments, timed chair stand, history of falls in the previous year, PASE score, MMSE score, smoking status, alcohol use, calcium supplement use, vitamin D supplement use, BMI, oral steroid use, antidepressant use, and number of comorbidities.
To explore whether greater number of depressive symptoms are associated with higher rates of bone loss, we used linear regression techniques to examine the association between number of depressive symptoms on the GDS score as a continuous predictor and change in BMD. We also performed analyses in which we compared the rate of change in BMD for subjects in three categories of GDS score: those who endorsed 0–2 depressive symptoms on the GDS, those who endorsed 2–5 symptoms, and those who endorsed ≥6 symptoms.
Additional secondary analyses examining the association between GDS score and rate of change in BMD were performed with substitution of annualized absolute change in BMD (grams per square centimeter) for annualized percentage rate of change in BMD as our outcome measure. As the findings from these secondary analyses were similar to those of our primary analyses, we have presented the primary results. All analyses were performed using SAS software (Version 9.1, SAS Institute Inc., Cary, NC).
Results
Characteristics of the study population
The cohort included 2,464 men, of whom 136 (5.5 %) scored ≥6 on the GDS. Of the men, 2,222 (90.2 %) were Caucasian. Baseline characteristics of the 2,464 men at the sleep visit according to the category of GDS score appear in Table 1. Men with a GDS score of ≥6 compared with men with a GDS score of <6 were older (p = 0.03), were more likely to report poor health status (p < 0.001), had more impairment of IADLs (p < 0.001), and were more likely to smoke (p < 0.01). They also had evidence of poorer physical functioning: they had slower walking speeds and were more likely to take longer to rise from a chair (p < 0.001).
Of the 3,135 men who attended the sleep visit, 671 were not included in this analysis due to missing BMD data or GDS scores. In general, the excluded men were more likely to report poor or fair health, have difficulty with IADLs and more comorbid medical conditions, and have poorer lower extremity function (results not shown).
Depression and rate of hip bone loss
The mean rates of bone loss at the total hip in men without vs. those with depression (GDS score <6 vs. ≥6) are shown in Table 2. On average, men with a GDS score ≥6 experienced a higher age-adjusted annualized percentage loss of bone density at the total hip than men with a GDS <6 (−0.69 vs. −0.39 %/year; p = 0.002). The effect of adjustment for possible mediators on the annualized percentage change in bone density is displayed in Table 3. In a base model of age, race, and clinic sites, results were similar to the age only-adjusted models. The addition of individual covariates did not significantly change the strength of the association between depressive symptoms and rate of bone loss, with the exception of timed chair stands and walking speed, each of which had a modest effect as manifested by a 10 % reduction in the difference in rate of change in BMD between men with and without depression.
Using the backwards elimination procedure, the following covariates were retained in the final model: number of impairments in IADLs, walking speed, smoking status, calcium supplement use, history of falls in previous year, and presence of comorbidities. Results were attenuated in this model: men with a GDS score ≥6 experienced an average 0.56 %/year loss of bone density compared to 0.40 % loss for men with GDS <6 (p = 0.10) (Table 3).
Results were similar at the femoral neck and trochanter, with significantly higher rates of bone loss in the base model for men with a GDS ≥6 compared to men with a GDS score of <6. At the femoral neck, inclusion of walking speed and chair stands in the model each modestly affected the strength of the association between depressive symptoms and rates of bone loss, similar to the findings at the total hip (results not shown). At the trochanter, walking speed was the only covariate that significantly affected the results (results not shown). In the final multivariable models using the backwards elimination procedure, mean rate of bone loss was higher for men with GDS ≥6 at the femoral neck (0.57 vs. 0.45 %; p = 0.37) and trochanter (0.48 vs. 0.34 %; p = 0.26), but associations were attenuated and no longer reached significance.
Number of depressive symptoms and rates of bone loss
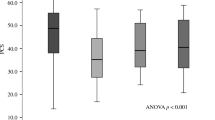
In a secondary analysis examining the number of depressive symptoms and rates of bone loss, there was evidence of a graded association between number of depression symptoms and higher rate of bone loss at the total hip, femoral neck, and trochanter in age-adjusted models. Subjects with GDS scores of 0–2 had lower average rates of bone loss than those with scores of 3–5, who in turn had lower average rates of bone loss that those with scores of ≥6 (Fig. 1a). In multivariable models, this trend persisted at the total hip but was attenuated at the femoral neck and trochanter (Fig. 1b). In linear regression models, for every 1 standard deviation increase in the GDS score (2.0) the average rate of loss of BMD at the total hip increased 0.09 % (p < 0.001) in the base model. In the multivariable model, the average rate of loss at BMD at the total hip increased 0.052 % (p = 0.028) for every 1 standard deviation increase in the GDS.
Discussion
Evidence of depression, based on the number of depressive symptoms (GDS of ≥6), was associated with an approximate 1.8-fold increase in the annualized rate of bone loss at the total hip, femoral neck, and trochanter, in this cohort of older men, due in part to poorer neuromuscular functioning among those men with depression. Our findings are consistent with some previous cross-sectional studies that have reported lower BMD in subjects with depression compared to subjects without depression [6, 22–26]. However, other cross-sectional studies [5, 7, 27] have not observed lower BMD in subjects with depression. Due to their limitations, cross-sectional studies may tend to over- or underestimate any association between depressive symptoms and bone density.
Little prospective data regarding rate of change in BMD in depressed subjects have been available, particularly in men. Previous studies including men are limited by small sample sizes and inadequate consideration of potential confounding factors. Whooley et al. [27] did not observe any association between depressive symptoms, baseline BMD, or rate of subsequent bone loss in a cohort of men aged 50 and older. However, that study had only four men with GDS ≥6 who had follow-up BMD, limiting the interpretation of the results. Using single-energy quantitative computerized tomography, Schweiger et al. measured change in lumbar spine BMD over a 24-month period in 18 depressed subjects and 21 healthy subjects, 40 years of age and older [28]. Controlling for age, gender, and initial BMD, subjects with major depression in that study had increased bone loss over the 2-year period. More data are available from studies limited to women. In a cohort of older women, we have previously reported an association between GDS scores ≥6 and rates of bone loss, an association that persisted after controlling for multiple potential confounding factors [4]. Milliken et al. found that higher scores on the Beck Depression Inventory were significantly associated with greater loss of BMD over 1 year at the femoral neck, but not the trochanter, in a cohort of 264 women aged 40–65 [29]. However, Spangler et al. did not observe an association between depressive symptoms and change in BMD over 3 years in the observational cohort of the Women’s Health Initiative [7].
Previous work has suggested that depression is associated with an increased risk of fractures in this cohort [9], as well as in others [1, 2, 30], although results have not always been consistent [7, 8]. The mechanism of an association between depression and fractures is unclear, but our finding of increased rates of bone loss among depressed subjects suggests that this may be a factor.
There are many potential explanations for a possible association between depressive symptoms and rates of change of BMD. Depression is associated with decreased physical activity, increased rates of smoking, weight loss, poorer health status, and medical comorbidities, all of which may predispose to increased rates of bone loss [31–34]. When individual covariates were examined, walking speed and time to rise from a chair without using arms did attenuate the difference observed in rates of bone loss between depressed and non-depressed men, suggesting that poorer neuromuscular functioning in depressed men may in part explain the observed association. No other covariates examined substantially impacted the association between depressive symptoms and rate of bone loss. In multivariable models that included impairment of IADLs, walking speed, smoking status, calcium use, history of previous falls, and comorbidities, the association between depressive symptoms and rate of bone loss was attenuated and no longer reached, possibly due to inadequate power.
Medications to treat depression have been postulated to have an effect on bone metabolism [35–37] and antidepressant use was more prevalent among men with GDS ≥6. In models controlling for age, depressive symptoms, and antidepressant use, depressive symptoms remained significantly associated with rates of bone loss, suggesting that antidepressant use alone does not explain the observed association.
Several potential mechanisms have been postulated for a direct effect of depression on bone metabolism. Elevated plasma cortisol levels have been observed in depressed subjects and have been attributed to an impaired hypothalamic–pituitary–adrenal system in depression [38]; these elevations in plasma cortisol levels have been postulated to lead to accelerated bone loss [23]. Upregulation of proinflammatory cytokines, such as Il-6 and TNF-alpha, appear to mediate resorption [39, 40] and are upregulated in depression [41–43].
These findings suggest that depressed men may be at risk for higher rates of bone loss, at least in part due to poorer neuromuscular functioning. Future research might focus on interventions to increase exercise in this population: structured physical activity has been shown to improve performance on measures of physical function, including gait speed and ability to rise from a chair [44, 45], and some evidence suggests that exercise can improve the symptoms of depression [46].
References
Sogaard AJ, Joakimsen RM, Tverdal A, Fonnebo V, Magnus JH, Berntsen GK (2005) Long-term mental distress, bone mineral density and non-vertebral fractures. The Tromso study. Osteoporos Int 16:887–897
Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS (1999) Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med 159:484–490
Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG (2010) Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporos Int 21:1627–1635
Diem SJ, Blackwell TL, Stone KL, Yaffe K, Cauley JA, Whooley MA, Ensrud KE (2007) Depressive symptoms and rates of bone loss at the hip in older women. J Am Geriatr Soc 55:824–831
Reginster JY, Deroisy R, Paul I, Hansenne M, Ansseau M (1999) Depressive vulnerability is not an independent risk factor for osteoporosis in postmenopausal women. Maturitas 33:133–137
Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T (2001) The association of bone mineral density and depression in an older population. J Am Geriatr Soc 49:732–736
Spangler L, Scholes D, Brunner RL, Robbins J, Reed SD, Newton KM, Melville JL, Lacroix AZ (2008) Depressive symptoms, bone loss, and fractures in postmenopausal women. J Gen Intern Med 23:567–574
Whitson HE, Sanders L, Pieper CF, Gold DT, Papaioannou A, Richards JB, Adachi JD, Lyles KW (2008) Depressive symptomatology and fracture risk in community-dwelling older men and women. Aging Clin Exp Res 20:585–592
Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, Ensrud KE, Barrett-Connor E, Cummings SR, Orwoll E (2007) Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res 22:211–219
Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568
Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585
Sheikh JI, Yesavage JA (1986) Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 5:165–173
Gerety MB, Williams JW Jr, Mulrow CD, Cornell JE, Kadri AA, Rosenberg J, Chiodo LK, Long M (1994) Performance of case-finding tools for depression in the nursing home: influence of clinical and functional characteristics and selection of optimal threshold scores. J Am Geriatr Soc 42:1103–1109
Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P (1994) Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411
Washburn RA, Ficker JL (1999) Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness 39:336–340
Ensrud KE, Nevitt MC, Yunis C, Cauley JA, Seeley DG, Fox KM, Cummings SR (1994) Correlates of impaired function in older women. J Am Geriatr Soc 42:481–489
Fitti JE, Kovar MG (1987) The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat 1:1–115
Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP (1983) Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment questionnaire. Arthritis Rheum 26:1346–1353
Teng EL, Chui HC (1987) The modified mini-mental State (3MS) examination. J Clin Psychiatr 48:314–318
Lohman TG, Roche AF, Martorell R (1988) Anthropometric standardization reference manual. Human Kinetics, Champaign
Ensrud KE, Walczak TS, Blackwell T, Ensrud ER, Bowman PJ, Stone KL (2004) Antiepileptic drug use increases rates of bone loss in older women: a prospective study. Neurology 62:2051–2057
Jacka FN, Pasco JA, Henry MJ, Kotowicz MA, Dodd S, Nicholson GC, Berk M (2005) Depression and bone mineral density in a community sample of perimenopausal women: Geelong Osteoporosis Study. Menopause 12:88–91
Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P (1996) Bone mineral density in women with depression. N Engl J Med 335:1176–1181
Schweiger U, Deuschle M, Korner A, Lammers CH, Schmider J, Gotthardt U, Holsboer F, Heuser I (1994) Low lumbar bone mineral density in patients with major depression. Am J Psychiatry 151:1691–1693
Wong SY, Lau EM, Lynn H, Leung PC, Woo J, Cummings SR, Orwoll E (2005) Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong). Osteoporos Int 16:610–615
Yazici KM, Akinci A, Sutcu A, Ozcakar L (2003) Bone mineral density in premenopausal women with major depressive disorder. Psychiatr Res 117:271–275
Whooley MA, Cauley JA, Zmuda JM, Haney EM, Glynn NW (2004) Depressive symptoms and bone mineral density in older men. J Geriatr Psychiatr Neurol 17:88–92
Schweiger U, Weber B, Deuschle M, Heuser I (2000) Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psychiatry 157:118–120
Milliken LA, Wilhelmy J, Martin CJ, Finkenthal N, Cussler E, Metcalfe L, Guido TA, Going SB, Lohman TG (2006) Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in postmenopausal women exercising for 1 year. J Gerontol A Biol Sci Med Sci 61:488–494
Mussolino ME (2005) Depression and hip fracture risk: the NHANES I epidemiologic follow-up study. Public Health Rep 120:71–75
Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, Tao JL, Cummings SR (1993) Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med 118:657–665
Chen JS, Cameron ID, Cumming RG, Lord SR, March LM, Sambrook PN, Simpson JM, Seibel MJ (2006) Effect of age-related chronic immobility on markers of bone turnover. J Bone Miner Res 21:324–331
Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, Hanlon JT, Nevitt MC, Whooley MA (2003) Central nervous system active medications and risk for fractures in older women. Arch Intern Med 163:949–957
Fitzpatrick LA (2002) Secondary causes of osteoporosis. Mayo Clin Proc 77:453–468
Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE (2007) Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med 167:1240–1245
Warden SJ, Haney EM (2008) Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: evidence from in vitro and animal-based studies. J Musculoskelet Neuronal Interact 8:121–132
Warden SJ, Robling AG, Haney EM, Turner CH, Bliziotes MM (2010) The emerging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5). Bone 46:4–12
Heuser I (1998) Anna-Monika-Prize paper. The hypothalamic–pituitary–adrenal system in depression. Pharmacopsychiatry 31:10–13
Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91
Nanes MS (2003) Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene 321:1–15
Dentino AN, Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ (1999) Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc 47:6–11
Kiecolt-Glaser JK, Glaser R (2002) Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res 53:873–876
Maes M (1999) Major depression and activation of the inflammatory response system. Adv Exp Med Biol 461:25–46
Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, Hadley EC, King AC, Kritchevsky SB, Maraldi C, Miller ME, Newman AB, Rejeski WJ, Romashkan S, Studenski S (2006) Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for elders pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci 61:1157–1165
Rejeski WJ, Marsh AP, Chmelo E, Prescott AJ, Dobrosielski M, Walkup MP, Espeland M, Miller ME, Kritchevsky S (2009) The lifestyle interventions and independence for elders pilot (LIFE-P): 2-year follow-up. J Gerontol A Biol Sci Med Sci 64:462–467
Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev 2009; CD004366
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) study is supported by the National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging, the National Center for Research Resources, and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. Dr. Haney is supported by a career development award from the NIAMS: K23 AR051926.
Conflicts of interest
None
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Diem, S.J., Harrison, S.L., Haney, E. et al. Depressive symptoms and rates of bone loss at the hip in older men. Osteoporos Int 24, 111–119 (2013). https://doi.org/10.1007/s00198-012-1975-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-1975-0