Abstract
Summary
Adherence to anti-osteoporosis medications is poor. We carried out a cohort study using a real-world population database to estimate the persistence of anti-osteoporosis drugs. Unadjusted 2-year persistence ranged from 10.3 to 45.4%. Denosumab users had a 40% lower risk of discontinuation at 2 years compared to alendronate users.
Purpose
The purpose of this study was to estimate real-world persistence amongst incident users of anti-osteoporosis medications.
Methods
This is a retrospective cohort using data from anonymised records and dispensation data (www.sidiap.org). Eligibility comprised the following: women aged ≥50, incident users of anti-osteoporosis medication (2012), with data available for at least 12 months prior to therapy initiation. Exclusions are other bone diseases/treatments and uncommon anti-osteoporosis drugs (N < 100). Follow-up was from first pharmacy dispensation until cessation, end of study, censoring or switching. Outcomes are 2- and 1-year persistence with a permissible gap of up to 90 days. Persistence with alendronate was compared to other bisphosphonates, strontium ranelate, selective oestrogen receptor modulators, teriparatide and denosumab. Cox models were used to estimate hazard ratios of therapy cessation according to drug used after adjustment for age, sex, BMI, smoking, alcohol drinking, Charlson co-morbidity index, previous fractures, use of anti-osteoporosis medication/s, oral corticosteroids and socio-economic status.
Results
A total of 19,253 women were included. Unadjusted 2-year persistence [95% CI] ranged from 10.3% [9.1–11.6%] (strontium ranelate) to 45.4% [43.1–47.8%] (denosumab). One-year persistence went from 35.8% [33.9%–37.7%] (strontium ranelate) to 65.8% [63.6%–68.0%] (denosumab). At the end of the first year and compared to alendronate users, both teriparatide and denosumab users had reduced cessation risk (adjusted HR 0.76, 95% CI 0.67–0.86 and 0.54, 95% CI 0.50–0.59 respectively) while at the end of the second year, only denosumab had a lower risk of discontinuation (adjusted HR 0.60, 95% CI 0.56–0.64).
Conclusions
Unadjusted 2-year persistence is suboptimal. However, both teriparatide and denosumab users had better 1-year persistence and only denosumab had 2-year better persistence compared to alendronate users. Unmeasured confounding by indication might partially explain our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regardless of the medical condition studied, adherence to the medications recommended is essential for successful disease control. Persistence is an important matter particularly in osteoporosis given that higher persistence rates have been associated with further reductions in hip and overall fracture risk [1, 2].
Despite this evidence, poor medication adherence is still considered a major issue. Low persistence rates have been extensively reported by both prospective and retrospective studies [3,4,5,6,7]; nearly 50% of patients do not follow their prescribed treatment regimen with early discontinuation of most of the therapies ranging from 50 to 78% [8,9,10].
Persistence is not only low but also seriously decreases from the first to the second year [11] and up to the fifth year of treatment [3].
In the last few years, a newly commercialised anti-osteoporosis drug (denosumab), which was not included in the aforementioned studies, has proved to have higher persistence rates compared to other anti-osteoporosis therapies (73–84%) [12,13,14,15] compared to other intravenous (i.v.) (27%) and oral bisphosphonates (28%) [7, 16].
Therefore, the aim of this study is to estimate the 1- and 2-year persistence and risk of discontinuation with each of the pharmacological treatments available in primary care (including denosumab) for post-menopausal osteoporosis in Catalonia (Spain) amongst incident users of such drugs between January and December 2012, using real-world data.
Methods
Study design and setting
We conducted a population-based cohort study using routinely collected data from de-identified primary care electronic medical records in the Sistema d‘Informació per al Desenvolupament de l‘Investigació en Atenció Primària (SIDIAP) database (http://www.sidiap.org).
Data source/measurements
The SIDIAP database contains electronic medical records from general practitioners and primary care nurses in 274 primary care practices for 5.8 million people in Catalonia (80% of the total population), and comprises extensive information on drug utilization (dispensation date/s, Anatomical Therapeutic Chemical (ATC) code/s and number of daily defined doses (DDDs) purchased) through linkage to the regional community pharmacy invoice data and clinical diagnosis using ICD-10 codes [17].
Participants
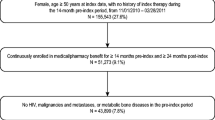
Eligible subjects were women of at least 50 years old initiating and purchasing at least 56 daily defined doses (DDDs) of any the following drugs: oral bisphosphonates (ATC M05BA subcodes), strontium ranelate (ATC M05BX03), selective oestrogen receptor modulators = SERMs (ATC G03XC), teriparatide (ATC H05AA02) or denosumab (ATC M05BX04) between 1 January and 31 December 2012 and for whom there was available data for at least 12 months prior to that first dispensation (i.e. index date).
The following exclusion criteria were applied: (1) use of the same drug during the 12 months prior to the index date; (2) history or concomitant incidence of malignant disease (ICD-10 codes: C00-C97 or D00-D49) or use of anti-neoplastics (ATC classes: L19), aromatase inhibitors (ATC L02BG) or cytostatic hormones (ATC classes L2) 12 months prior to the index date; (3) history or concomitant incidence of Morbus Paget (ICD-10 codes: M88) or HIV infection (ICD-10 codes: B20-B24) 12 months prior to index date or (5) drugs with less than 100 users eligible in the study which were also excluded for statistical power issues.
Follow-up
Each patient was studied from at least 12 months prior to the index date (run-in/wash-out period) until end of follow-up, which is defined as the earliest of death or end of data availability, switching or treatment cessation.
Study outcomes
The primary outcome of interest in this study was 2-year persistence with each of the study drugs, with 1-year persistence being a secondary outcome. For those drugs that have two or more different dosing frequencies available (e.g. daily, weekly and monthly for risedronate), persistence was estimated separately for each dosing frequency. Patients were assumed to be persistent with any of the analysed drugs, when a new pack of the analysed drug was dispensed within 90 days (maximum permissible gap) after the end of days’ supply from the previous prescription. In this analysis, a different number of days per pack dispensed were used according to the national medicine catalogue and the estimated number of daily defined doses (DDDs) extrapolated from the World Health Organization ATC/DDD catalogue: 28 days per pack dispensed were considered for daily and weekly BP, strontium ranelate, selective oestrogen receptor modulators and teriparatide; 30 days per pack dispensed for monthly BPs; and 182 days per pack dispensed for denosumab.
A sensitivity analysis was carried out extending the permissible gap to 180 days in order to capture any variations in drug persistence.
Both 1- and 2-year persistence with each of the studied drugs were compared to that amongst users of the recommended first-line therapy (weekly alendronate), defined as the reference group.
Statistical methods
Survival analyses were performed where the study outcome was time from index date (treatment initiation) to 2-year (primary) or 1-year (secondary outcome) cessation or censoring for each drug user group. In this survival analysis, failure (the event) was defined as non-persistence with each drug of interest, irrespective of whether the patient terminated treatment completely or restarted treatment after the permissible gap. For this analysis, censoring was considered at end of follow-up, death or date of treatment switching (to an alternative anti-osteoporosis medication). The survival probability (i.e. persistence estimate) at 1 and 2 years was presented with 95% confidence bands.
Kaplan-Meier estimates of cumulative incidence of therapy discontinuation stratified by osteoporosis drug used were plotted. In order to account for potential confounders, multivariable Cox regression models were fitted adjusting for a list of pre-specified confounders as assessed at index date: age, gender, body mass index, smoking, alcohol drinking, Charlson co-morbidity index, previous number of fractures (0, 1, 2 or ≥3), previous use of anti-osteoporosis medication/s, socio-economic status (measured using the ecologic MEDEA socio-economic scale [18]) and use of oral corticosteroids. Hazard ratios (HR) and 95% confidence intervals for risk of discontinuation according to drug use were estimated, using weekly alendronate (the most commonly dispensed anti-osteoporosis medication in our setting) as the reference group.
Multiple imputations with chained equations (MICE) were used to impute variables with missing values (body mass index, smoking, alcohol drinking and socio-economic status) in the multivariable regression models. All the statistical analyses were carried out using STATA SE for Mac V.12.0.
Sample size/power
For the primary study outcome—accepting an alpha risk of 0.05 and in a two-sided log-rank test, and assuming 50% persistence at 2 years for alendronate users (reference group)—754 subjects in the alternative/comparison (other than alendronate) group and 3762 alendronate users are required to ensure 95% power to detect as significant a HR ≤80%. A 10% dropout rate was anticipated for this estimation.
Results
Participants and baseline characteristics
A total of 19,253 patients were identified as incident users of any of the studied drugs between 1st January and 31st December 2012. Baseline characteristics of these patients are reported in Table 1. Overall, most women included were under 75 years old, had a previous diagnosis of osteoporosis (57%) and were using bone loss medication (71%) and calcium/vitamin D supplements (55%).
Two-year persistence with anti-osteoporosis medications (primary outcome)
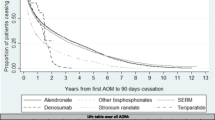
Kaplan-Meier (unadjusted) analyses confirmed that denosumab users had the highest persistence at the end of the second year of treatment (45.44% [95% CI 43.12 to 47.77]), followed by alendronate (28.88% [95% CI 28.03 to 29.74]) users (Table 2 and Fig. 1). Incidence rates (IR) of therapy discontinuation in the first 2 years after therapy initiation ranged from 39.12/100 person-years [95% CI 36.75 to 41.65] amongst denosumab users to 106.8/100 py [95% CI 102.44 to 111.40] amongst users of strontium ranelate (see Table 3 for full details and all study drug/s).
Multivariable (Cox) models confirmed that compared to alendronate users—reference group—2-year cessation risk was significantly lower for denosumab users, even after adjustment for pre-identified confounders: adjusted HR 0.60 [95% CI 0.56 to 0.64]. Conversely, ibandronate, risedronate and strontium ranelate users had an increased risk of cessation compared to alendronate users: adjusted HR of 1.27 [95% CI 1.19 to 1.37], of 1.46 [95% CI 1.36 to 1.57] and of 1.61 [95% CI 1.53 to 1.68] respectively. No significant differences in 2-year risk of cessation were found for raloxifene, bazedoxifene and teriparatide users compared to those using weekly alendronate (see Fig. 2).
One-year persistence with anti-osteoporosis medication
When analysing the estimates of persistence by drug (Table 2), denosumab, teriparatide and bazedoxifene users reported the highest persistence (65.8%, 95% CI 63.55 to 67.99; 57.54%, 95% CI 53.51 to 61.5; and 48.47%, 95% CI 45.18 to 51.76 respectively). In contrast, risedronate and strontium ranelate users had the lowest persistence (33.4%, 95% CI 30.45 to 36.45 and 35.8%, 95% CI 33.89 to 37.74 respectively).
Incidence rates (IR) of therapy discontinuation were higher amongst users of risedronate and strontium ranelate. Conversely, those taking denosumab had the lowest IR of therapy discontinuation compared with the rest of anti-osteoporosis medications (Table 3).
Figure 3 reports the estimated adjusted risk of discontinuation at the end of the first year compared with weekly alendronate. After adjusting for potential confounders, women who were prescribed denosumab reported the lowest risk of cessation compared to weekly alendronate users (adjusted HR 0.54 [95% CI of 0.50 to 0.59]), followed by those using teriparatide (adjusted HR 0.76 [95% CI 0.67 to 0.86]. Conversely, users of ibandronate, risendronate and strontium ranelate had an increased risk of 1-year therapy cessation.
Sensitivity analysis: 180-day gap permitted
A sensitivity analysis was carried out to determine the persistence at the end of the second year with a permissible gap between prescriptions of 180 days. No major differences were found when analysing the drug persistence compared with the previous 90-day permissible gap (data not shown).
Discussion
Key results
In a population-based study using a source population that covers over five million subjects, a total of 19,267 women were identified as incident users of anti-osteoporosis medication between January and December 2012.
Women using denosumab reported the highest 1- and 2-year persistence rates (and the lowest incidence rates of therapy discontinuation): about two thirds and almost half of the women who started denosumab in 2012 were persistent at the end of the first and second years after therapy initiation. This remained significant in multivariable adjusted models: a 46 and 40% significantly lower risk of 1- and 2-year cessation was observed amongst denosumab users when compared to users of weekly alendronate use respectively. Teriparatide users had also a significantly improved 1-year persistence, but 2-year persistence was similar to that amongst users of weekly alendronate.
In contrast, a significantly higher risk of discontinuation was reported for ibandronate, risedronate and strontium ranelate users at both 1 and 2 years when compared to alendronate users. Regarding the risk of cessation, no differences were found between alendronate and any of raloxifene and bazedoxifene users at 1 and 2 years or for teriparatide users at 2 years; however, persistence with these medications at the end of the second year was lower than those reported for alendronate users.
Comparison with other studies
In our population, overall persistence with anti-osteoporosis therapies was found to be very low; only 47 and 26% persisted with the prescribed treatment at 1 and 2 years respectively.
Drug persistence is an important issue thoroughly analysed in the last years. Low persistence rates with these medications has been previously reported [3,4,5,6,7, 19, 20] in prospective and retrospective studies across the USA and Europe; however, methodological differences regarding the refill gap allowed or the list of drugs included make the cross-study comparison difficult.
As in our study, two other reports analysed the persistence rates allowing the 90-day refill gap between prescriptions [3, 7]. The first study carried out in the Netherlands [3], analysed a total of 8610 subjects of the IADB database and found an overall persistence rate of 67.2%. Differences regarding the definition of the event, such as not counting those subjects who discontinued their treatment for a valid reason, could explain the higher persistence rates found in their study. Although this approach would create a more realistic picture of persistence, the lack of information on drug indication in their databases may have introduced a selection bias, hindering the interpretation of their results assuming that only those subjects who were truly non-persistent would be included. On the other hand, the Grand Study [7], which analysed 4147 women in Germany from the (IMS® DA) database, reported persistence rates of approximately 50% at the end of the first year and of 22% at the end of the second year, which is more in accordance to what we found.
None of the aforementioned studies included denosumab [3, 4, 6, 7, 19, 20] and only one included teriparatide [5]. Reasons for this were either the studies were published before the commercialization of the drug [6, 19, 20] or they included only oral BP [6, 7].
High persistence rates at 1 year have been previously reported for teriparatide [5, 21, 22], which is in line with our results; those subjects using teriparatide reported the second highest persistence and the second lowest risk of discontinuation after denosumab compared to weekly alendronate. The recommended treatment duration for teriparatide goes from 18 to 24 months, which could be the reason underlying the observed high risk of cessation in the second year for this drug.
The persistence of denosumab has been demonstrated in the Denosumab Adherence Preference Satisfaction (DAPS) study. The DAPS study showed that patients treated with denosumab had a significantly high rate of persistence compared with weekly alendronate at 1 year (the adjusted rate ratio of 0.50, representing a 50% relative risk reduction of cessation with denosumab) [23]. In Germany, persistence with denosumab was evaluated using a retrospective analysis on 2386 post-menopausal women; 73% were persistent at 12 months and 58% at 24 months using permissible gap of 60 days [12]. In addition, the real-world persistence to denosumab has been demonstrated in other studies in Hungary and in Sweden, reporting 12-month persistence rates of 84% [13] and 83% [14]. Moreover, prospective observational studies carried out in Austria, Germany and Greece showed that the overall 12-month persistence to denosumab is more than 90% [15]. Our results confirm the higher persistence rates amongst denosumab users and expand what has been previously published comparing denosumab with the rest of anti-osteoporosis medications currently available in primary care: Denosumab users reported not only higher persistence rates but also the lowest risk of cessation at the end of the first and second years, compared to weekly alendronate.
Both teriparatide and denosumab are not considered first-line therapies in the treatment of osteoporosis and are often used to treat subjects in more severe cases. Disease severity, such as already existing osteoporotic fractures, may affect adherence: in a meta-analysis carried out in 2007, the perceived disease severity was strongly associated with a better adherence [24]. Furthermore, higher persistence of anti-osteoporosis medications has been reported (75% at the end of the first year) in patients with fractures [25] compared to persistence rates found in the general population [3,4,5,6,7, 19, 20].
At last, strontium ranelate users reported low persistence rates and an increased cessation which could be partly explained by the regulatory warnings on strontium ranelate safety issued by the European Medicine Agency in 2014, which led to a restriction in use of this anti-osteoporosis drug.
Limitations
The present study is largely descriptive in nature, and as such, it did not allow assessment of causality or drug effects, but to observe and describe associations between drug used and the proposed outcomes (1- and 2-year persistence). Therefore, confounding by indication (i.e. higher disease severity in users of denosumab/teriparatide compared with users of alendronic acid) was likely for the analyses of comparative persistence, despite attempts to adjust for observed potentially unbalanced variables/baseline characteristics. The lack of information on key markers of disease severity like bone mineral density (which was missing for >99% of the included population) and the fact that drugs with less convenient posology (daily injections of teriparatide) have (in some of our analyses) better persistence than weekly oral alendronate indicate that unresolved confounding might partially explain our findings.
Other limitations also need to be considered: first, treatment dates were based on date of filling the prescription at the pharmacy, which is not necessarily the date of treatment administration. However, misclassification is expected to be at random, which should bias our study results (estimates of comparative persistence) to the null. Secondly, we were not able to differentiate in the analysis between the physician’s and the patient’s decision to abandon or switch treatment, as both would lead to treatment discontinuation as defined in the proposed study. Furthermore, four covariates had missing information: body mass index, socio-economic status, smoking, and alcohol drinking, and out of these the greatest percentage was for the body mass index and alcohol consumption information. Multiple imputation was used to minimize the effect of missingness in the resulting models. Given that this study is based on primary care data, we were unable to assess the persistence with zoledronic acid and cannot draw any conclusion comparing it to any of the other medications analysed. Finally, we were unable to do further sensitive analysis with shorter treatment gaps (30 or 60 days) given that, in Spain, repeated prescriptions are collected from GP practices every 2 months and hence refill gaps of 2 months or less would not identify therapy cessation.
Generalizability
The study had broad inclusion criteria, which allowed for the inclusion of virtually all post-menopausal women (aged 50 or older at index date) in the target population treated with each of the analysed drugs. The results should therefore be generalizable to the entire population treated with the analysed drugs for osteoporosis in Catalonia (Spain). Generalizability to other jurisdictions is uncertain.
Conclusion
Anti-osteoporosis drugs are commonly used in Catalonia, with a total of 19,267 identified as incident drug users in the year 2012.
Persistence at 1 and 2 years with anti-osteoporosis drugs is overall suboptimal. Despite also having low persistence rates, users of denosumab and teriparatide had a better persistence at the end of the first year compared to users of alendronate (first-line treatment). However, at the end of the second year, only denosumab users had a better persistence compared to users of alendronate.
Finally, bazedoxifene and raloxifene users had equivalent compliance to that observed amongst alendronate users, while users of strontium ranelate, ibandronate and risedronate were the three groups with poorest 1- and 2-year persistence. Unmeasured confounding due to more severe disease (i.e. lower bone mineral density or fragility fractures) amongst users of denosumab and teriparatide might partially explain our findings.
References
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Gallagher AM, Rietbrock S, Olson M, van Staa TP (2008) Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res 23:1569–1575
van Boven JF, de Boer PT, Postma MJ, Vegter S (2013) Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 31:562–570
Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD (2012) Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 166:735–741
Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P (2012) Persistence and compliance of medications used in the treatment of osteoporosis—analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther 50:315–322
Netelenbos JC, Geusens PP, Ypma G, Buijs SJ (2011) Adherence and profile of non-persistence in patients treated for osteoporosis—a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 22:1537–1546
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T (2012) GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int 23:223–231
Compston JE, Seeman E (2006) Compliance with osteoporosis therapy is the weakest link. Lancet 368:973–974
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
Carbonell-Abella C, Pages-Castella A, Nogués X, Javiad MK, Arden NK, Cooper C, Diez-Perez A, Prieto-Alhambra D (2013) Persistence with different antiosteoporosis medications: A population-based cohort study. Osteoporos Int 24(Supp 1):S87–S384
Sicras Mainar A, Navarro Artieda R, Gutiérrez Nicuesa L, Sorio Vilela F, Intorcia M (2011) Continuance of treatment for osteoporosis in postmenopausal patients in the primary care setting. Farm Aten Primaria 9:46–53
Hadji P, Intorcia M, Thellmann K, Steinle T, Eisen C, Schimd T (2013) GRAND 3: the German retrospective cohort analysis on non-adherence in osteoporotic patients 3: persistence analysis of female patients treated with denosumab. Osteoporos Int 24(Suppl 1):S87–S384
Lakatos P, Tóth E, Cina Z, Lang Z, Psachoulia E, Intorcia M (2013) Persistence & compliance to treatment for osteoporosis in postmenopausal women in Hungary: a retrospective cohort study. Value Health 16:A567–A568
Karlsson L, Lundkvist J, Intorcia M, Psachoulia E, Ström O (2013) Treatment persistence in Swedish women initiating denosumab for treatment of postmenopausal osteoporosis. Value Health 16:A567
Hadji P, Papaioannou NA, Gielen E, Tepie MF, Zhang E, Kalouche-Khalid L, Fahrleitner-Pammer A (2014) 12-month persistence with Denosumab (Dmab) in women with postmenopausal osteoporosis (pmo): interim results of a 24-month prospective observational study in Germany, Austria, Greece and Belgium. Osteoporos Int 25(Suppl 2):S159–S440
Hadji P, Claus V, Kostev K, Intorcia M, Steinle T (2010) GRAND—the German retrospective cohort analysis on non-adherence in osteoporosis: analysis of persistence with intravenous bisphosphonates in women. Osteoporos Int 21(Suppl 1):S25–S388
Garcia-Gil Mdel M, Hermosilla E, Prieto-Alhambra D, Fina F, Rosell M, Ramos R, Rodriguez J, Williams T, Van Staa T, Bolibar B (2011) Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform Prim Care 19:135–145
Borrell C, Marí-Dell’olmo M, Serral G, Martínez-Beneito M, Gotsens M, Members MEDEA (2010) Inequalities in mortality in small areas of eleven Spanish cities (the multicenter MEDEA project). Health Place 16:703–711
Yeaw J, Benner JS, Walt JG, Sian S, Smith DB (2009) Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15:728–740
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242
Arden NK, Earl S, Fisher DJ, Cooper C, Carruthers S, Goater M (2006) Persistence with teriparatide in patients with osteoporosis: the UK experience. Osteoporos Int 17:1626–1629
Ziller V, Zimmermann SP, Kalder M, Ziller M, Seker-Pektas B, Hellmeyer L, Hadji P (2010) Adherence and persistence in patients with severe osteoporosis treated with teriparatide. Curr Med Res Opin 26:675–681
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL, Investigators DAPS (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
DiMatteo MR, Haskard KB, Williams SL (2007) Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care 45:521–528
Klop C, Welsing PM, Elders PJ, Overbeek JA, Souverein PC, Burden AM, van Onzenoort HA, Leufkens HG, Bijlsma JW, de Vries F (2015) Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int 26:1831–1840
Acknowledgements
We thank all the health professionals involved in registering data in computerised medical records for SIDIAP (Information System for Development of Primary Care Research). We thank as well Francisco Sorio Vilela and Laura Canals from Amgen for their contribution. Amgen provided comments on the design of the study protocol and the analysis plan. The final protocol and analysis plan were mutually agreed by SIDIAP and Amgen, based on the principle of the “best science known in the research field.” Amgen also provided comments on the publication prior to its submission to the journal. However, SIDIAP alone decided whether to incorporate Amgen comments in the submitted publication. Co-funded with FEDER funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Scientific approval was obtained from the SIDIAP Scientific Committee, and ethics approval was granted by the relevant board (CEIC IDIAP Jordi Gol). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
DPA’s research team has received unrestricted research funding from Bioiberica, Amgen and Servier Laboratoires; DPA’s department has received fees for speaker services from Amgen. C.T reports personal fees from Fundació Jordi Gol i Gurina, during the conduct of the study, and personal fees from Boehringer Ingelheim, outside the submitted work. DML reports fees for speaker or consultant services from Amgen, Lilly, MSD and Servier. CR, ASC, CCA and MSA report no conflict of interest.
Funding
Unrestricted research grant from Amgen. Partial funding of researchers (DPA) from NIHR Musculoskeletal Biomedical Research Unit Oxford, University of Oxford, UK.
Rights and permissions
About this article
Cite this article
Reyes, C., Tebe, C., Martinez-Laguna, D. et al. One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 28, 2997–3004 (2017). https://doi.org/10.1007/s00198-017-4144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4144-7