Abstract
A cross-sectional survey was conducted in Shanghai, eastern China, to evaluate the prevalence of loss of muscle mass corresponding to sarcopenia in Chinese men and women and compare the results with the prevalence in other populations. We also analyzed the differences between men and women, and assessed the effect of lean mass and fat mass of different regions on bone mass. A total of 1766 men and 1778 women aged 18–96 years participated in this study. Bone mineral density of spine and femur, and lean mass and fat mass of several body regions were measured by dual-energy X-ray absorptiometry. Class 1 and class 2 sarcopenia were defined as the appendicular lean mass (ALM) index (ALM/height2) 1 and 2 standard deviations below the sex-specific means for young adults. Mean values for ALM index were 7.93 for men and 6.04 kg/m2 for women, aged 18–40 years. The reference values for classes 1 and 2 sarcopenia were 7.01 and 6.08 kg/m2 in men and 5.42 and 4.79 kg/m2 in women. The prevalence of sarcopenia was 4.8 % in women and 13.2 % in men aged 70 years and older, which is lower than that in Caucasian populations, but the same as that in Japanese and Koreans in Asia. Men demonstrated greater declines in muscle mass with aging than women, partly due to the protective effect of fat mass on lean mass in women. Leg lean mass was the strongest factor on femur bone mass; however, trunk lean mass was the strongest factor on spine bone mass. Maintaining a healthy weight is important for the elderly in order to avoid osteoporosis and sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragility fractures are common in the elderly due to low bone mass and muscle deterioration with advancing age. More than 90 % of fragility fractures in osteoporotic patients were reported as the result of a fall [1]. Fractures severely affect mobility and independent living. Osteoporosis is a skeletal disorder characterized by low bone density and deterioration of bone microarchitecture. Sarcopenia, or muscle wasting, is the degenerative loss of skeletal muscle mass and atrophy of type II fast-twitch muscle fibers in senescence, causing a plethora of disabilities and lifestyle-related diseases [2]. These two health problems often occur concurrently and lead to an increased risk of fragility fracture in aging populations [3].
Many potential mechanisms for sarcopenia, as well as the relationship between sarcopenia and bone mass, have been investigated, but most studies have focused on Caucasian populations [4–7 ]; few studies have been conducted in Asia, particularly in China.
We therefore performed the present study in Chinese men and women to determine reference values for sarcopenia and calculate the prevalence of sarcopenia among Chinese people. We also compared the prevalence of sarcopenia among the Chinese with that of Caucasian populations and other races in Asia. In addition, we compared the bone mass of different body composition profiles (normal, obesity, sarcopenia, and sarcopenia–obesity) and identified factors of body composition that affect bone mass and lean mass in this community-dwelling population of elderly people.
Materials and methods
Subjects
Healthy Chinese men (n = 1766) and women (n = 1778) aged 18–96 years participated in this study (Fig. 1). The young adults (423 men and 442 women) aged 18–40 years were included to establish reference values. Participants aged 41 years or older (1343 men and 1336 women) were evaluated for the presence and characteristics of sarcopenia. All of the participants were from Shanghai, China, and were recruited at Shanghai Huadong Hospital, affiliated with Fudan University, when undergoing physical examinations between January 2005 and December 2009. All of the subjects participated in daily physical exercise, either outdoors or at home, including housework. Individuals who were confined to a wheelchair or a bed were excluded from the study. All participants were in good health according to clinical medical evaluations. None of the participants were suffering from chronic diseases, such as hyperthyroidism, hyperparathyroidism, renal failure, malabsorption syndrome, alcoholism, chronic colitis, multiple myeloma, leukemia, or chronic arthritis. Stroke, Parkinson’s disease, peripheral neuropathy, and cognitive impairment were also excluded. Likewise, none of the participants were taking any medications that were likely to affect bone or soft tissue metabolism, such as anti-osteoporotic (e.g. glucocorticoids, heparin, warfarin, thyroxine, sex hormone, bisphosphonate, SERMs, calcitonin, PTH analogue, or calcitriol) or weight-controlling drugs. Candidates on diets for weight loss or weight gain were excluded from the study. All of the subjects provided written informed consent before participating in the study. The program was approved by the Fudan University affiliated Huadong Hospital ethics committee.
Measures of whole-body DXA
Body weight and body height were measured while the participants were not wearing shoes and the results were recorded. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Regional bone mineral density (BMD), including lumbar spine (L1–4) and left femoral neck (FN) were measured using dual-energy X-ray absorptiometry (DXA; Hologic Delphi A, Hologic Inc.). Body composition was measured with whole body scan of the same machine operated in slow scan mode. Manual DXA analysis software was used to analyze all of the DEXA scans. The system software provides estimates of the following three compartments: fat mass, lean mass, and bone mass for the whole body and specific regions: arms, legs, head, and trunk. To minimize interobserver variation, all scans and analyses were conducted by the same investigator. The day-to-day coefficients of variance (CVs) of these observations were 0.86 % in the lumbar spine BMD, 1.86 % in the FN BMD, 0.95 % in the total body BMD, 0.74 % in lean mass, and 1.5 % in fat mass. The densitometer was standardized by a standard phantom prior to each measurement.
Definition of sarcopenia, osteoporosis, and obesity
Sarcopenia was based on appendicular lean mass (ALM; kg) measurements[8], which corresponds to the sum of the two upper and lower limb muscular masses, measured in kilograms, and was normalized for height [skeletal muscle index (SMI) = ALM/height2 (kg m−2)]. The cutoff values to define class 1 and class 2 sarcopenia in each gender were respectively defined as one and two standard deviations (SD) below the sex-specific means of the reference data for young people aged 18–40 years in this study.
According to the World Health Organization (WHO) definition [9] and the BMD reference data established by young people aged in their twenties in this study, subjects with a BMD that was 2.5 SD lower than the peak mean of the same gender (T score ≤ −2.5) were determined to be osteoporotic.
Obesity was defined according to total body fat mass assessed by DXA. The cutoff to define obesity was also based on previous work [8]. Participants were classified as obese if their percentage of body fat (fat % = fat mass/body weight × 100) was above the 60th percentile of the present study sample of the same gender, which corresponded to 40 % body fat.
Statistical analysis
SPSS version 14.0 was used for the statistical analyses. The means and SDs were calculated for all anthropometric data. Initial analyses described the participants’ characteristics according to age and gender. Continuous variables were expressed as means ± SDs. The two-sample U test was used for the comparison of lean mass and fat mass between men and women. The chi-squared test was used for the comparison of the prevalence of sarcopenia between men and women. The correlations between SMI and body composition variables were investigated using Pearson’s correlation test for normally-distributed variables or Spearman’s correlation for non-normally-distributed variables. The participants were divided into four body composition groups according to the definition of sarcopenia and obesity: healthy/normal, purely sarcopenic, purely obese, and sarcopenic–obese. Differences of bone mass among the four groups were evaluated using analysis of variance (ANOVA). Associations between bone mass and lean mass or fat mass of different regions were assessed using multiple linear regression analysis. BMDs of the lumbar spine and the FN were dependent variables, while lean mass and fat mass of different regions were independent variables. The analysis adjusted age, body weight and BMI in order to assess the respective effects of lean mass and fat mass on bone. All statistical tests were two-tailed, and P < 0.05 was considered significant.
Results
Characteristics of anthropometry and densitometer measurements of study subjects
All subjects were divided into 10-year age subgroups for cross-sectional analysis by sex, beginning at age 18–20 years for the youngest groups. All subjects older than 90 years were included in the oldest group. Basic anthropometry and densitometer measurements of body composition of the study participants are listed in Table 1. The highest value for height occurred in the 21–30-years age group both men and women, and a significant decrease in height was found above 50 years old in women and above 60 years old in men. Clear declines in BMD with age were evident in the spine and the femur in both sexes. For men, the peak BMD values of the lumbar spine and FN were 0.99 g/cm2 (SD 0.13) and 0.90 g/cm2 (SD 0.14), respectively, and both occurred in the 21–30-years age group. For women, the peak BMD values of the lumbar spine and FN were 0.99 g/cm2 (SD 0.12) and 0.79 g/cm2 (SD 0.09), respectively, occurring in the 31–40-years age group and 21–30-years age group, respectively. Compared to the peak value, the BMD of the FN in the 71–80-years, 81–90-years and 90 years and older age groups decreased by 24.05, 25.32, and 29.11 %, respectively, in women, and decreased by 21.11, 24.44, and 26.67 %, respectively, in men. In addition, the BMD of the spine increased after the age of 60 years in both genders, owing to hyperostosis of the spine.
The peak ALM values were 16.15 kg (SD 1.93) for women and 23.78 kg (SD 3.50) for men, both of which occurred in the 21–30-years age group. Compared to the peak value, the ALM in the 71–80-years, 81–90-years and 90 years and older age groups decreased by 8.54, 15.73, and 15.11 %, respectively, in women, and decreased by 15.71, 21.08, and 24.53 %, respectively, in men. However, the patterns of SMI were different from that of ALM. The SMI demonstrated its peak value in the 18–20-years age group for men and the 21–30-years age group for women, and then decreased slightly. SMI increased again between 41 and 60 years of age in both genders, which may be due to the decrease in body height with age.
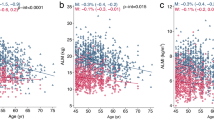
The changes in average values for appendicular and trunk lean mass and fat mass are shown in Fig. 2. As expected, lean mass of both the appendicular region and the trunk were almost significantly higher in men than in women in each age group (P < 0.01, two-sample U test). Fat % and fat mass of the appendicular region were significantly higher in women than in men in each age group (P < 0.01). Trunk fat mass was the same for both men and women before 70 years; after age 70, trunk fat mass was gradually higher in women than in men. In both genders, aging was accompanied by an increase in fat mass of different regions and a decrease in lean mass of different region. However, more significant changes were observed in the ALM of men and the trunk fat mass of women.
Prevalence of sarcopenia and osteoporosis in elderly Chinese
The SMI values in young men and women aged 18–40 years were 7.93 ± 0.93 and 6.04 ± 0.62 kg m−2, respectively. Therefore, the cutoff values for class 1 sarcopenia in Chinese men and women were 7.01 and 5.42 kg m−2, respectively. Similarly, the cutoff values of class 2 sarcopenia in Chinese men and women were 6.08 and 4.79 kg m−2, respectively. The prevalences of class 1 and class 2 sarcopenia were 30.1 and 11.2 %, respectively, in men over 50 years old and 34.0 and 13.2 %, respectively, in men over 70 years old. The prevalences of class 1 and class 2 sarcopenia were 14.1 and 3.7 %, respectively, in women over 50 years old and 16.5 and 4.8 %, respectively, in women over 70 years old. The prevalences of class 1 and class 2 sarcopenia were significantly different between men and women in each age group (P < 0.01, chi-squared test). According to the diagnostic criteria of osteopenia and osteoporosis, the prevalences of osteopenia and osteoporosis in men over 50 years old were 65.6 and 5.8 %, respectively, and 68.1 and 6.7 %, respectively, in men over 70 years old. The prevalences of osteopenia and osteoporosis in women over 50 years old were 64.9 and 34.4 %, respectively, and 68.0 and 42.3 %, respectively, in women over 70 years old. Table 2 shows comparisons of large-scale studies which evaluated the prevalence of sarcopenia in different countries. The differences in prevalence of sarcopenia between these studies may be due to the definition of sarcopenia and cohort selection. There are two definitions for sarcopenia: (1) ALM divided by height squared (ALM/ht2) [4, 10–16] and (2) ALM as a percentage of body weight (ALM/wt) [5, 14, 17]. The prevalence of sarcopenia using the definition of ALM/wt was higher than that of ALM/ht2 [14]. The other reason may be the different cohort of each study; for example, some studies selected community-dwelling people whether active or not, but the subjects in our study were all in good health and able to walk. The prevalence of sarcopenia in our study is thus a little lower than in others [4, 10, 13].
Correlation of SMI with age, BMI, lean mass, fat mass, and percent of fat mass
The values for SMI, age, BMI, lean mass, fat mass, and percent of fat mass in men and women over 50 years are shown in Table 3. The relationship between SMI and lean mass is self-evident, so we only analyzed the correlation between SMI and fat mass of different body regions.
In both men and women, SMI showed significant negative correlations with age (r = −0.154 to −0.433, P < 0.01) and significant positive correlations with BMI, total lean mass, total fat mass, and appendicular and trunk fat mass (r = 0.333–0.827, P < 0.01). In women, SMI was also positively related to age of menarche (r = 0.073, P < 0.01) and negatively related to years since menopause (r = −0.133, P < 0.01). SMI showed a positive relationship with percent of total fat mass, percent of appendicular fat mass, and percent of trunk fat mass in women (r = 0.078–0.297, P < 0.01). However, in men, SMI showed a negative relationship with percent of appendicular fat mass (r = −0.124, P < 0.01) and was not related to percent of total fat mass or percent of trunk fat mass.
Body composition and bone mass
Comparisons of body composition and bone mass among the four body composition groups of men and women are shown in Table 4. In subjects with pure sarcopenia, BMI, lean mass, fat mass, fat mass %, and total body and regional BMD (lumbar spine and FN) were significantly lower than that of the other three groups (P < 0.001) in men. The same results were seen in women (P < 0.05), with the exception of lean mass and BMD of the spine and total body. The purely obese group showed a higher BMI, more lean mass and fat mass, higher SMI, and greater BMD of the lumbar spine and the FN than any other group in both sexes (all P < 0.05, except lumbar spine in women). The sarcopenia–obese group had higher bone mass of the spine, the femur, and the total body than that of the purely sarcopenia group in men (all P < 0.001, except total BMD). In women, the BMD of the femur was higher in the sarcopenia–obese group than the purely sarcopenic group, while the BMD of the lumbar spine and the total body was lower in the sarcopenia–obese group than the purely sarcopenic group, but these results were not statistically significant.
Regression analysis of lean mass, fat mass of different regions with BMD of lumbar spine and FN, adjusting age, weight and BMI in men and women
The relationship between lean mass and fat mass of different regions (appendicular, trunk, and head) with BMD of the lumbar spine and FN were studied by multiple linear regression. The analysis, which adjusted age, weight, and BMI, was shown in Table 5. Trunk lean mass explained 7.4 % of the lumbar spine BMD variance in men, and trunk lean mass accounted for 13.5 % of the lumbar spine BMD variance in women. Left leg lean mass explained 25 % and trunk lean mass explained 2.1 % of FN BMD variance in men, and left leg lean mass explained 20.4 % and trunk lean mass explained 1.5 % of FN BMD in women. Leg lean mass was significantly associated with femur bone mass, and trunk lean mass was significantly associated with spine bone mass and femur bone mass. Based on our model, fat mass, unlike lean mass, was not a significant predictor of either femur or spine bone mass.
Discussion
This cross-sectional study was performed to evaluate the loss of muscle mass corresponding to sarcopenia among Chinese men and women and to compare the differences in sarcopenia prevalence and characteristics between elderly Chinese and that of other races. Specifically, we aimed to analyze the reasons for the differences, examine whether obesity changes the relationship between sarcopenia and bone mass, and, finally, investigate the effect of body regional lean mass and fat mass on bone mass. We reached several major conclusions. First, we defined the cutoff for class 1 and class 2 sarcopenia as 7.01 and 6.08 kg m−2, respectively, in Chinese men and 5.42 and 4.79 kg m−2, respectively, in Chinese women. We established the prevalences of class 1 and class 2 sarcopenia over 70 years old as 34.0 and 13.2 %, respectively, in men, and 16.5 and 4.8 %, respectively, in women. Second, the prevalence of sarcopenia in women is lower than that of men in this study, and men lose more skeletal muscle mass with age than women. The prevalence of sarcopenia in Chinese elderly people in this study was lower than that in Caucasian populations, but the same as the prevalence in Japanese and Korean populations. Third, obesity had a positive effect on bone mass in both elderly men and women. An obese person tends to have higher bone mass than a non-obese person, whether sarcopenia is present or not. Fourth, leg lean mass was significantly associated with femur bone mass, while trunk lean mass was significantly associated with spine bone mass. Trunk lean mass also contributed to femur bone mass. Fat mass was not as significant as lean mass on either FN or lumbar spine BMD in the elderly.
Sarcopenia, or muscle wasting, and osteoporosis, or fragile bone disease, are significant health burdens among elderly populations that become more evident with increasing age. Sarcopenia results in declines in activities of daily living, quality of life, and self-rated health, and an increased risk of falls and related skeletal fractures. Moreover, a loss of more than 40 % of total muscle mass is associated with death in elderly with chronic inflammatory conditions [18].
Many previous studies of sarcopenia were limited to subjects of Caucasian and African–American ethnicities. Baumgartner et al. [4] first established cutoff values for sarcopenia (ALM/height2) of 7.26 kg m−2 for men and 5.45 kg m−2 for women, and reported that the prevalence of sarcopenia increased from 13 to 24 % in persons under 70 years of age to more than 50 % in persons older than 80 years. They further reported that the prevalence of sarcopenia was slightly greater in Hispanics than in non-Hispanic whites. Thereafter, several large-scale studies were launched to investigate the prevalence of sarcopenia in the West [4–7, 10–14]. In recent years, many surveys were conducted in Asian countries that examined sarcopenia in Asian ethnicities [15–17]. In our current study, the cutoff values for sarcopenia were defined as 6.08 and 4.79 kg m−2 in Chinese men and women, respectively, and the prevalence rates for individuals over 70 years old were 13.2 % in men and 4.8 % in women.
The prevalence of sarcopenia in Chinese elderly people was lower than that in Caucasians, but the same as that in Japanese and Koreans in Asia. This difference might originate from ethnic differences [19, 20, 21]. Another consideration is that young people in the East are slimmer than those in the West, so the peak ALM value is relatively lower in the East; moreover, one study showed that total physical activity levels have fallen sharply among adults in China during the last 20 years due to the change of lifestyle and economic well-being [22], which may impair the peak ALM of Chinese people in recent years; therefore, the lean mass decrease in elderly Chinese seems to be small compared to the recently depressed peak ALM. In addition, the cohort we selected included individuals who all had independent movement, and we excluded those who were confined to a house or a wheelchair, so the prevalence of sarcopenia was relatively lower in our study population than in the general population.
It is difficult to compare the prevalence of sarcopenia in different races in various countries, mostly because of practical difficulties in assessing muscle mass. On average, 5–13 % of elderly people aged 60–70 years are affected by sarcopenia, and the prevalence increases to 11–50 % for those aged 80 years and above [23]. The broad range of sarcopenia prevalence is partly due to the heterogeneity of study populations, but also due to the different techniques used to assess muscle mass. DXA is currently considered the gold standard for such measurements, but, depending on the actual technique used in different studies and on the cutoff values chosen, the assessment of muscle mass may vary considerably [24].
Longitudinal studies of body composition in older adults show a tendency toward a loss of lean mass and a gain in fat over time in healthy elderly persons [25, 26]. The tendency toward lean mass loss was more significant in men than in women, and, conversely, fat gain was more significant in women than in men. In our study, differences in lean mass and fat mass of both appendicular and trunk regions were observed across different age groups. First, women had a higher percentage of body fat and fat mass than men, while men had higher lean mass than women. Second, the age-associated loss of appendicular and trunk lean mass were greater for men than for women, whereas the loss of ALM was more than the loss of trunk lean mass in both sexes. Third, the increase in fat mass was greater in women than in men, but age-associated increases in trunk fat mass were more rapid than similar increases in appendicular fat mass for both men and women. Our findings are in agreement with several previous studies which demonstrated that men lose more skeletal muscle mass with age, even though they have greater skeletal muscle mass than women at all ages [27]. In our study, the prevalence of sarcopenia over 70 years of age was higher in men (13.2 %) than in women (4.8 %). In a similar study in the USA, the prevalence based on total skeletal mass determined by DXA was 10 % for men and 8 % for women between 60 and 69 years old and 40 and 18 %, respectively, for men and women over 80 years old [28].
These important findings provide direct evidence for differences in muscle mass in older men and women. The mechanisms underlying such gender-related differences with aging remain in question. However, fat mass and hormonal factors may play a role. Fat mass may have several age-related effects on lean mass. Obese people may have good nutrition with high protein intake, which is a protective factor against sarcopenia; however, obese people are often not as physically active as non-obese people, and obesity leads to functional limitations and a sedentary lifestyle, which, in turn, result in a loss of muscle mass and poor muscle strength. Additionally, excess accumulation of fatty acids around the muscle fibers may interfere with their functioning and thereby reduce muscle quality [29]. A menopause-associated decline in estrogen is well recognized in women, and estrogen may also exhibit anabolic effects on muscle [30]. Therefore, fat mass may have some protective effects on lean mass owing to the conversion of androgens to estrogens by fat, but this would only account for improvements of lean mass and strength in women, not men [31].
On the other hand, as demonstrated in our study, height loss is common with advancing age, and is greater in women than in men; from our study, a significant decrease in height was found above 50 years old in women and above 60 years old in men; on average, women over the age of 70 years lost 10 cm in height, while men of the same age lost 8 cm. An older adult who has lost more height would have a higher ALM/height2 value and would, therefore, be less likely to be classified as sarcopenic. This is another possible reason why sarcopenia occurs less frequently in women than in men.
The concurrence of both obesity and sarcopenia, a condition known as “sarcopenic obesity,” occurs in about 6 % of community-dwelling elderly Americans [32]. It is more often associated with the development of physical disabilities than either sarcopenia or obesity alone [33, 34]. However, our results showed that obesity may offer some protection against osteoporosis, even in sarcopenic elderly men and women in China, which is due to the different effects of fat mass on bone mass and muscle activity.
Obesity is believed to be inversely correlated with fracture risk because of the relatively higher BMD of obese individuals. Some studies have also reported that fat mass of the hip can prevent hip fracture due to fat pads, which has implications for fall risk and fractures [35]. However, a recent study of postmenopausal women suggested that obesity is a risk factor for certain fractures, particularly those of the ankle and upper leg [36]. Although higher BMI is generally associated with higher BMD, obesity also has some profound adverse effects on bone health: for example, reduced physical activity, low vitamin D status [37], and some metabolic diseases. Our study also found that the important factor which decides BMD of lumbar and femur is not fat mass but lean mass. Therefore, the effects of obesity on bone health are double-edged and the final effects depend on different situations.
The relationship between muscle mass and bone mass may be due to the site-specific effects of loading on bone. Our study found that leg lean mass is the strongest factor associated with femur BMD in men and women. Furthermore, other studies reported that leg lean mass is associated with femur BMD, independent of its skeletal loads and other lifestyle, nutritional, and hormonal factors [38]. This suggests that common factors, possibly genetic factors, might also influence the coupled maintenance of leg muscle mass and femur BMD. However, trunk lean mass is the strongest factor associated with spine BMD in both sexes, and trunk lean mass is also positively correlated with femur BMD.
Our study has some limitations. First, it is limited to the evaluation of lean mass by DXA. We cannot assess muscle function, such as muscle strength and physical performance. Second, our study does not account for other potential determinants of lean mass and bone mass, including lifestyle habits, vitamin D status, or sex hormone levels. Finally, we did not evaluate possible mechanisms of action of body composition variables on bone density.
Weight loss potentially contributes to the development of sarcopenia in aging populations, since lean mass is lost during weight loss, but fat mass is gained during weight gain. Men experience age-related weight loss earlier in life than women and would more likely lose more lean mass than women over their lifespan. Lean mass is associated with muscle strength, bone density, and physical functioning. However, lean mass was closely associated with body weight and fat mass in our study, and it appears that avoidance of weight loss would be of substantial value in preventing the loss of lean mass and related disability in old age.
In conclusion, although the obese elderly may have a unique etiology underlying sarcopenia (sarcopenic obesity), fatness is related to higher lean and bone mass, and weight loss generally causes muscle and bone loss in the elderly. Therefore, maintaining a healthy weight is important for the elderly in order to avoid osteoporosis and sarcopenia.
References
Grisso JA, Kelsey JL, Strom BL, Chiu GY, Maislin G et al (1991) Risk factors for falls as a cause of hip fracture in women. The Northeast Hip Fracture Study Group. N Engl J Med 324:1326–1331
Marcell TJ (2003) Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 58:M911–M916
Walsh MC, Hunter GR, Livingstone MB (2006) Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17:61–67
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D et al (2011) Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci 26:1–8
Foley RN, Wang C, Ishani A, Collins AJ, Murray AM (2007) Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 27:279–286
Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S et al (1990) Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 52:214–218
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Janssen I (2006) Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc 54:56–62
Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W et al (2003) Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc 51:1120–1124
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C et al (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95:1851–1860
Bouchard D, Dionne I, Brochu M (2009) Sarcopenic/obesity and physical capacity in older men and women: data from the nutrition as a determinant of successful aging (NuAge)—the Quebec longitudinal study. Obesity (Silver Spring) 17:2082–2088
Woods J, Iuliano S, King S, Strauss BJ, Walker KZ (2011) Poor physical function in elderly women in low-level aged care is related to muscle strength rather than to measures of sarcopenia. Clin Interv Aging 6:67–76
Lau E, Lynn H, Woo J, Kwok TC, Melton LJ (2005) Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. Gerontol A Biol Sci Med Sci 60:213–216
Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H et al (2010) A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol 110:57–65
Kim M, Baek K, Song K, Kang M, Park C et al (2011) Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the fourth Korea national health and nutrition examination surveys (KNHANES IV) 2009. J Clin Endocrinol Metab 96:3250–3256
Abellan G (2009) Epidemiology and consequences of sarcopenia. J Nutr Health Aging 13:708–712
Flegal KM, Carroll MD, Ogden CL, Johnson CL (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727
Mokdad AH, Stroup DF, Giles WH (2003) Public health surveillance for behavioral risk factors in a changing environment. Recommendations from the behavioral risk factor surveillance team. MMWR Recomm Rep 52:1–12
Yoshiike N, Seino F, Tajima S, Arai Y, Kawano M et al (2002) Twenty-year changes in the prevalence of overweight in Japanese adults: the National Nutrition Survey 1976–95. Obes Rev 3:183–190
Ng S, Norton E, Popkin B (2009) Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med 68:1305–1314
Morley JE (2008) Sarcopenia: diagnosis and treatment. J Nutr Health Aging 12:452–456
Blake GM, Fogelman I (2010) An update on dual-energy x-ray absorptiometry. Semin Nucl Med 40:62–73
Forbes GB (2003) Some adventures in body composition, with special reference to nutrition. Acta Diabetol 40:S238–S241
Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB et al (2005) Weight change and the conservation of lean mass in old age. The Health, Aging and Body Composition Study. Am J Clin Nutr 82:872–878
Roberts SB, Williamson DF (2002) Causes of adult weight gain. J Nutr 132:3824S–3825S
Melton J, Khosla S, Crowson S, Connor M, Fallon W et al (2004) Epidemiology of sarcopenia. J Am Geriatr Soc 48:625–630
Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P, Kooi ME (2006) Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 14:357–367
Floter A, Nathorst-Boos J, Carlstrom K, Ohlsson C, Ringertz H et al (2005) Effects of combined estrogen/testosterone therapy on bone and body composition in oophorectomized women. Gynecol Endocrinol 20:155–1560
Roubenoff R, Hughes VA (2000) Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 55:M716–M724
Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D et al (2004) Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 12:1995–2004
Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V (2008) Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 18:388–395
Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A et al (2004) Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes 28:234–241
Pang MY, Eng JJ, McKay HA, Dawson AS (2005) Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int 16:1769–1779
Juliet E, Nelson B, Roland C, Cyrus C, Steven B et al (2011) Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 124:1043–1050
Orwoll E, Nielson C, Marshall L, Lambert L, Holton K et al (2009) Vitamin D deficiency in older men. J Clin Endocrinol Metab 94:1214–1222
Baird J, Kurshid MA, Kim M, Harvey N, Dennison E et al (2011) Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos Int 22:1323–1334
Acknowledgments
Research project of the Shanghai Municipal Health Bureau.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cheng, Q., Zhu, X., Zhang, X. et al. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metab 32, 78–88 (2014). https://doi.org/10.1007/s00774-013-0468-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0468-3