Abstract
Summary
Although obesity and osteoporosis are important public health problems, the effect of fat mass on bone mass remains controversial. This study demonstrated that fat mass was inversely related to bone mineral content, and abdominal obesity was significantly associated with bone mineral content independent of total fat mass.
Introduction
Obesity and osteoporosis, two disorders of body composition, have become increasingly important public health problems throughout the world. However, the effect of fat mass on bone mass remains controversial. This study investigates the effect of fat mass and regional fat distribution on bone mass within a community-dwelling cohort.
Methods
A total of 3,042 subjects (1,284 men, 362 premenopausal women, and 1,396 postmenopausal women) were studied. Fat mass, percent fat mass, lean mass, percent lean mass, and bone mineral content (BMC) were measured by dual energy X-ray absorptiometry.
Results
Fat mass and percent fat mass decreased significantly across increasing tertiles of BMC in all three subgroups (men, premenopausal and postmenopausal women). In contrast, lean mass and percent lean mass increased significantly across tertiles of BMC in men, and a similar trend was also identified in postmenopausal women. Interestingly, although correlation analysis showed a positive association between fat mass and BMC (p < 0.05), this association became negative after controlling for age and weight (p < 0.05). Finally, in premenopausal and postmenopausal women, subjects with the lowest waist circumference (WC) had the highest BMC in the higher three quartiles of percent fat mass after adjusting for age and weight (p < 0.05), indicating that abdominal obesity is associated with BMC independent of total fat mass.
Conclusion
This study demonstrated that fat mass was inversely related to BMC after removing the mechanical loading effect in Korean men and women. Moreover, abdominal obesity as measured by WC was significantly associated with BMC independent of total fat mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and osteoporosis, two disorders of body composition, have become increasingly important public health problems throughout the world, and Korea is no exception. According to the Korean National Health and Nutrition Examination Survey in 2008, the prevalence of obesity among Korean men and women was 35.6% and 26.5%, respectively [1, 2]. We have previously reported that the crude prevalence of osteoporosis according to the World Health Organization (WHO) criteria [3] in a rural community in Korea (40–79 years old) was 13.1% for men and 24.3% for women. This finding is similar to the numbers reported from Western countries [4, 5].
Although both obesity and osteoporosis generally become more prevalent with advancing age, complex relationships exist between these two conditions. Higher body weight or body mass index (BMI) has been known to be a protective factor against bone loss in both men and women [6–10]. In contrast, a reduction in body weight causes bone loss, although the mechanism of bone loss is not clear [11]. Increasing body weight imparts greater physical loading to bone, which induces an anabolic effect on the bone remodeling process. Indeed, higher mechanical loading has been shown to activate osteocytes, dendritic resident cells, which transduce the signal into anabolic responses, such as the expression of c-fos, insulin-like growth factor-I (IGF-I), and osteocalcin [12]. In this setting, the increasing body weight seems to be protective against osteoporosis. However, overweight status and obesity represent consequences of a gain in fat mass as well as an increase in lean mass. Identification of the specific roles fat mass plays in bone mass regulation is important. Recent publications provided evidence that fat tissue, once considered as a depot for energy substrate, is a metabolically active organ. The fat cells secrete cytokines that regulate a host of physiological processes directly related to carbohydrate and fat metabolism and to the development of cardiovascular disease (CVD) and type 2 diabetes (DM) [12]. For instance, leptin, a key factor regulating appetite and body weight, has been shown to inhibit bone formation through hypothalamic relay of the beta-adrenergic pathway [13]. In addition, adiponectin, an emerging adipokine correlated with insulin sensitivity, increased osteoblast proliferation and differentiation while inhibiting osteoclastogenesis in vitro [14]. Therefore, an increase in fat mass not only increases the risk of CVD but can also negatively regulate bone metabolism. However, these potentially unfavorable effects can be complicated by a concomitant increase in mechanical loading, resulting in the inconsistent results seen in many previous studies. A number of studies have shown that both fat mass and lean mass can contribute to an increase in bone mass, thereby reducing the risk of fractures [10, 15, 16]; others have demonstrated a detrimental effect of fat mass on bone mass after controlling for body weight [12, 17, 18]. Additionally, when considering total fat mass, subcutaneous fat was beneficial to bone mass, whereas visceral fat was deleterious [19].
In this community-based, cross-sectional study, we investigated the effect of fat mass and regional fat distribution on bone mass in a Korean population. Using an approach to isolate the effects of fat mass, we found that fat mass, especially abdominal fat mass, negatively regulates bone mass.
Methods
Study population
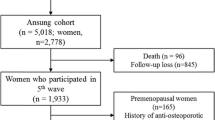
The study population consisted of 1,564 men and 1,862 women >40 years old from a prospective community-based cohort (Ansung cohort). The Ansung cohort was established for the Korean Health and Genome Study in 2001 to investigate the frequency and determinants of chronic diseases in Korea. The Ansung cohort participants include residents in a rural setting 100 km south of Seoul. The farming community of Ansung had a population of 123,906 in 2000. Details of the design of this cohort and the methods used have been previously described [3]. Among the 5,018 subjects of the Ansung cohort, 1,284 males, 362 premenopausal women, and 1,396 postmenopausal females who completed a dual energy X-ray absorptiometry (DXA) between 2006 and 2008 were included in the present study regardless of other underlying co-morbidities. Subjects with a history of estrogen use, glucocorticoid therapy, or medication use for osteoporosis were excluded from the study. Trained staff collected all data using standardized protocols. Study procedures were in accordance with institutional guidelines and approved by an institutional review board. Informed consent was obtained from all study participants.
Measurements of body composition and anthropometric parameters
Height and body weight were measured by standard methods while wearing light clothing. BMI was calculated as weight divided by height squared (kg/m2). Waist circumference (WC) was measured at the narrowest point between the lower limit of the ribcage and the iliac crest. Hip circumference was measured as the maximal circumference over the buttocks. Whole body bone mineral content (BMC, g), bone mineral density (BMD, g/cm2) at the lumbar spine, femoral neck, and total hip, lean mass, and fat mass were measured using DXA (Lunar Prodigy, GE Medical System). The precision error (%CV) was 1.7% for lumbar spine BMD, 1.8% for femoral neck BMD, and 1.7% for total hip BMD. Percent fat mass was calculated as fat mass divided by weight. The details of the method have previously been described [3].
Biochemical parameters
Total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and high-sensitivity C-reactive protein (hsCRP) were measured enzymatically using an autoanalyzer (ADVIA® 1650 Chemistry System; Siemens, Deerfield, IL, USA). The coefficients of variation (%CV) for total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride were 3.0%, 4.0%, 4.0%, and 5.0%. For the evaluation of glucose metabolism, plasma glucose and insulin levels were measured after a 12-h fast. Plasma glucose levels were determined using the hexokinase method. Plasma insulin levels were measured by radioimmunoassay (Linco Research, St Charles, MO, USA). The coefficients of variation for glucose, hsCRP, and insulin were 2.9%, 7.0%, and 10%. The homeostasis model assessment of the insulin resistance (HOMA-IR) was calculated and normalized by logarithmic transformation owing to a skewed distribution.
Statistical analysis
All data were analyzed using SPSS for Windows (Version 16.0, SPSS, Chicago, IL, USA). The values of all variables were presented as mean ± standard deviation (SD) or standard error of the mean (SEM). We compared baseline characteristics among men, premenopausal, and postmenopausal women using analysis of variance (ANOVA). Comparisons of mean values of variables among groups were performed with analysis of covariance (ANCOVA) test adjusted for age and weight. Multiple comparison analyses in each group were performed with post hoc analyses, and the least significant difference test using the t-test was applied in all the multiple comparison analyses. Correlation analyses between body composition parameters and BMC were performed with Pearson’s correlation analysis and partial correlation analysis controlled for age and weight. All tests were two-tailed, and p < 0.05 was considered significant.
Results
The baseline characteristics of the subjects are shown in Table 1. The three groups (men, premenopausal women, and postmenopausal women) have been separately analyzed based on sex and menopausal status. Men exhibited a lower fat mass and a higher lean mass compared to the premenopausal and postmenopausal women. However, the WC and waist-to-hip ratio for men were in between the premenopausal and postmenopausal women. Postmenopausal women showed higher total cholesterol, LDL cholesterol, and triglyceride levels than premenopausal women. Men were less insulin-resistant than premenopausal or postmenopausal women. No significant difference in insulin sensitivity was identified between premenopausal and postmenopausal women.
Differences in the body composition and metabolic profiles across tertiles of BMC were initially examined (Table 2). The ANCOVA test adjusted for age and weight revealed that fat mass and percent fat mass decreased significantly across increasing tertiles of BMC in all three groups. In contrast, lean mass and percent lean mass increased significantly across increasing tertiles of BMC in men. The 3rd tertile of BMC demonstrated the largest lean mass and percent lean mass in postmenopausal women. No significant difference was noted across tertiles in premenopausal women. Notably, indices of abdominal obesity (i.e., WC and waist-to-hip ratio) were lowest in the 3rd tertile of BMC in men and postmenopausal women. HOMA-IR also decreased significantly in men and postmenopausal women across the tertiles of BMC. However, consistent changes in the inflammatory marker, hsCRP, were only observed in postmenopausal women. Collectively, these results suggest that gaining fat mass, abdominal obesity, and increasing insulin resistance are associated with reduced BMC, while the accumulation of lean mass is positively associated with BMC.
To further explore the interaction of fat mass and bone mass, Pearson’s correlation analysis and partial correlation analysis were performed to determine if variables were significantly related with BMC before and after adjusting for age and weight. As shown in Table 3, both lean mass and fat mass were positively associated with BMC in all three groups. However, after controlling for age and weight, the relationship between fat mass and BMC changed from a positive association to a negative association still with statistical significance. This result suggests that fat mass, which apparently affects BMC in a positive way, actually negatively regulates BMC after removing the physical effect of loading. However, partial correlation analyses between fat mass and BMD failed to show an inverse relationship in women (data not shown). In men, fat mass was negatively correlated with femoral neck and total hip BMD (r = −0.130 and −0.112, respectively). WC and waist-to-hip ratio also exhibited negative correlations with BMD after adjusted for age and weight (data not shown).
HOMA-IR was negatively associated with total BMC in men and postmenopausal women after controlling for age and weight. The total cholesterol to HDL cholesterol ratio, suggested as one component of the Framingham risk score (FRS) [20], was also inversely associated with total BMC in men and premenopausal women after adjusting for age and weight. In premenopausal women, the HDL cholesterol and TG have a positive and negative association with BMC, respectively, after adjusting for age and weight. In both premenopausal and postmenopausal women, hsCRP was negatively associated with BMC after adjustment for age and weight (Table 3).
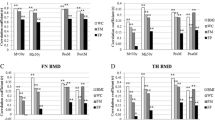
Having identified a negative role of fat mass on BMC, we then studied whether abdominal obesity measured by WC or truncal fat was related to BMC in groups stratified by percent fat mass after controlling for age and weight. In men, within a given quartile of percent fat mass, the BMC did not differ within the tertiles of WC (Fig. 1a). However, in women, negative association between WC and BMC was observed within the 1st and 2nd quartiles or higher three quartiles of percent fat mass in premenopausal (Fig. 1b) or postmenopausal women (Fig. 1c), respectively. Similar associations were also observed when we stratified by truncal fat obtained from DXA instead of WC. As shown in Fig. 1d, within the given stratum except the 2nd quartile, men with the highest truncal fat mass had the lowest BMC. Postmenopausal women with the highest truncal fat mass also showed the lowest BMC within the 3rd and 4th quartiles (Fig. 1f), although we were unable to find similar association in premenopausal women (Fig. 1e). These results suggest that within a given fat mass, the higher abdominal fat plays an important role in negatively regulating bone mass, especially in postmenopausal women.
Finally, the lack of significant association between BMD and fat mass in women prompted us to study whether the bone area is related with body size or body composition parameters, thereby masking the effects of fat mass on bone mass. As shown in Table 4, when we evaluated the correlation between whole body bone area obtained from DXA and height or BMI as a surrogate for body size, we found a positive correlation between these parameters. In addition, bone area is also significantly correlated with body composition parameters including fat mass and bone mass (Table 4).
Discussion
In a Korean community-based, cross-sectional study, we demonstrated that in the absence of its physical loading effect, fat mass has a negative association with BMC. Additionally, we found that among the fat mass, abdominal fat plays an important role in negatively regulating bone mass.
In an analysis that compared the mean values of fat and lean body mass across tertiles of BMC, both the fat mass and percent fat mass decreased as the BMC increased after adjusting for age and weight. The interaction between adiposity and bone mass has long been the subject of interest in epidemiological studies. In one study on a cohort of 140 healthy postmenopausal women, a real BMD at any site was positively related to weight, BMI, and fat mass (r ≈ 0.5) and less closely related to lean mass (r ≈ 0.2) [10]. The same group reported that BMD was dependent on both fat and lean mass in premenopausal women; however, male BMD was related only to lean mass [15]. Furthermore, baseline fat mass and changes in fat mass were predictive of changes in BMD over 10 years in postmenopausal women [21]. Another study on 921 African–American, Asian, Latino, and Caucasian women 20 to 25 years old found that fat mass was positively correlated with BMD at all skeletal sites [22]. The early postmenopausal intervention cohort (EPIC) study has also shown that fat mass is the principal soft tissue determinant of BMD at baseline and the determinant for the rate of change in BMD over 2 years [8]. It has been suggested that fat mass exerts an anabolic effect on bone, in addition to skeletal loading, which is associated with increased concentrations of free sex hormones, and increased insulin, amylin, and leptin levels [23–25].
These results contradict our study results that demonstrated a negative role of fat mass on BMC. This discrepancy might originate from the interference of the mechanical loading effect of fat tissue on the bone mass, which was not completely excluded in the previous studies. Indeed, it is difficult to exclude and control the effect of weight in these studies due to the strong collinearity between fat mass and body weight. To overcome this limitation, we carried out ANCOVA test analyzed with a general linear model using age and body weight as a covariate, whereas other previous studies used multivariable linear regression analyses. Using this approach, we demonstrated that the positive relationship between fat mass and bone mass became negative after adjusting for age and weight. Lean mass, however, maintains a positive relationship with bone mass after adjusting for age and weight. Our results are supported by a previous study by Zhao et al. [18] who also demonstrated an inverse relationship between fat mass and total BMC, lumbar spine, or femoral neck BMD by multivariate linear regression analyses after incorporating body weight as a covariate in both Chinese and Caucasians. In another study of a large cohort of Chinese by Hsu et al. [17], a negative relationship between percent fat mass and total hip BMC was identified, and the risks of osteoporosis, osteopenia, and nonspine fractures were significantly higher for subjects with higher percents of fat mass, independent of body weight.
Interestingly, we found sex difference in many of the correlations. It has been well established that for a given BMI, men have more lean mass, whereas women have higher adiposity [26]. In addition, men have more visceral adipose tissue, while women have more peripheral or subcutaneous adipose tissue. These differences, as well as differences in sex hormones and adipokines, may contribute to the gender difference in insulin resistance [26]. We speculate that this differential fat distribution depending on gender may have resulted in sex difference in many of the correlations.
In this study, the total BMC was analyzed according to tertiles of WC stratified by percent fat mass after controlling for age and weight. Women with higher WC demonstrated lower BMC. Truncal fat mass obtained from DXA was also negatively associated with BMC in men and postmenopausal women. These results suggests that within a given fat mass, higher abdominal fat is important in negatively regulating bone mass, which is particularly important in postmenopausal women. It has been known that abdominal obesity is highly associated with insulin resistance and metabolic syndrome [27]. In support of this notion, we were also able to find a negative relationship between BMC and HOMA-IR. Abdominal fat is comprised of visceral and subcutaneous fat. Although we have not evaluated the composition of visceral vs. subcutaneous fat in this study, previous studies have demonstrated that visceral fat has been shown to be distinct from subcutaneous fat in its effect on bone metabolism [29, 30]. Indeed, Gilsanz et al. [19] found that the visceral fat exerts negative effects on femoral bone mass and strength, while the subcutaneous fat has positive effects. In another study conducted in 461 healthy Korean subjects, the visceral fat area on abdominal computed tomography scan was inversely associated with BMD, whereas the subcutaneous fat area showed no significant association [28]. The relative role of visceral vs. subcutaneous fat in the regulation of bone mass should be further studied.
In this study, BMC was used as a dependent variable for bone mass instead of BMD. Although BMD has been widely accepted as a surrogate measure for osteopenia and osteoporosis, it does not provide adequate correction for the bone and body size [31]. Since DXA measures areal, not volumetric, BMD, it may artificially overestimate the relative bone mass for people with bones larger than average and underestimate bone mass for those with smaller bones [31]. Moreover, the negative correlation between fat mass and BMC disappeared when we substituted BMD for BMC in women. The latter finding seems to result from the fact that bone area is highly correlated with body size as measured by height or BMI (Table 4), thereby obliterating the correlation between fat mass and bone mass. In addition, the bone area is also significantly correlated with fat mass or lean mass. Therefore, we suggest that it would be more reasonable to use BMC for the analysis of the interactions between body composition parameters and bone mass.
The present study cannot overcome the limitation of its cross-sectional nature, and therefore, the inverse relationship between bone mass and fat mass or abdominal fat in this study could not be interpreted as a causal relationship. In addition, this study was conducted within a single community, and therefore it cannot be generalized for the whole Korean population. Future longitudinal studies will be helpful in clarifying the relationship regarding changes in bone mass and fat mass.
Our findings have clinical implications suggesting that gaining fat mass may not be protective in the maintenance of bone mass and that losing fat mass may not increase the risk for osteoporosis. In conclusion, fat mass was inversely correlated to bone mass after removing the mechanical loading effect in both men and women within a Korean community-based-cohort. Furthermore, in women, a high WC, as a surrogate for abdominal obesity, has an additional impact on bone mass in subjects with high fat mass.
References
Korea National Statistical Office (2010) Statistics Korea
Weaver CM (2010) Role of dairy beverages in the diet. Physiol Behav 100:63–66
Shin CS, Choi HJ, Kim MJ et al (2010) Prevalence and risk factors of osteoporosis in Korea: a community-based cohort study with lumbar spine and hip bone mineral density. Bone 47:378–387
Cheng H, Gary L, Curtis J, Saag K, Kilgore M, Morrisey M, Matthews R, Smith W, Yun H, Delzell E (2009) Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int 20:1507–1515
Looker A, Melton L III, Harris T, Borrud L, Shepherd J (2010) Prevalence and trends in low femur bone density among older US adults: NHANES 2005–2006 compared with NHANES III. J Bone Miner Res 25:64–71
Wardlaw GM (1996) Putting body weight and osteoporosis into perspective. Am J Clin Nutr 63:433S–436S
Edelstein SL, Barrett-Connor E (1993) Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol 138:160–169
Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C (1999) Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early postmenopausal intervention cohort (EPIC) study group. J Bone Miner Res 14:1622–1627
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31:547–555
Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, Lim TM, Cundy TF (1992) Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab 75:45–51
Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T (2003) Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg 13:383–388
Zhao L, Jiang H, Papasian C, Maulik D, Drees B, Hamilton J, Deng H (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23:17–29
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Williams G, Wang Y, Callon K, Watson M, Lin J, Lam J, Costa J, Orpe A, Broom N, Naot D (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150:3603
Reid I, Plank L, Evans M (1992) Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab 75:779–782
Khosla S, Atkinson EJ, Riggs BL, Melton LJ III (1996) Relationship between body composition and bone mass in women. J Bone Miner Res 11:857–863
Hsu Y, Venners S, Terwedow H, Feng Y, Niu T, Li Z, Laird N, Brain J, Cummings S, Bouxsein M (2006) Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr 83:146
Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW (2007) Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92:1640–1646
Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD (2009) Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94:3387–3393
Expert Panel on Detection E, Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285:2486–2497
Wu F, Ames R, Clearwater J, Evans MC, Gamble G, Reid IR (2002) Prospective 10-year study of the determinants of bone density and bone loss in normal postmenopausal women, including the effect of hormone replacement therapy. Clin Endocrinol (Oxf) 56:703–711
Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB (2005) The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone 37:474–481
Cornish J, Reid IR (2001) Effects of amylin and adrenomedullin on the skeleton. J Musculoskelet Neuronal Interact 2:15–24
Goulding A, Taylor RW (1998) Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int 63:456–458
Reid IR, Evans MC, Cooper GJ, Ames RW, Stapleton J (1993) Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol 265:E655–E659
Geer EB, Shen W (2009) Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6(Suppl 1):60–75
Alberti K, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062
Choi HS, Kim KJ, Kim KM, Hur NW, Rhee Y, Han DS, Lee EJ, Lim SK (2010) Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int 87:218–225
Wajchenberg B (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21:697
Heiss CJ, Sanborn CF, Nichols DL, Bonnick SL, Alford BB (1995) Associations of body fat distribution, circulating sex hormones, and bone density in postmenopausal women. J Clin Endocrinol Metab 80:1591–1596
Prentice A, Parsons T, Cole T (1994) Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr 60:837–842
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, J.H., Choi, H.J., Kim, M.J. et al. Fat mass is negatively associated with bone mineral content in Koreans. Osteoporos Int 23, 2009–2016 (2012). https://doi.org/10.1007/s00198-011-1808-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1808-6