Abstract
Osteoporosis is a bone disease characterized by reduced bone mass, which leads to increased risk of bone fractures, and poses a significant risk to public health, especially in the elderly population. The traditional Chinese medicinal herb Epimedii has been utilized for centuries to treat bone fracture and bone loss. Icariin is a prenylated flavonol glycoside isolated from Epimedium herb, and has been shown to be the main bioactive component. This review provides a comprehensive survey of previous studies on icariin, including its structure and function, effect on bone metabolism, and potential for clinical application. These studies show that icariin promotes bone formation by stimulating osteogenic differentiation of BMSCs (bone marrow-derived mesenchymal stem cells), while inhibiting osteoclastogenic differentiation and the bone resorption activity of osteoclasts. Furthermore, icariin has been shown to be more potent than other flavonoid compounds in promoting osteogenic differentiation and maturation of osteoblasts. A 24-month randomized double-blind placebo-controlled clinical trial reported that icariin was effective in preventing postmenopausal osteoporosis with relatively low side effects. In conclusion, icariin may represent a class of flavonoids with bone-promoting activity, which could be used as potential treatment of postmenopausal osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is the most common type of bone disorder, resulting from a disruption of balance between bone formation and resorption. It is characterized by degeneration of bone microstructure, reduction of bone mass, and leads to a considerably higher increased risk of fractures [1]. The estimated number of individuals with osteoporosis and osteopenia, the precursor to osteoporosis, continues to increase, especially with the aging of the population [2]. Osteoporosis poses a serious public health problem, particularly threatening postmenopausal women and senior citizens. The total number of bone fractures due to osteoporosis reported in the USA exceeded 2 million in 2005, costing nearly $17 billion, with indirect and direct costs expected to increase substantially annually between 2005 and 2025. Currently, bone mineral density (BMD) scans which utilize dual-energy X-ray absorption (DEXA) and provide two-dimensional information for an area of the bone, are the most commonly used method for the diagnosis of osteoporosis. Quantitative computed tomography (QCT) has the advantage of detecting the volumetric, rather than areal, BMD, allowing measurements of bone geometric parameters and regional bone strength, leading to improved sensitivity and accuracy of BMD measurements. Finite element analysis (FEA) utilizes high-resolution images and three dimensional models to analyze the changes in bone strength under different conditions, and to predict the load and location of potential fractures [3]. Other than BMD, several characteristics of the bone, including degree of mineralization, hydroxyapatite crystal size, collagen structure, heterogeneity of bone microstructure, connectivity of trabeculae, and microdamage, are of clinical significance.
Bone remodeling occurs continually and is mediated through the coupled cycle of bone resorption and bone formation. Bone loss and the resultant osteopenia and osteoporosis occur due to an imbalance in this process. At the cellular level, osteoclasts promote bone resorption by secreting acids and enzymes that disassemble and digest bone mineral and proteins, while osteoblasts promote bone formation by creating a protein matrix consisting primarily of collagen that is soon calcified, resulting in mineralized bone. Strategies to prevent and treat osteoporosis include general preventive measures (such as diet and exercise) and pharmacologic interventions [4]. Drugs commonly used in clinical settings are divided into two categories: (1) bone resorption inhibitors, such as hormone (estrogen) therapy, estrogen agonists, calcitonin, bisphosphonates, and isoflavones, which exert their effect by interfering osteoclast resorption activity; and (2) bone formation promoters, such as fluoride and parathyroid hormone, which enhance bone mass and strength through stimulating osteoblast activity. However, clinical use of most anti-resorption drugs is limited due to the adverse side effects of suppressing bone formation in the long term [5]. For example, although short-term (3 years or less) bisphosphonate use appears to be well-tolerated in children and adolescents, adverse side effects after long-term use of bisphosphonates have been reported, including the upper gastrointestinal bleeding, acute phase response, hypocalcaemia and secondary hyperparathyroidism, musculoskeletal pain, osteonecrosis of the jaw, and ocular events [6]. The most commonly reported adverse reaction of Calcitonin (Miacalcin, Fortical, Nasal calcitonin) is nasal irritation [7]. Subcutaneous injections of teriparatide (Forteo) and abaloparatide (Tymlos), the parathyroid hormone medications currently FDA-approved for osteoporosis treatment, are highly effective in building the bone and reducing fractures, although teriparatide is very costly (approximately $850 per month) and cannot be used for more than 2 years due to the tumorigenic potential observed in rats [8]. Similarly, abaloparatide, like teriparatide, is subject to a total treatment duration limit of 24 months, due to the potential risk for osteosarcoma incidence, especially in susceptible individuals [9, 10].
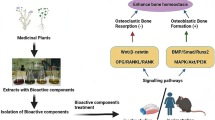
More researchers are turning to traditional Chinese medicines to search for preventive or alternative therapies to treat osteoporosis. Increasingly, natural products, particularly flavonoids, are being explored for their therapeutic potentials in reducing bone loss and maintaining bone density. Icariin, 2-(4′-methoxylphenyl)-3-rhamnosido-5-hydroxyl-7-glucosido-8-(3′-methyl-2-butylenyl)-4-chromanone, is the most abundant flavonoid constituent in Herba Epimedii, and has been shown to be effective for bone regeneration and repair [11]. H. Epimedii is a centuries-old traditional Chinese medical plant used in the treatment of fractures, joint disease, and gonadal dysfunctions [12]. Naturally isolated icariin is fast becoming an attractive alternative in the prevention and treatment of osteoporosis. Commercially purified icariin used in most research studies is commonly extracted using high-performance liquid chromatography (HPLC). Evidence is steadily accumulating that icariin may play a dual role in bone health by stimulating bone formation while simultaneously inhibiting bone resorption [13]. Importantly, icariin can be steadily and locally released, using biomaterials, making it an attractive osteoinductive candidate for bone tissue engineering [14,15,16]. Angiogenesis also plays an important role in bone regeneration, and it has been reported that icariin could increase angiogenesis via stimulating endothelial cell migration, proliferation, and tubule genesis in vivo [17]. In this review, we update and summarize the current model of the bone remodeling cycle, the role of icariin in bone formation and bone resorption, its pharmacokinetics and pharmacological effects, and compare its efficacy to other flavonoids.
Icariin: activation of bone remodeling
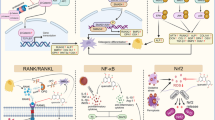
Bone remodeling is comprised of two basic physiological processes—osteoblastogenesis and osteoclastogenesis [18, 19]—which comprise a highly coordinated, dynamic, and continual physiological cycle of bone formation and bone resorption [20]. The process of bone remodeling is respectively executed by two distinct lineage cells—osteoblasts and osteoclasts. Osteoblasts are derived from bone marrow-derived mesenchymal stem cells (BMSCs) and mature into specialized, terminally differentiated osteocytes [21]. The group of osteoblasts and the bone matrix form the osteon, the basic structural unit of mature bones. Osteoclasts, originating from monocyte/macrophage hematopoietic progenitors in the bone marrow, are multinucleated cells which secrete acids and collagenolytic enzymes to resorb the bone [22]. Osteoclast differentiation and activity are dependent on the receptor activator of nuclear factor kappa-B (NF-kB) ligand (RANKL), secreted by osteoblasts and BMSCs [23] (Fig. 1).
Icariin stimulates bone formation by promoting osteoblastogenesis and the bioactivity of the osteoblasts. Icariin, a prenylated flavonol glycoside isolated from Epimedium herb, increases the number of BMSC-derived osteoblasts and augments the maturation of pre-osteoblasts, meanwhile inhibits the adipocytic transdifferentiation of primary osteoblasts. Icariin-mediated osteogenesis is the result event of multiple signaling transduction pathways, including up-regulation of BMP, NO, MAPK, Wnt pathways, and down-regulation of ERK and JNK pathways. Icariin also can exert the osteogenic effect by inhibiting the ROS generation
Normal bone remodeling requires balance between bone formation and bone resorption, but this balance is disrupted under certain circumstances, such as estrogen deficiency following menopause or in some autoimmune diseases [24, 25]. Estrogen plays a role in bone remodeling, through ERs (estrogen receptors) in the osteoblasts and osteoclasts, with ERα mediating the predominant effect [26]. Estrogen can enhance the proliferation and maturation of primary osteoblasts, inhibit their apoptosis, and enhance their bone-inducing activity [27], while it also suppresses osteoclastogenesis. Inhibition of the ER signaling pathway resulted in bone loss in a mice model [28]. Estrogen is also associated with osteoimmunology, and its deficiency leads to augmentation of activated T cell activity, osteoclastogenesis, and increased bone resorption [29]. Icariin and its derivatives can restore the balance of bone remodeling through an estrogen mimetic effect which regulates osteogenic progenitor cell fate commitment, proliferation, maturation, and matrix mineralization [13].
Icariin stimulates the osteogenic differentiation of BMSCs into osteoblasts
BMSCs are multipotent stem cells located within the bone marrow stroma and have the potential of giving rise to several cell linages, including osteoblasts, chondrocytes, adipocytes, cardiomyocytes, and endothelial cells [30]. Thus, BMSCs provide a primary source of osteoblasts. Icariin treatment of pre-osteoblastic MC3T3-E1 cells and mouse primary osteoblasts in vitro promotes the expression of osteoblast marker genes, Runx2 (runt-related transcription factor 2), and Id-1 (inhibitor of DNA-binding 1) [31]. Icariin also enhances the self-renewal ability, and augments the osteogenic differentiation of BMSCs in vitro and in vivo in female Sprague-Dawley rats (6 months old) [32, 33]. However, it should be noted that the skeleton of 6-month-old rats is still growing and has not reached its peak bone mass; therefore, 9-month-old ovariectomized female rats are recommended as a more accurate model for simulating postmenopausal osteoporosis [34].
Multiple signaling pathways are involved the osteogenic differentiation, including BMP (bone morphogenetic protein), NO (nitric oxide), MAPK (mitogen-activated protein kinase), and the canonical Wnt/β-catenin pathways [31, 35,36,37,38]. BMP-4 is induced under icariin treatment and subsequently initiates the BMP signaling pathway, as established in multiple cell models, including human MSCs and mouse-derived pre-osteoblastic MC3T3-E1 and C3H10T1/2 MSCs, and wild-type C57BL/6N mouse primary osteoblasts [31]. The BMP-2/Smad4 signal transduction pathway is reported to be activated by icariin in both human osteoblastic hFob1.19 and murine osteoblastic MC3T3-E1 cell lines [39, 40]. Treatment with NO pathway inhibitors diminishes the osteogenic effect of icariin in rat primary bone marrow stromal cells in vitro [35]. In MC3T3-E1 cells, icariin induces ERK (extracellular signal-regulated kinase) and JNK (c-Jun N terminal kinase) activation, but p38 kinase activity is not affected [36]. Blocking estrogen-mediated signaling attenuates icariin’s bone-inducing effects [36]. Icariin can also induce β-catenin mRNA expression, β-catenin subcellular redistribution, and enhances the GSK-3β (glycogen synthase kinase-3) phosphorylation levels in rat BMSCs [37]. The mechanism of activation of these signaling pathways perhaps arises from the estrogen mimetic properties of icariin [41, 42] and induced production of estrogen by icariin [43].
It appears that icariin also promotes human BMSCs differentiation by epigenetic regulation. ROS (reactive oxygen species) are one of the factors controlling human BMSCs differentiation, and excessive ROS promotes adipogenic differentiation, and is clinically associated with corticosteroid-induced osteonecrosis [44]. Intracellular ROS levels, MMP (mitochondrial membrane potential), P-gp (P-glycoprotein) activity, methylation of ABCB1 (ATP-binding cassette subfamily B member 1), and other important indices of oxidative stress are significantly up-regulated in BMSCs of patients with steroid-associated osteonecrosis [45]. However, treatment with icariin alleviates oxidative stress and reduces CpG island hypermethylation of ABCB1 in BMSCs isolated from steroid-associated osteonecrosis of femoral head (ONFH) patients [45]. One study suggested that icariin can also protect DNA from excessive oxidative stress in an AAPH (2,2′-azobis(2-amidinopropane) dihydrochloride)-induced oxidative damage of DNA model [46], although apparently, the 10−5–10−4 M concentration adopted in the study is cytotoxic, and the extent to which icariin protects DNA from oxidative damage is debatable in cell and animal models. The osteogenic differentiation of human BMSCs induced by icariin is dose-dependent [32, 47], and the optimal concentration in vitro ranges from 10−9 to 10−5 M, with concentrations above 10−5 M resulting in cytotoxicity [32].
Icariin suppresses adipocytic transdifferentiation of primary osteoblasts
BMSCs are multipotent and have the potential of giving rise to both adipocytes and osteoblasts [48]. Icariin increases the number of mature osteoblasts simultaneously through an alternative pathway, by suppressing the adipocytic transdifferentiation of primary osteoblasts [49, 50]. Adipogenesis-related genes, peroxisome proliferator-activated receptor γ (Pparg), and CCAAT/enhancer-binding protein β (Cebpb) genes are down-regulated when the primary osteoblasts are treated with icariin, and inhibition of adipogenic transdifferentiation leads to further osteoblastic differentiation [49].
Icariin stimulates the bone formation activity of osteoblasts
Icariin also can facilitate the maturation of primary osteoblasts and bone remodeling activity of osteoblasts. Icariin treatment induces expression of terminal differentiation markers, ALP (alkaline phosphatase) and Col I (collagen type I), and mineralization of osteoblasts [36, 51,52,53]. In addition, icariin attenuates cell apoptosis and preserves cell viability in rat calvarial osteoblasts exposed to hypoxic conditions (2% oxygen) by counteracting the effects of oxidative stress [53]. In the glucocorticoid-induced osteoporosis Sprague-Dawley rat model, the bone mass increase observed with icariin (125 mg/kg, daily for 12 weeks, i.g.) treatment was comparable to that of alendronate (0.3 mg/kg daily for 12 weeks, i.g.), significantly increasing ALP, a bone formation marker, and reducing CTX (carboxy-terminal collagen cross-links), a bone resorption marker. Furthermore, icariin displays a robust anti-apoptotic effect and a concentration of 10−7 M was shown to completely annihilate dexamethasone-induced apoptosis in osteocytes [51].
Icariin regulates bone homeostasis mainly by activating the ER and ERK signaling pathways, simultaneously inducing the mRNA expression of OPG (osteoprotegerin) and activating the Wnt signaling pathway. Icariin has been shown to activate ER by phosphorylation at Ser 118 and Ser 167 and prevent glucocorticoid-induced apoptosis in osteocytes by activating ERK signaling via ER. OPG, a decoy receptor of RANKL, also known as OCIF (osteoclastogenesis inhibitory factor) or TNFRSF11B (tumor necrosis factor receptor superfamily member 11B), belongs to the TNF superfamily and plays an essential role in restraining bone loss by counteracting the effects of RANKL [54, 55]. The anabolic response of trabecular bone was diminished in OPG knockout C57BL/6J mice when they were orally administered icariin (300 mg/kg) every day for 8 weeks [56]. Another report shows that icariin, 5 mg/kg per day by local injection over the calvaria surface of OPG-deficiency mice, induces bone formation and reverses the osteopenia phenotype [57]. In vitro experiments on BMSCs were conducted using the bone marrow of the femur and bilateral tibia of C57/BL6 mice, and showed that treatment with 50 μM icariin for 2 days induces the activation of Wnt/β-catenin and BMPs/Smads/Runx2 signaling, and augments the expression of signaling pathway associated genes, including BMP2, BMP4, RUNX2, OC, Wnt1, Wnt3a, AXIN2, DKK1, TCF1, and LEF1 [57]. Although the dosages adopted by the two research groups are incomparable due to different administration protocols, it is reasonable to speculate that icariin functions in a dosage-dependent manner, mediated by both OPG and ERs.
Icariin: inhibition of bone resorption
Icariin inhibits osteoclastogenesis by suppressing osteoclastic differentiation
Inhibition of osteoclastogenesis partially contributes to the anti-osteoporosis efficacy of icariin and its derivatives. Tartrate-resistant acid phosphatase (TRAP) is commonly utilized as an osteoclast differentiation marker. Icariin treatment of osteoclast precursor cells (isolated from 8-month-old female imprinting control region (ICR) mice) leads to a significant decrease of TRAP-positive multinuclear cells in a dose-dependent manner [58], and icariin directly quells the RANKL-induced differentiation of hemopoietic cells from which osteoclasts are formed [59]. Icariin and its derivatives increase the anti-osteoblastogenic mRNA expression of ALP, OC (osteocalcin), COL-1 (typeIcollagen), and OPG, suppressing that of RANKL in primary osteoblasts, resulting in an indirect suppression of osteoclastic differentiation [60]. Icariin also prevents mRNA expression of inflammatory cytokines and inflammation-induced progenitor osteoclast differentiation [58]. Besides regulating osteoclastic differentiation, icariin induces a pause in the cell cycle of precursor osteoclast cells, leading to apoptosis. Icariin treatment of RAW 264.7 (a mouse-derived cell line that has the potential of differentiation into osteoclast upon RANKL induction) leads to G2/M cell cycle arrest, inhibition of proliferation, and apoptosis, in an ER-dependent manner [61] (Fig. 2).
Icariin inhibits bone resorption by suppressing osteoclastogenesis and the bioactivity of the osteoclasts. Icariin acts upon monocyte/macrophage-derived cells (pro-osteoclasts) and inhibits their differentiation by regulation of RANKL, OPG, ALP, and Col-1. Bone resorptive activity of osteoclasts could be inhibited by icariin. Meanwhile, another way of reducing bone loss could be through the alleviation of inflammatory signaling pathways by icariin
Icariin inhibits the osteoclast activity
Icariin (10−8 M) has an inhibitory effect on osteoclast activity by suppressing inflammatory signaling pathways, such as p38, ERK, NF-κB, and JNK in primary osteoclasts isolated from 8-month-old female ICR mice [58]. A notable decrease of osteoclastic resorption area was observed after the osteoclasts isolated from Sprague-Dawley fetal neonatal rats were treated with icariin and its derivatives at concentrations of 10−5–10−8 nM [60], indicating that icariin undermines osteoclastic activity by restraining motility and bone resorption. In vitro experiments in 1~2-day-old Japanese white rabbits show that icariin can also suppress the activities of osteoclasts by eliciting the decline of superoxide anion (·O2−) generation, decreasing size and number of actin rings, and intracellular calcium concentration [62].
Icariin: another way of reducing bone loss by regulating immune response?
Bone remodeling and the immune system are closely linked. Normal bone remodeling is disrupted in autoimmune diseases such as arthritis, and activated T cells can directly lead to the production of OPGL, a ligand of OPG, and subsequent bone loss [63]. Inflammatory signaling pathways also induce primary osteoclast maturation and bone resorption activity [64]. Clinically, bone loss is closely associated with estrogen deficiency in ovariectomized mice, as well as in postmenopausal women. Icariin can increase biosynthesis of estrogen and activate the ER-mediated signaling pathways [36, 43]. Since estrogen and ERs are also involved in modulating immune responsiveness [65], it is reasonable to speculate that icariin could regulate the immune response, and exert its effect on bone formation via an ER-dependent way.
Immune responses activated through the LPS (lipopolysaccharide) pathway also result in osteoclastogenesis and bone loss. Icariin treatment was shown to prevent immune-related bone loss, reduce RANKL expression levels, and enhance OPG expression levels in a New Zealand rabbit model of antigen-induced arthritis [66]. Icariin can inhibit the proliferation of CD4+ T cells stimulated with mitogens, or specific antigen ovalbumin, and can suppress Th1 and Th17 cell differentiation, and inhibit cytokine production in mice [67, 68]. These studies indicate that icariin can potentially prevent inflammatory bone loss by inhibiting the immune response.
Clinical trials and extended applications of icariin
Novel therapeutic methods based on icariin are being developed
The use of icariin in the prevention and treatment of osteoporosis has been studied [69,70,71,72]. A 24-month randomized double-blind placebo-controlled clinical trial in healthy, late postmenopausal women, was conducted to explore the efficacy of icariin in preventing bone loss. Results show that, compared to the placebo group (n = 50), the intervention group (n = 50, a daily dose of 60 mg icariin, 15 mg daidzein, and 3 mg genistein) had a significantly reduced bone loss. Treatment with icariin maintained BMD at 12 months (femoral neck 1.1%, p = 0.285; lumbar spine 1.0%, p = 0.158) and 24 months (femoral neck 1.6%, p = 0.148; lumbar spine 1.3%, p = 0.091) [69]. A long-term (up to 12~24 months) administration of icariin products resulted in an improved BMD in the lumbar spine and femoral neck in a time-dependent manner. However, the effect of icariin in maintaining BMD was not impressive, as the improvement in BMD was less than half of that shown to occur in studies of similar duration, investigating estrogen replacement or treatment with bisphosphonates, and small compared to the effects of PTH (parathyroid hormone) treatment. However, there is no incidence of breast cancer and cardiovascular events in the postmenopausal women after icariin treatment for 2 years [69]. In contrast, long-term compliance with ERT (estrogen replacement therapy) was poor, due to its malignant effect in reproductive organs [70, 71] and potential risks for cardiovascular diseases [73]. A recent longitudinal follow-up study validates these controversial side effects and shows that ERT resulted in an increase in cancellous bone volume, together with an increase in endometrial thickness [74]. These findings position icariin as an attractive alternative therapy due to its low side effects. In another 24-month randomized double-blind and controlled clinical trial in osteoporosis patients (consisting of 36 males and 324 females, aged 50~70, treatment group n = 360, control group n = 120), Epimedium total flavone capsule showed higher efficacy in terms of symptom relief, measured by scores for back pain and leg pains (90.83 to 75.00%), and ratio of BMD enhancement (47.38 to 34.23%), compared to Gusongbao capsule (approved to prevent and treat osteoporosis by the State Food and Drug Administration, China, 2010) [72]. Adverse events reported in this study included rash, constipation, diarrhea, cardiopalmus, tinnitus, and gastrointestinal dysfunction, with an incidence rate of 6.67% compared to the control group of 5.00% [72]. Though icariin has been tested in animal models of bone loss [33, 51, 75,76,77], to date, no studies have been reported on icariin tested in a non-rodent model. FDA guidelines (1994) recommend potential therapeutic agents to be tested in at least two animal species, including one rodent species and a second, non-rodent model. In addition, further clinical trials on a larger scale and novel delivery systems are needed to explore the efficacy of icariin and its derivatives on bone formation and regeneration in humans, as well as any possible occurrences of side effects with long-term usage.
Thus, novel therapeutic methods utilizing icariin are being developed for bone regeneration and treatment of osteoporosis. An enzymatic hydrolysis method using β-glucosidase has been developed to produce icariside II, the main effective component in vivo after administration of icariin [78]. Further research focuses on the development of drug delivery systems for icariin. Multiple materials have been adopted as scaffolds to deliver icariin, including chitosan/nano-sized hydroxyapatite, porous PHBV (poly 3-hydroxybutyrate- co-3-hydroxyvalerate), gelatin/hyaluronic acid composite microspheres, and small intestine submucosa [14, 79,80,81]. The evidences from these studies are limited, and further exploration for the novel delivery systems of icariin should be addressed in the future.
Icariin improves the bioactivity and biocompatibility of bone implant materials
Periprosthetic osteolysis (PIO), which can lead to implant instability and failure, is a major orthopedic problem after total joint arthroplasty (TJA) [82, 83]. Icariin effectively induces bone formation and inhibits bone resorption in a murine macrophage cell line (RAW264.7) induced by titanium (Ti) particles [84]. In addition, icariin exhibits bone-protecting effect, increases bone mass, and decreases bone loss in titanium-particle-induced osteolytic sites in a C57BL/6 mouse calvarial model [85]. Icariin was shown to improve the biocompatibility of Ti substrates in another pilot study. Ti nanotubes were loaded with icariin and sealed with a chitosan/gelatin multilayer coating. The fabricated Ti nanotube adjusted the icariin release profile, modulated the biocompatibility of Ti substrates, and elicited bone formation efficacy in primary osteoblasts isolated from 3-day-old Sprague-Dawley (SD) rats [86]. These studies show that icariin has the potential of being developed as a complementary or substitutive therapy for revision surgery. Research also shows that an expanded application of icariin could enhance the bioactivity and biocompatibility of porous b-TCP (b-tricalcium phosphate) ceramic disks. Porous b-TCP ceramics have been widely utilized as bone substitution material in clinic, and a 3-month investigation showed that icariin loaded onto b-TCP ceramics induced new bone formation after intramuscular implantation of Wistar Albino rats [87].
Pharmacological effects and pharmacokinetic properties of icariin
The metabolites of icariin in human are icaritin and desmethylicaritin [88]; it is the metabolites themselves that bind to the ER and exert the bone-protective effect [13]. Sprague-Dawley rats (ovariectomized at 6 weeks) were administered with a standard extract of traditional Chinese medicinal plant Epimedium (300 mg/kg body weight), and icariin, icariside I, icariside II, icaritin, and desmethylicaritin were detected in their sera [89]. An additional metabolite, demethylicaritin, was detected in rats, showing that the metabolism of icariin is species-specific [89], and that a comprehensive metabolite profile needs to be further established in human. The pharmacokinetic profile showed that icariin and icariside II reached t max 0.5–1 h, icariside I, icaritin, and desmethylicaritin peaked at t max 8 h, and micromolar levels of icaritin (clinically irrelevant high dosage) were detectable 72 h after administration in rats [89]. After administration of an aqueous decoction of H. Epimediumii, icaritin but not demethylicaritin was detectable from 1 h, reaching a peak at 8 h (1.51 ± 1.6 nM) in sera of human volunteers [88]. These data are preliminary, and the clinical pharmacological effects and pharmacokinetic properties of icariin and its derivatives have not yet been fully elucidated, warranting further research.
The intestinal absorption mechanisms of icariin-related flavonoids were examined in the human intestinal Caco-2 cell model and the perfused rat intestinal model. Results show that flavonoids were absorbed by intrinsic permeation and transporter-mediated efflux [90]. The report also shows that heating processes help preserve the bioactivity of flavonoids [90].
Comparison of bone-protective efficacy between icariin and other flavonoids
Certain flavonoid compounds including epimedin and genistein have the potential to promote osteogenesis, and these bioactive constituents exert bone-protective effect in a similar mechanism to icariin. Epimedin is also isolated from H. Epimedii and promotes osteoblast proliferation and maturation in an ER-dependent manner [91, 92]. Genistein is a flavonoid rich in soy and exhibits a similar bone-protecting effect in an ER-dependent manner [93]. One report suggested that icariin is more potent than genistein in terms of osteogenic potential [94].
Conclusions and prospects
Osteoporosis is a bone disease where bone loss generates porous and weak bone structure, caused by the disruption of the balance of bone formation and resorption. Icariin is an osteoinductive flavonoid compound with a mimetic property of estrogen that induces bone formation and inhibits bone resorption. At the in vitro level, icariin stimulates the osteogenic differentiation of BMSCs and suppresses the adipogenic transdifferentiation of primary osteoblasts; at the same time, icariin inhibits bone loss by suppressing osteoclastogenic differentiation, immune response, and bone resorption activity. Concurrently, the efficacy of induction of osteogenesis and inhibition of bone resorption has been studied at the in vivo level. However, the limitations of animal models in evaluating the therapeutic efficacy and pharmacological properties of icariin should be taken into consideration. For example, due to the small size of rodents, their skeleton consists of a proportionally smaller portion of cancellous bone mass and a larger portion of cortical bone mass, compared to their human counterparts [95]. Meanwhile, species differences at the cellular and biochemical levels may also negatively influence the usefulness of animal models of osteoporosis. There are also characterized differences between human and mouse physiology regarding the actions of estrogens and estrogen analogs [96]. Animal studies use very high dosage of icariin, which make the results clinically irrelevant; therefore, information generated from skeletal studies of rodents should be approached with extreme caution. The anti-osteoporotic activity of icariin and its derivatives needs further verification using other mammalian models, primates, or human clinical data. Meanwhile, the continued investigation of the pharmacological effects and pharmacokinetic properties of icariin and its derivatives in humans, contributes to the development of icariin-related therapies.
Icariin is a potentially useful treatment for bone regeneration, in view of its osteogenic bioactivity. Pharmacological evidence based on animal models has been accumulating about the mechanisms by which icariin regulates osteoblastogenesis and osteoclastogenesis, but the efficacy of prevention and treatment of osteoporosis is lacking of strong evidence in clinical trials. Furthermore, icariin provides a plausible and intriguing prospect for treating autoimmune-induced bone loss, and further research on the molecular mechanisms of icariin regulating immunity may help expand its application in treating bone diseases.
Abbreviations
- AAPH:
-
2,2′-azobis(2-amidinopropane) dihydrochloride
- ALP:
-
alkaline phosphatase
- ABCB1:
-
ATP-binding cassette subfamily B member 1
- BMP:
-
bone morphogenetic protein
- BMSC:
-
bone marrow-derived mesenchymal stem cells
- Cebpb:
-
CCAAT/enhancer-binding protein β
- CTX:
-
carboxy-terminal collagen cross-links
- Col-1:
-
type I collagen
- ERs:
-
estrogen receptors
- ERK:
-
extracellular signal-regulated kinase
- GSK-3β:
-
glycogen synthase kinase-3
- Id-1:
-
inhibitor of DNA-binding 1
- JNK:
-
c-Jun N terminal kinase
- LPS:
-
lipopolysaccharide
- MMP:
-
mitochondrial membrane potential
- NO:
-
nitric oxide
- OC:
-
osteocalcin
- ONFH:
-
osteonecrosis of femoral head
- OPG:
-
osteoprotegerin
- ·O2−:
-
superoxide anion
- P-gp:
-
P-glycoprotein
- ROS:
-
reactive oxygen species
- Pparg:
-
peroxisome proliferator-activated receptor γ
- Runx2:
-
runt-related transcription factor 2
- RANKL:
-
receptor activator of nuclear factor kappa-B ligand
- TRAP:
-
tartrate-resistant acid phosphatase
References
Nih Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Alexander IM (2009) Pharmacotherapeutic management of osteoprosis and osteopenia. Nurse Pract 34:30–40
de Bakker CM, Tseng WJ, Li Y, Zhao H, Liu XS (2017) Clinical evaluation of bone strength and fracture risk. Curr Osteoporos Rep 15:32–42
Anonymous (2010) Management of osteoporosis in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause (New York, NY) 17:25–54 quiz 55–26
Bone H (2012) Future directions in osteoporosis therapeutics. Endocrinol Metab Clin N Am 41:655–661
Papapetrou PD (2009) Bisphosphonate-associated adverse events. Hormones 8:96–110
Chesnut CH, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, Leboff M, Maricic M, Miller P (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 109:267
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 32:426
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316:722
Jolette J, Attalla B, Varela A, Long GG, Mellal N, Trimm S, Smith SY, Ominsky MS, Hattersley G (2017) Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1-34). Regul Toxicol Pharmacol 86:356–365
Zhang X, Liu T, Huang Y, Wismeijer D, Liu Y (2014) Icariin: does it have an osteoinductive potential for bone tissue engineering? Phytother Res 28:498–509
Qin L, Zhang G, Shi Y, Lee K, Leung P (2005) Prevention and treatment of osteoporosis with traditional herbal medicine. In: Deng HW, Liu YZ, Guo CY, Chen D (eds) Current topics in osteoporosis. World Scientific Publisher, Singapore, pp 513–531
Ming LG, Chen KM, Xian CJ (2013) Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J Cell Physiol 228:513–521
Fan J, Bi L, Wu T, Cao L, Wang D, Nan K, Chen J, Jin D, Jiang S, Pei G (2012) A combined chitosan/nano-size hydroxyapatite system for the controlled release of icariin. J Mater Sci Mater Med 23:399–407
Zhang X, Xu M, Song L, Wei Y, Lin Y, Liu W, Heng BC, Peng H, Wang Y, Deng X (2013) Effects of compatibility of deproteinized antler cancellous bone with various bioactive factors on their osteogenic potential. Biomaterials 34:9103–9114
Zhao J, Ohba S, Komiyama Y, Shinkai M, Chung UI, Nagamune T (2010) Icariin: a potential osteoinductive compound for bone tissue engineering. Tissue Eng A 16:233
Chung BH, Kim JD, Kim CK, Kim JH, Won MH, Lee HS, Dong MS, Ha KS, Kwon YG, Kim YM (2008) Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem Biophys Res Commun 376:404–408
Seeman E (2009) Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19:219
Myneni VD, Mezey E (2016) Regulation of bone remodeling by vitamin K2. Oral Dis 23:1021–1028
Sims NA, Gooi JH (2008) Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol 19:444–451
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498
Bar-Shavit Z (2007) The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem 102:1130–1139
Matsuo K, Irie N (2008) Osteoclast-osteoblast communication. Arch Biochem Biophys 473:201
Fennen M, Pap T, Dankbar B (2016) Smad-dependent mechanisms of inflammatory bone destruction. Arthritis Res Ther 18:279
Ginaldi L, De MM (2016) Osteoimmunology and beyond. Curr Med Chem 23:3754–3774
Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S (1998) Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol 54:105–112
Manolagas SC, O’Brien CA, Almeida M (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9:699–712
Khalid AB, Krum SA (2016) Estrogen receptors alpha and beta in bone. Bone 87:130–135
Xu F, Mcdonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol 6:121
Abdallah BM, Al-Shammary A, Khattab HM, Aldahmash A, Kassem M (2016) Bone marrow stromal stem cells for bone repair: basic and translational aspects. Recent Advances in Stem Cells. Springer International Publishing, pp 213-232
Zhao J, Ohba S, Shinkai M, Chung UI, Nagamune T (2008) Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun 369:444–448
Fan JJ, Cao LG, Wu T, Wang DX, Jin D, Jiang S, Zhang ZY, Bi L, Pei GX (2011) The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules 16:10123–10133
Nian H, Ma MH, Nian SS, Xu LL (2009) Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine 16:320
Jee WS, Yao W (2001) Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1:193
Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN, Zhou J, Ma HP, Xian CJ, Chen KM (2014) Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K–AKT–eNOS–NO–cGMP–PKG. Bone 66:189–198
Song L, Zhao J, Zhang X, Li H, Zhou Y (2013) Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur J Pharmacol 714:15
Fu S, Yang L, Hong H, Zhang R (2016) Wnt/β-catenin signaling is involved in the icariin induced proliferation of bone marrow mesenchymal stem cells. J Tradit Chin Med 36:360–368
Wei Q, Zhang J, Hong G, Chen Z, Deng W, He W, Chen MH (2016) Icariin promotes osteogenic differentiation of rat bone marrow stromal cells by activating the ERα-Wnt/β-catenin signaling pathway. Biomed Pharmacother 84:931–939
Liang W, Lin M, Li X, Li C, Gao B, Gan H, Yang Z, Lin X, Liao L, Yang M (2012) Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med 30:889–895
Cao H, Ke Y, Zhang Y, Zhang CJ, Qian W, Zhang GL (2012) Icariin stimulates MC3T3-E1 cell proliferation and differentiation through up-regulation of bone morphogenetic protein-2. Int J Mol Med 29:435
Zhang ZB, Yang QT (2006) The testosterone mimetic properties of icariin. Asian J Androl 8:601
Liu J, Ye HY (2005) Determination of rat urinary metabolites of icariin in vivo and estrogenic activities of its metabolites on MCF-7 cells. Pharmazie 60:120–125
Yang L, Lu D, Guo J, Meng X, Zhang G, Wang F (2013) Icariin from Epimedium brevicornum Maxim promotes the biosynthesis of estrogen by aromatase (CYP19). J Ethnopharmacol 145:715–721
Sun Z, Yang S, Ye S, Zhang Y, Xu W, Zhang B, Liu X, Mo F, Hua W (2013) Aberrant CpG islands’ hypermethylation of ABCB1 in mesenchymal stem cells of patients with steroid-associated osteonecrosis. J Rheumatol 40:1913–1920
Sun ZB, Wang JW, Xiao H, Zhang QS, Kan WS, Mo FB, Hu S, Ye SN (2015) Icariin may benefit the mesenchymal stem cells of patients with steroid-associated osteonecrosis by ABCB1-promoter demethylation: a preliminary study. Osteoporos Int 26:187
Zhao F, Tang YZ, Liu ZQ (2007) Protective effect of icariin on DNA against radical-induced oxidative damage. J Pharm Pharmacol 59:1729–1732
Chen KM, Ma HP, Ge BF, Liu XY, Ma LP, Bai MH, Wang Y (2007) Icariin enhances the osteogenic differentiation of bone marrow stromal cells but has no effects on the differentiation of newborn calvarial osteoblasts of rats. Pharmazie 62:785–789
Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME (2006) Playing with bone and fat. J Cell Biochem 98:251
Zhang D, Fong C, Jia Z, Liao C, Yao X, Yang M (2016) Icariin stimulates differentiation and suppresses adipocytic transdifferentiation of primary osteoblasts through estrogen receptor-mediated pathway. Calcif Tissue Int 99:1–12
Zhang J, Li Y, Sun J, Liu C, Zhang D (2011) Synergistic or antagonistic effect of MTE plus TF or icariin from Epimedium koreanum on the proliferation and differentiation of primary osteoblasts in vitro. Biol Trace Elem Res 143:1746–1757
Feng R, Feng L, Yuan Z, Wang D, Wang F, Tan B, Han S, Li T, Li D, Han Y (2013) Icariin protects against glucocorticoid-induced osteoporosis in vitro and prevents glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem Biophys 67:189–197
Ma XN, Zhou J, Ge BF, Zhen P, Ma HP, Shi WG, Cheng K, Xian CJ, Chen KM (2013) Icariin induces osteoblast differentiation and mineralization without dexamethasone in vitro. Planta Med 79:1501–1508
Ma HP, Ma XN, Ge BF, Zhen P, Zhou J, Gao YH, Xian CJ, Chen KM (2014) Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Prolif 47:527
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309
Hofbauer LC, Heufelder AE (2001) Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med 79:243
Zheng D, Peng S, Yang S-H, Shao Z-W, Yang C, Feng Y, Wu W, Zhen W-X (2012) The beneficial effect of icariin on bone is diminished in osteoprotegerin-deficient mice. Bone 51:85–92
Li XF, Xu H, Zhao YJ, Tang DZ, Xu GH, Holz J, Wang J, Cheng SD, Shi Q, Wang YJ (2013) Icariin augments bone formation and reverses the phenotypes of osteoprotegerin-deficient mice through the activation of Wnt/ β -catenin-BMP signaling. Evid Based Complement Alternat Med 2013(2013–11-4):652317
Hsieh TP, Sheu SY, Sun JS, Chen MH (2011) Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis. Phytomedicine 18:176
Chen KM, Ge BF, Liu XY, Ma PH, MB L, Bai MH, Wang Y (2007) Icariin inhibits the osteoclast formation induced by RANKL and macrophage-colony stimulating factor in mouse bone marrow culture. Die Pharmazie 62:388–391
Huang J, Yuan L, Wang X, Zhang TL, Wang K (2007) Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci 81:832
Zhang D, Zhang J, Fong C, Yao X, Yang M (2012) Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie 94:2514–2522
Huang J, Zhang JC, Zhang TL, Wang K (2007) Icariin suppresses bone resorption activity of rabbit osteoclasts in vitro. Chin Sci Bull 52:890–895
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, Mccabe S (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304
Arron JR, Choi Y (2000) Osteoimmunology: bone versus immune system. Nature 408:535–536
Carlsten H (2005) Immune responses and bone loss: the estrogen connection. Immunol Rev 208:194–206
Chen CW, Dai QP, Fan TY, Chen YQ, Che T (2016) Icariin prevents cartilage and bone degradation in experimental models of arthritis. Mediat Inflamm 2016:1–10
Li X, Hu Y, He L, Wang S, Zhou H, Liu S (2012) Icaritin inhibits T cell activation and prolongs skin allograft survival in mice. Int Immunopharmacol 13:1
Shen R, Deng W, Li C, Zeng G (2015) A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int Immunopharmacol 24:224
Zhang G, Qin L, Shi Y (2007) Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J Bone Miner Res 22:1072
Castelo-Branco C, Figueras F, Sanjuan A, Vicente JJ, Mj MDO, Pons F, Balasch J, Vanrell JA (2000) Long-term compliance with estrogen replacement therapy in surgical postmenopausal women: benefits to bone and analysis of factors associated with discontinuation. Menopause 6:307–311
Pilon D, Castilloux AM, Lelorier J (2001) Estrogen replacement therapy: determinants of persistence with treatment 1. Obstet Gynecol 97:97–100
Min LU, Wang L, Luo Y (2013) Treatment of primary osteoporosis with epimedium total flavone capsule: a multicenter clinical observation on 360 cases. Chin J Osteoporos 19:279–274
Valdiviezo C, Lawson S, Ouyang P (2013) An update on menopausal hormone replacement therapy in women and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 20:148–155
Khastgir G, Studd J, Holland N, Alaghband-Zadeh J, Fox S, Chow J (2001) Anabolic effect of estrogen replacement on bone in postmenopausal women with osteoporosis: histomorphometric evidence in a longitudinal study. J Clin Endocrinol Metab 86:289–295
Wei H, Zili L, Yuanlu C, Biao Y, Cheng L, Xiaoxia W, Yang L, Xing W (2011) Effect of icariin on bone formation during distraction osteogenesis in the rabbit mandible. Int J Oral Maxillofac Implants 40:413
Zhang G, Qin L, Hung WY, Shi YY, Leung PC, Yeung HY, Leung KS (2006) Flavonoids derived from herbal Epimedium brevicornum Maxim prevent OVX-induced osteoporosis in rats independent of its enhancement in intestinal calcium absorption. Bone 38:818
Chen KM, Ge BF, Ma HP, Zheng RL (2004) The serum of rats administered flavonoid extract from Epimedium sagittatum but not the extract itself enhances the development of rat calvarial osteoblast-like cells in vitro. Pharmazie 59:61–64
Xia Q, Xu D, Huang Z, Liu J, Wang X, Wang X, Liu S (2010) Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 81:437–442
Xia L, Li Y, Zhou Z, Dai Y, Liu H, Liu H (2013) Icariin delivery porous PHBV scaffolds for promoting osteoblast expansion in vitro. Mater Sci Eng C 33:3545–3552
Li M, Gu Q, Chen M, Zhang C, Chen S, Zhao J (2017) Controlled delivery of icariin on small intestine submucosa for bone tissue engineering. Mater Sci Eng C 71:260
Yan H, Zhou Z, Huang T, Peng C, Liu Q, Zhou H, Zeng W, Liu L, Ou B, He S (2016) Controlled release in vitro of icariin from gelatin/hyaluronic acid composite microspheres. Polym Bull 73:1–12
Hallab NJ (2016) Biologic responses to orthopedic implants: innate and adaptive immune responses to implant debris. Spine 41(Suppl 7):S30
Urban RM, Hall DJ, Della VC, Wimmer MA, Jacobs JJ, Galante JO (2012) Successful long-term fixation and progression of osteolysis associated with first-generation cementless acetabular components retrieved post mortem. J Bone Joint Surg (Am Vol) 94:1877
Cui J, Zhu M, Zhu S, Wang G, Xu Y, Geng D (2014) Inhibitory effect of icariin on Ti-induced inflammatory osteoclastogenesis. J Surg Res 192:447
Wang J, Tao Y, Ping Z, Zhang W, Hu X, Wang Y, Wang L, Shi J, Wu X, Yang H (2016) Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep 6:23827
Zhang Y, Chen L, Liu C, Feng X, Wei L, Shao L (2016) Self-assembly chitosan/gelatin composite coating on icariin-modified TiO 2 nanotubes for the regulation of osteoblast bioactivity. Mater Des 92:471–479
Zhang X, Guo Y, Li DX, Wang R, Fan HS, Xiao YM, Zhang L, Zhang XD (2011) The effect of loading icariin on biocompatibility and bioactivity of porous β-TCP ceramic. J Mater Sci Mater Med 22:371–379
Shen P, Wong SP, Yong EL (2007) Sensitive and rapid method to quantify icaritin and desmethylicaritin in human serum using gas chromatography–mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 857:47–52
Wong SP, Shen P, Lee L, Li J, Yong EL (2009) Pharmacokinetics of prenylflavonoids and correlations with the dynamics of estrogen action in sera following ingestion of a standardized Epimedium extract. J Pharm Biomed Anal 50:216
Chen Y, Zhao YH, Jia XB, Hu M (2008) Intestinal absorption mechanisms of prenylated flavonoids present in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo). Pharm Res 25:2190
Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM (2005) Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomed Int J Phycol Phycochem 12:189–193
Xiao HH, Fung CY, Mok SK, Wong KC, Ho MX, Wang XL, Yao XS, Wong MS (2014) Flavonoids from Herba epimedii selectively activate estrogen receptor alpha (ERα) and stimulate ER-dependent osteoblastic functions in UMR-106 cells. J Steroid Biochem Mol Biol 143:141
Qi S, Zheng H (2017) Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res 177:281–287
Ma HP, Ming LG, Ge BF, Zhai YK, Song P, Xian CJ, Chen KM (2015) Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J Cell Biochem 112:916–923
Turner RT, Maran A, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD (2001) Animal models for osteoporosis. Rev Endocr Metab Disord 2:117
Turner RT (1999) Mice, estrogen, and postmenopausal osteoporosis. J Bone Miner Res 14:187–191
Funding
This work was supported by the Natural Science Foundation of China (81371989), Guangdong Science and Technology Department Project (2015A030313776, 2016A050503008), and the Shenzhen Municipal Science and Technology Innovation Committee Project (JSGG20150331154931068, JCYJ20160301151248779, JCYJ20160229172757249, CXZZ20151015151249563, and CXZZ20150401152251209).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, D., Yang, D. et al. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int 29, 535–544 (2018). https://doi.org/10.1007/s00198-017-4255-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4255-1