Abstract
Summary
The purpose of the study is to investigate the relationship between sarcopenia and body composition and osteoporosis in cohorts of three different races with a total of 17,891 subjects. Lean mass and grip strength were positively associated with bone mineral densities (BMDs). Subjects with sarcopenia were two times more likely to have osteoporosis compared with normal subjects.
Introduction
The relationship between sarcopenia and osteoporosis is not totally clear. First, the present study assessed this relationship by using two different definitions for sarcopenia. Second, we examined the associations of body composition (including muscle mass as a major and important component) and muscle strength on regional and whole-body BMDs.
Methods
In total, 17,891 subjects of African American, Caucasian, and Chinese ethnicities were analyzed. Sarcopenia was defined by relative appendicular skeletal muscle mass (RASM) cut points and also by the definition of the European Working Group on Sarcopenia in Older People (low RASM plus low muscle function). Multiple regression analyses were conducted to examine the association of fat mass, lean mass (including muscle mass), and grip strength with regional and whole-body BMDs. Multivariate logistic regression analysis was performed to explore the association between sarcopenia and osteopenia/osteoporosis.
Results
BMDs were positively associated with lean mass and negatively associated with fat mass, after controlling for potential confounders. Grip strength was significantly associated with higher BMDs. Each standard deviation (SD) increase in RASM resulted in a ~37 % reduction in risk of osteopenia/osteoporosis (odds ratio (OR) = 0.63; 95 % confidence interval (CI) = 0.59, 0.66). Subjects with sarcopenia defined by RASM were two times more likely to have osteopenia/osteoporosis compared with the normal subjects (OR = 2.04; 95 % CI = 1.61, 2.60). Similarly, subjects with sarcopenia (low muscle mass and low grip strength) were ~1.8 times more likely to have osteopenia/osteoporosis than normal subjects (OR = 1.87; 95 % CI = 1.09, 3.20).
Conclusions
High lean mass and muscle strength were positively associated with BMDs. Sarcopenia is associated with low BMD and osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aging process is accompanied by the progressive decline in bone mineral density (BMD), muscle mass, and muscle strength. The reduction of bone mass is a key feature of osteoporosis, which is defined in an individual with a BMD T-score at least 2.5 standard deviations (SDs) below the normal T-score in young adults [1]. Low BMD and bone architecture deterioration can increase the risk of fracture, posing a major clinical and public health burden.
The age-associated loss of muscle mass and strength is referred to as sarcopenia, which has a multifactorial basis including disuse, changing endocrine function, chronic diseases, inflammation, insulin resistance, and nutritional deficiencies [2]. Sarcopenia could result in adverse outcomes such as physical disability, poor quality of life, escalated costs of health care, and increased mortality. The prevalence of sarcopenia is reported to be 5–13 % in adults between 60 and 70 years of age and 11–50 % in people >80 years old [3]. Even with a conservative estimate of prevalence, sarcopenia affects more than 50 million people worldwide today [4], and it has been estimated that the direct public health cost for sarcopenia was approximately $18.5 billion in the USA alone in 2000 [5]. The most widely used criterion for diagnosis of sarcopenia is based on the relative appendicular skeletal muscle mass (RASM), which is measured by the appendicular lean mass divided by height squared (kg/m2) [2, 6]. However, there is debate about the value of this definition of sarcopenia based on muscle mass alone. For example, the European Working Group on Sarcopenia in Older People (EWGSOP) requires both low muscle mass and low muscle function (strength or physical performance) for the diagnosis of sarcopenia [4]. The rationale behind this latter definition is that muscle strength does not depend solely on muscle mass, and the relationship between muscle mass and muscle strength is not linear [4, 7]. Therefore, defining sarcopenia based solely on muscle mass is of limited clinical value.
The pathophysiology and etiology of sarcopenia and osteoporosis, and the relationship between them, are complicated and multifactorial. Recent studies have shown that muscle and bone share some common genetic, nutritional, lifestyle, and hormonal determinants [8], and that body composition and muscle strength are correlated with bone density [9]. In elders, decreased muscle mass and increased fat mass may contribute to difficulties with physical function. Many studies indicate that sarcopenia is an important predictor of poor physical function after consideration of the body weight or fat mass of the individual [10]. It has been hypothesized that certain determinants may influence bone through body composition and various determinants of BMD also influence body fat mass (FM) or muscle mass [11]. Two main components of body composition are FM and lean mass (LM), but it is unclear whether LM or FM is a better predictor of BMD. Many studies have shown that LM is positively correlated with whole-body and/or regional area BMD [11, 12, 9, 13, 14], and that RASM is also significantly associated with regional BMD [14, 15]. However, another study found that only FM is associated with BMD [16], and several recent studies reported that, after controlling for the mechanical loading effects of body weight on bone mass, there is an inverse association between FM and BMD [17–19]. There are two potential explanations for these inconsistent findings. First, LM and FM are primary components of body weight, and they are highly correlated with one another. When both FM and LM are included into a regression model, the regression coefficients for FM and LM should be interpreted with caution because of this correlation which results in multicollinearity. Second, differences in experimental design, study sample size, demographic characteristics (including age, sex, and ethnicity), and menstrual status (pre-, peri-, or postmenopause) may contribute to these inconsistent or contradictory results, posing difficulty in comparing findings across studies.
To date, the relationship between BMD and sarcopenia, defined by low RASM as well as by the more stringent EWGSOP criterion, has not been thoroughly studied. In the current study, we therefore assessed this relationship by using three large cohorts of different ethnicities based on two different definitions for sarcopenia. Also, we concurrently examined the associations of body composition and muscle strength on regional and whole-body BMDs.
Methods
Subjects
The study population consisted of three samples. The first sample was composed of 4274 healthy unrelated African American (AA) subjects (2383 males, 1891 females) from two cities (New Orleans and Baton Rouge, LA) in the southern USA. The second sample, collected in the Midwestern (Omaha, NE and Kansas City, MO) and the southern (New Orleans and Baton Rouge, LA) USA, consisted of 8604 unrelated healthy Caucasians (2947 males, 5657 females). The third sample consisted of 5013 unrelated healthy Chinese subjects (1677 males and 3336 females), recruited from two cities (Xi’an, Shanxi, and Changsha, Hunan) in central of People’s Republic of China.
Through the Louisiana Osteoporosis Study (LOS), Kansas City Osteoporosis Study (KCOS), and Chinese Osteoporosis Study (COS), we are building a large research cohort and database for human complex disease studies. In total, the study consisted of 17,891 participants of three different ethnicities. All participants signed informed consent documents before entering the studies. The comprehensive exclusion criteria were consistent and communicated elsewhere [20]. Briefly, none of the subjects had chronic diseases, medications, or treatments which may affect bone and/or soft tissue metabolism.
Measurement
A Hologic 4500 dual-energy X-ray absorptiometry (DXA) scanner (Hologic Inc., Bedford, MA, USA) was used to measure BMD (g/cm2) at whole body (WB), lumbar spine (LS), femoral neck (FN), total radius (TR), total FM, and total LM. DXA can give regional measures for the limbs, and limb lean tissue mass (appendicular) can be used as a good proxy for muscle mass [21]. Appendicular lean mass (aLM) was calculated as the sum of LM in the arms and legs. BMD for spine reflected the combined BMD of L1–L4. The Hologic Spine Phantom was scanned daily to monitor the long-term stability of scanning. DXA quality assurance measurements were performed at all study cities to ensure scanner reliability, and identical scan protocols were used for all participants. Weight was measured in light indoor clothing using a calibrated balanced beam scale, and height was measured using a calibrated stadiometer without shoes. BMI (kg/m2) was calculated as weight divided by height squared. Percentage fat mass (PFM) was defined as the ratio of total body FM divided by body weight. RASM was calculated as a LM divided by height squared. Grip strength was evaluated with the Jamar 1 hand-held dynamometer (TEC Inc., Clifton, NJ). Two measurements of maximum strength were taken at both hands. The maximum grip strength value was recorded in kilograms.
All participants completed an interviewer-assisted comprehensive questionnaire to assess information about demographic, occupational, and lifestyle information; reproductive history; disease history; consumption of alcohol; cigarette smoking; and physical activity. Subjects were defined as nonsmokers (never smoked or quit smoking at least 5 years prior to study). Subjects were considered to consume alcohol if they drank any volume of alcohol at least once per week. Regular exercise was defined by any type of exercise at least once per week.
Diagnosis of osteoporosis and sarcopenia
For the current study, osteoporosis and osteopenia were defined using WHO criteria. Specifically, osteoporosis was defined as a T-score at the FN of at least 2.5 SDs below the peak BMD of a young female adult reference group (T-score ≤ −2.5), and osteopenia was defined as FN BMD of 1.0–2.5 SDs below the young female adult reference group (−2.5 < T-score < −1) [22]. These criteria were developed based on well-established normative data from the Third National Health and Nutrition Examination Survey (NHANES III) reference database on Caucasian women aged 20–29 years [23, 24].
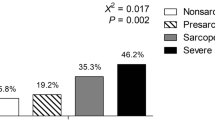
Sarcopenia was defined using two distinct approaches. The first approach defined sarcopenia as RASM (aLM/height2) being more than 2 SDs below the mean of young male and female reference groups. The current objective cut points for sarcopenia in men and women are RASM ≤7.26 kg/m2 and RASM ≤5.45 kg/m2, respectively [6, 2, 25]. These criteria for sarcopenia are based on previous studies including subjects of Caucasian and AA ethnicity. Because of ethnic difference in body composition, such criterion appears not to be applicable to Chinese subjects. A previous study established the cutoff values of 6.08 and 4.79 kg/m2 for sarcopenia for healthy Chinese men and women, respectively [26], and these criteria were used for the diagnosis of sarcopenia in our Chinese sample. The second approach for defining sarcopenia was based on the new European consensus definition of the EWGSOP in which both low muscle mass and low muscle function were used for the diagnosis of sarcopenia, with differentiation into three conceptual stages. The presarcopenia stage is characterized only by low muscle mass. The sarcopenia stage is characterized by low muscle mass plus either low muscle strength or low physical performance. The severe sarcopenia stage was defined by low muscle mass plus low muscle strength plus low physical performance [4]. Low muscle strength was defined as grip strength in men being ≤29 kg if BMI was ≤24, ≤30 kg if BMI was 24.1–28, and ≤32 kg if BMI was >28. In women, the corresponding numbers for low muscle strength were ≤17 kg if BMI was ≤23, ≤17.3 kg if BMI was 23.1–26, ≤18 kg if BMI was 26.1–29, and ≤21 kg if BMI is >29 [27].
Statistical analysis
The means and SDs were calculated for anthropometric measures. Differences in basic characteristics by race and sex were compared using analysis of variance (ANOVA) for continuous variables. And the subsequent comparisons were performed by Tukey’s honestly significant difference (HSD) to locate the source of significances. Pearson’s chi-squared test (χ 2) was used to compare differences for categorical variables.
In order to assess the associations between body composition and BMDs independently from the confounding effect of body weight, the entire cohort was divided into 10-kg strata of body weight. Only those strata containing over 200 subjects were included in statistical analysis to ensure appropriate statistical power. Partial correlation analyses, stratified by weight strata and controlling for age, height, race, and gender, were conducted to determine the strength of the relation between BMDs and body composition variables.
Multiple regression analysis was conducted to examine the association of FM, LM, and grip strength with regional and whole-body BMDs. For the entire cohort, race and sex were included in the regression models to test interactions using product terms. Regression models were also stratified by race and sex/menopause status with lifestyle risk factors adjusted. Body weight was not included in the multiple regression analyses to avoid multicollinearity since LM and FM are the primary components of weight. The variance inflation factor (VIF) was used to examine multicollinearity of variables in each model. If the largest VIF is >10, then multicollinearity exists.
Multivariate logistic regression analysis was also performed to examine the association between sarcopenia and osteopenia/osteoporosis. In the multivariate logistic regression model, the dependent variable is sarcopenia and the independent variable is osteopenia/osteoporosis, which is a dummy variable. Other covariates were adjusted in the model including age, gender, height, weight, race, city, smoking, alcohol drinking, and regular exercise. The results were expressed as odds ratios (ORs) and 95 % confidence interval (CI).
All statistical analyses were conducted using the Statistical Analysis System 9.3 (SAS Institute, Cary, NC, USA). All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Descriptive statistics
Table 1 shows the basic characteristics, anthropometric measurements, and regional and total body BMDs of the study population which was stratified into six subgroups by race and gender. There are 4274 unrelated healthy AA subjects [1891 females and 2383 males, aged 46.60 ± 11.8 years (mean ± SD), ranging 18.1–97.5], 8604 unrelated healthy Caucasians [2947 males, 5657 females, aged 49.3 ± 15.0 years (mean ± SD), ranging 18.1–95.2], and 5013 unrelated healthy Chinese subjects [1677 males and 3336 females, aged 36.0 ± 18.3 years (mean ± SD), ranging 18.0–88.0]. The proportion of premenopausal women and postmenopausal women is 39.0 and 21.0 %, respectively. All the subjects signed informed consent documents before entering the studies. In brief, subjects with diseases, treatments, or conditions that would be apparent, nongenetic, causes for abnormal bone mass or FM were excluded.
Within each ethnic grouping, men had lower absolute and relative FM, a greater LM, and greater RASM than women. As expected, regional and whole-body BMDs were greater in men than in women. AAs had the greatest LM and RASM, followed by Caucasians and Chinese in both men and women. Such differences among the three race groups remained for FM and PFM in women. However, in men, Caucasians had the greatest FM and PFM. Mean BMD differed significantly between the three race groups at whole-body and regional skeletal sites (p < 0.001) except for the FN, with values being highest in AAs and lowest in Chinese. Grip strength was higher in men than in women and was the highest in AAs and lowest in Chinese.
For women aged >50, the prevalence of osteopenia in AAs, Caucasians, and Chinese was 26.63, 53.00, and 54.65 %, respectively, and the prevalence of osteoporosis is 2.71, 8.83, and 14.97 %, respectively. For women aged <50, the prevalence of osteopenia in AAs, Caucasians, and Chinese was 10.19, 25.32, and 40.53 %, respectively, and the prevalence of osteoporosis is 0.46, 0.85, and 0.79 %, respectively. For men aged >65, the prevalence of osteopenia in AAs, Caucasians, and Chinese was 25.00, 37.43, and 47.67 %, respectively, and the prevalence of osteoporosis is 1.92, 3.89, and 7.56 %, respectively. For men aged <65, the prevalence of osteopenia in AAs, Caucasians, and Chinese was 8.28, 19.94, and 16.81 %, respectively, and the prevalence of osteoporosis is 0.17, 1.22, and 0.86 %, respectively. Based on the low RASM definition of sarcopenia, the prevalence of sarcopenia in AAs, Caucasians, and Chinese was 1.82, 3.87, and 1 %, respectively. According to the EWGSOP definition, the prevalence of sarcopenia in AAs, Caucasians, and Chinese is 1.40, 3.23, and 0.8 %, respectively. The established cutoff values for RASM by Baumgartner et al. (24) were limited to Caucasian and AA ethnicities, with separate criteria used for Chinese (25).
Correlation between bone mass and other body composition variables
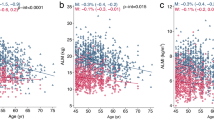
Table 2 shows partial correlation coefficients, controlling for age, race, sex, and height, between body composition, grip strength, and BMDs. LM and FM were significantly positively correlated with BMD when body weight was not controlled. In each 10-kg stratum of weight, LM was positively associated with BMDs, whereas FM and PFM were negatively correlated with BMDs. These findings suggest that body weight may confound the relationship between BMD and FM if not controlled in the model. Significant positive associations between aLM, LM, and BMDs were found in the entire cohort and in each of the 10-kg strata. Grip strength was significantly correlated with BMD at all skeletal sites.
Multiple regression analysis of body composition and BMD
Table 3 displays the regression coefficients, adjusted for various covariates, for whole-body and regional BMDs with LM, FM, and grip strength. LM was the dominant independent contributor to whole-body and regional BMDs in the total sample and the three subgroups (all p < 0.001). In the total sample, FM was significantly negatively associated with whole-body BMD (p < 0.001). FM did not predict LS or FN BMDs in pre- and postmenopausal women, or TR in postmenopausal women. Grip strength was significantly associated with higher BMDs at all sites (p < 0.001).
Significant race × FM interactions were found for whole-body, LS, and TR BMD (p < 0.001) when all subjects were included in the regression model. Significant sex × LM interactions were also found for whole-body and FN BMD (p < 0.001). Significant race × LM and sex × FM interactions (p < 0.001) were found for whole-body and TR BMD, respectively. No significant interaction was detected in any of the three subgroups.
Weight was not included in the regression model due to the very high multicollinearity with LM and FM (VIF > 10). VIFs in the models shown in Table 3 were less than 5 (data not shown), indicating that collinearity is unlikely in these models.
Sarcopenia and ORs of osteoporosis
The association between osteopenia/osteoporosis and sarcopenia is shown in Table 4. Each SD increase in RASM resulted in a 37 % reduction in the risk of osteopenia/osteoporosis (OR = 0.63; 95 % CI = 0.59, 0.66). Using the RASM-only definition of sarcopenia, after adjustment with covariates, the OR of osteopenia/osteoporosis in subjects having sarcopenia was two times higher than that in those without sarcopenia (OR = 2.04; 95 % CI = 1.61, 2.60). When sarcopenia was defined by EWGSOP, the OR of osteopenia/osteoporosis in subjects with presarcopenia was two times higher than that in normal subjects (OR = 2.09; 95 % CI = 1.60, 2.72), whereas the OR in subjects with sarcopenia (low muscle mass and low grip strength) was 1.87 times more than that in normal subjects (OR = 1.87; 95 % CI = 1.09, 3.20). There were significant linear trends for higher ORs of osteopenia/osteoporosis with sarcopenia than those of normal subjects in men and premenopausal women (p values <0.05).
Discussion
In the present study, with three diverse race cohorts that totaled 17,891 subjects aged from 18 to 97 years, we observed that subjects with sarcopenia were two times more likely to have osteoporosis compared with normal subjects. Each SD increase in RASM resulted in a 36 % reduction in the risk of osteopenia/osteoporosis. LM consistently showed significant positive correlations with whole-body and regional BMD. LM was positively associated with BMD at all skeletal sites. Grip strength was also associated with BMD at all sites. FM, in contrast, was negatively associated with BMDs.
The present study found that subjects with sarcopenia, based on the definition of Baumgartner et al. or EWGSOP [25, 4], were more likely to have osteopenia/osteoporosis than normal subjects. A similar association between osteoporosis and sarcopenia was found by several previous studies [28–30]. None of these previous studies, however, examined both genders. Moreover, the present study showed that grip strength, an important factor of sarcopenia, is significantly associated with BMDs at all skeletal sites, which may reflect the mechanical loading that muscle contractions and movements place on bone. Also, it can be attributed to the fact that bone and muscle share genetic, lifestyle, and hormonal determinants. It is suggested that improving muscle strength will be beneficial to muscle mass as well as bone health. The mechanism underlying this association needs to be further investigated to improve prevention and treatment of sarcopenia and osteoporosis [31].
Our study confirmed that LM is an important contributor to BMD and has a stronger effect on BMD than FM regardless of skeletal size, race, or sex/menopause status. Our findings are consistent with most previous studies on relationships between body composition and BMD [32, 33, 9, 34–37]. The association between LM and BMD could be explained by several factors. As a surrogate of skeletal muscle mass, LM has greater effects on BMD than FM because bone mass is mainly determined by muscular contraction as well as by the static mechanical load of muscle weight [9, 38]. Importantly, both muscle cells and osteoblasts derive from a common mesenchymal precursor, and some common genes may regulate bone and muscle via endocrine and cytokine signaling [39]. Muscle mass and bone mass also share dietary and hormonal determinants [40]. The positive correlation between LM and BMD indicates that larger LM may lead to higher BMD. Consequently, varied forms of physical activity may have a positive influence on both BMD and LM and force on muscles, thereby promoting the accumulation of bone materials under the periosteum [41, 42]. Our study also demonstrated that grip strength was positively correlated with LM as well as BMD.
The relationship between FM and BMD has been controversial. Some studies have emphasized the positive association between FM and BMD [11, 33, 43, 34, 44–46, 36]. These studies considered that body weight is not appropriate to enter into regression models, since body weight, consisting of LM and FM, may cause possible collinearity on the relationship between FM and BMD. In contrast, controlling for body weight, other studies found an inverse association between FM and BMD [17, 47, 18, 48–50, 19, 51, 52, 36]. In order to investigate the relationship between FM and BMD, it is necessary to control for the mechanical loading effects of body weight. In our study, partial correlation, performed in 10-kg strata of weight, showed that FM and PFM were indeed both negatively associated with whole-body and regional BMDs. Furthermore, multivariate regression analyses, in which body weight was not included in the model to avoid the possible collinearity, also found that FM had a significant negative effect on BMD. Differing from most previous studies, our study investigated the relationship between fat and bone density in subjects of three races, both genders, and pre- and postmenopausal women. Although the underlying mechanism for the negative effect of FM on BMD is unclear, several possible mechanisms have been suggested [53]. First, the contribution of adipose tissue to inflammatory processes may be responsible, in part, for the negative relationship between fat and bone [50]. Proinflammatory cytokines produced by adipocytes could stimulate osteoclast activity, thereby contributing to bone loss [54]. Second, both adipocytes and osteoblasts originate from a common progenitor, bone marrow mesenchymal stem cells (MSCs). Studies have shown that the preferential differentiation of MSCs into adipocytes, rather than into osteoblasts, may lead to fat accumulation and decreased BMD [54, 55].
In the present study, we found that the independent contribution of grip strength to BMD was consistently present at all BMD sites and explained a large proportion of the variation in BMD, findings that are consistent with a previous study [56]. Grip strength reflects the maximal loading effect on forearm and directly exerted forces on both the strength of the muscle and the bone simultaneously through physical activities. Although grip strength and LM were highly correlated, there may be an independent effect of grip strength over that explained by LM. Additional research is needed to further elucidate the contribution of grip strength to bone.
Several limitations should be noted in our study. First, as a cross-sectional study, the present study cannot establish the cause-and-effect relationship between sarcopenia and osteoporosis. Second, muscle strength was measured only by grip strength. We did not evaluate the effect of isometric strength of leg on BMD. The EWGSOP recommends also using physical performance, e.g., walking speed, for the diagnosis of sarcopenia; EWGSOP defines sarcopenia as low RASM plus either low grip strength or low walking speed. However, walking speed was not available in the current study; thus, our definition of sarcopenia did not reflect complete implementation of the EWGSOP criteria. Third, although we adjusted for several lifestyle factors in statistical analysis, there are some important biomarkers that were not considered, such as specific hormone, leptin, and lipid levels. Fourth, a wider distribution of BMD and RASM, including frail elderly individuals, may be needed to fully understand the complex relationship between osteoporosis and sarcopenia. Despite these limitations, the present study was conducted in three different races, with a large sample size, so that the findings would be more robust across populations of different ethnicities.
In summary, the present study found that LM and grip strength were positively associated with BMD at all skeletal sites measured, and that FM was negatively associated with BMD. Additionally, subjects with sarcopenia were more likely to have osteoporosis compared with nonsarcopenic subjects. Further studies are needed to reveal the underlying mechanisms of for the associations between body composition and bone health.
References
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res 9(8):1137–1141. doi:10.1002/jbmr.5650090802
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. Int Working Group Sarcopenia J Am Med Directors Assoc 12(4):249–256. doi:10.1016/j.jamda.2011.01.003
von Haehling S, Morley JE, Anker SD (2010) An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachex Sarcopenia Muscle 1(2):129–133. doi:10.1007/s13539-010-0014-2
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 39(4):412–423. doi:10.1093/ageing/afq034
Roth SM, Metter EJ, Ling S, Ferrucci L (2006) Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol 18(6):625–630. doi:10.1097/01.bor.0000245722.10136.6d
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2003) Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51(11):1602–1609
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A: Biol Med Sci 61(10):1059–1064
Coin A, Perissinotto E, Enzi G, Zamboni M, Inelmen EM, Frigo AC, Manzato E, Busetto L, Buja A, Sergi G (2008) Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr 62(6):802–809. doi:10.1038/sj.ejcn.1602779
Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB (2001) Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the health, aging, and body composition study. J Bone Miner Res Off J Am Soc Bone Miner Res 16(7):1343–1352. doi:10.1359/jbmr.2001.16.7.1343
Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B (2009) Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutri 89(6):1895–1900. doi:10.3945/ajcn.2008.26950
Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P (2001) Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res Off J Am Soc Bone Miner Res 16(11):2142–2151. doi:10.1359/jbmr.2001.16.11.2142
Genaro PS, Pereira GA, Pinheiro MM, Szejnfeld VL, Martini LA (2010) Influence of body composition on bone mass in postmenopausal osteoporotic women. Arch Gerontol Geriatr 51(3):295–298. doi:10.1016/j.archger.2009.12.006
Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C (2001) Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology 47 (4):207–212. doi:52800
Sherk VD, Palmer IJ, Bemben MG, Bemben DA (2009) Relationships between body composition, muscular strength, and bone mineral density in estrogen-deficient postmenopausal women. J Clin Densitometry Off J Int Soc Clin Densitometr 12(3):292–298. doi:10.1016/j.jocd.2008.12.002
Blain H, Jaussent A, Thomas E, Micallef JP, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Rossi M, Picot MC (2010) Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Exp Gerontol 45(9):679–684. doi:10.1016/j.exger.2010.04.006
Wu F, Ames R, Clearwater J, Evans MC, Gamble G, Reid IR (2002) Prospective 10-year study of the determinants of bone density and bone loss in normal postmenopausal women, including the effect of hormone replacement therapy. Clin Endocrinol 56(6):703–711
Taes YE, Lapauw B, Vanbillemont G, Bogaert V, De Bacquer D, Zmierczak H, Goemaere S, Kaufman JM (2009) Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab 94(7):2325–2331. doi:10.1210/jc.2008-2501
Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW (2007) Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92(5):1640–1646. doi:10.1210/jc.2006-0572
Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V (2007) Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab 92(1):143–147. doi:10.1210/jc.2006-0794
Deng HW, Shen H, Xu FH, Deng HY, Conway T, Zhang HT, Recker RR (2002) Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res Off J Am Soc Bone Miner Res 17(4):678–686. doi:10.1359/jbmr.2002.17.4.678
Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D (2002) Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutri 76(2):378–383
Prevention and management of osteoporosis (2003). World Health Organization technical report series 921:1–164, back cover
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42(3):467–475. doi:10.1016/j.bone.2007.11.001
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R (1998) Updated data on proximal femur bone mineral levels of US adults. Osteopo Int: J Estab Result Coop Between Europ Found Osteoporo Natl Osteoporo Found USA 8(5):468–489
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Cheng Q, Zhu X, Zhang X, Li H, Du Y, Hong W, Xue S, Zhu H (2013) A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metab 32(1):78–88. doi:10.1007/s00774-013-0468-3
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A: Biol Med Sci 56(3):M146–M156
Verschueren S, Gielen E, O’Neill TW, Pye SR, Adams JE, Ward KA, Wu FC, Szulc P, Laurent M, Claessens F, Vanderschueren D, Boonen S (2013) Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteopo Int: J Estab Result Coop Between Europ Found Osteoporo Natl Osteoporo Found USA 24(1):87–98. doi:10.1007/s00198-012-2057-z
Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75(2):175–180. doi:10.1016/j.maturitas.2013.03.016
Di Monaco M, Vallero F, Di Monaco R, Tappero R (2011) Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr 52(1):71–74. doi:10.1016/j.archger.2010.02.002
Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA (2005) Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Asp Med 26(3):181–201. doi:10.1016/j.mam.2005.01.004
Douchi T, Kuwahata R, Matsuo T, Uto H, Oki T, Nagata Y (2003) Relative contribution of lean and fat mass component to bone mineral density in males. J Bone Miner Metab 21(1):17–21. doi:10.1007/s007740300003
Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB (2005) The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone 37(4):474–481. doi:10.1016/j.bone.2005.04.038
Park JH, Song YM, Sung J, Lee K, Kim YS, Kim T, Cho SI (2012) The association between fat and lean mass and bone mineral density: the Healthy Twin Study. Bone 50(4):1006–1011. doi:10.1016/j.bone.2012.01.015
Gnudi S, Sitta E, Fiumi N (2007) Relationship between body composition and bone mineral density in women with and without osteoporosis: relative contribution of lean and fat mass. J Bone Miner Metab 25(5):326–332. doi:10.1007/s00774-007-0758-8
Shin D, Kim S, Kim KH, Park SM (2013) Importance of fat mass and lean mass on bone health in men: the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). Osteopo Int: J Estab Result Coop Between Europ Found Osteoporo Natl Osteoporo Found USA. doi:10.1007/s00198-013-2412-8
Cui LH, Shin MH, Kweon SS, Park KS, Lee YH, Chung EK, Nam HS, Choi JS (2007) Relative contribution of body composition to bone mineral density at different sites in men and women of South Korea. J Bone Miner Metab 25(3):165–171. doi:10.1007/s00774-006-0747-3
Turner CH (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23(5):399–407
Karasik D, Kiel DP (2008) Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res Off J Am Soc Bone Miner Res 23(6):788–802. doi:10.1359/jbmr.080218
Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C (1996) Do genetic factors explain associations between muscle strength, lean mass, and bone density? a twin study. Am J Physiol 270(2 Pt 1):E320–E327
Lekamwasam S, Weerarathna T, Rodrigo M, Arachchi WK, Munidasa D (2009) Association between bone mineral density, lean mass, and fat mass among healthy middle-aged premenopausal women: a cross-sectional study in southern Sri Lanka. J Bone Miner Metab 27(1):83–88. doi:10.1007/s00774-008-0006-x
Andreoli A, Monteleone M, Van Loan M, Promenzio L, Tarantino U, De Lorenzo A (2001) Effects of different sports on bone density and muscle mass in highly trained athletes. Med Sci Sports Exerc 33(4):507–511
MacInnis RJ, Cassar C, Nowson CA, Paton LM, Flicker L, Hopper JL, Larkins RG, Wark JD (2003) Determinants of bone density in 30- to 65-year-old women: a co-twin study. J Bone Miner Res Off J Am Soc Bone Miner Res 18(9):1650–1656. doi:10.1359/jbmr.2003.18.9.1650
Szulc P, Beck TJ, Marchand F, Delmas PD (2005) Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—the MINOS study. J Bone Miner Res Off J Am Soc Bone Miner Res 20(5):721–729. doi:10.1359/JBMR.041230
Bogl LH, Latvala A, Kaprio J, Sovijarvi O, Rissanen A, Pietilainen KH (2011) An investigation into the relationship between soft tissue body composition and bone mineral density in a young adult twin sample. J Bone Miner Res Off J Am Soc Bone Miner Res 26(1):79–87. doi:10.1002/jbmr.192
Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, Lim TM, Cundy TF (1992) Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab 75(1):45–51
Yu Z, Zhu Z, Tang T, Dai K, Qiu S (2009) Effect of body fat stores on total and regional bone mineral density in perimenopausal Chinese women. J Bone Miner Metab 27(3):341–346. doi:10.1007/s00774-009-0036-z
Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X (2006) Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutri 83(1):146–154
Kim JH, Choi HJ, Kim MJ, Shin CS, Cho NH (2012) Fat mass is negatively associated with bone mineral content in Koreans. Osteopo Int: J Estab Result Coop Between Europ Found Osteoporo Natl Osteoporo Found USA 23(7):2009–2016. doi:10.1007/s00198-011-1808-6
Yoo HJ, Park MS, Yang SJ, Kim TN, Lim KI, Kang HJ, Song W, Baik SH, Choi DS, Choi KM (2012) The differential relationship between fat mass and bone mineral density by gender and menopausal status. J Bone Miner Metab 30(1):47–53. doi:10.1007/s00774-011-0283-7
Kim CJ, Oh KW, Rhee EJ, Kim KH, Jo SK, Jung CH, Won JC, Park CY, Lee WY, Park SW, Kim SW (2009) Relationship between body composition and bone mineral density (BMD) in perimenopausal Korean women. Clin Endocrinol 71(1):18–26. doi:10.1111/j.1365-2265.2008.03452.x
Blum M, Harris SS, Must A, Naumova EN, Phillips SM, Rand WM, Dawson-Hughes B (2003) Leptin, body composition and bone mineral density in premenopausal women. Calcif Tissue Int 73(1):27–32
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res 23(1):17–29. doi:10.1359/jbmr.070813
Cao JJ (2011) Effects of obesity on bone metabolism. J Orthop Surg Res 6:30. doi:10.1186/1749-799X-6-30
Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2(1):35–43. doi:10.1038/ncprheum0070
Chan DC, Lee WT, Lo DH, Leung JC, Kwok AW, Leung PC (2008) Relationship between grip strength and bone mineral density in healthy Hong Kong adolescents. Osteopo Int: J Estab Result Coop Between Europ Found Osteoporo Natl Osteoporo Found USA 19(10):1485–1495. doi:10.1007/s00198-008-0595-1
Acknowledgments
We appreciate all study participants who provided with phenotypic information. We would like to thank all staff who provided the clinical expertise, collected, and managed the data. We appreciate suggestions from Dr. Jian Li. This work was partially supported by grants from the National Institutes of Health (P50AR055081, R01AG026564, R01AR050496, RC2DE020756, R01AR057049, and R03TW008221) and startup funds from Tulane University and University of Missouri-Kansas City.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, H., Liu, Y., Tian, Q. et al. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int 27, 473–482 (2016). https://doi.org/10.1007/s00198-015-3241-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3241-8