Abstract
Summary
The prevalence of sarcopenia increases with age. The diagnosis of sarcopenia relies in part on normative data on muscle mass, but these data are lacking. This study provides population-based reference data on muscle mass in young men, and these results may be used clinically for the diagnosis of sarcopenia in men.
Introduction
The ageing population increases the prevalence of sarcopenia. Estimation of normative data on muscle mass in young men during the peak of anabolic hormones is necessary for the diagnosis of sarcopenia in ageing males. The purposes of this study were to provide population-based reference data on lean body mass (LBM) in young men during the time of peak levels of GH/IGF-1 and testosterone and further to apply the reference data on a population-based sample of men aged 60–74 years to estimate the prevalence of sarcopenia.
Methods
This is a cross-sectional, population-based single-centre study. Our participants are from random selection of 783 men, aged 20–29 years, and 600 men, aged 60–74 years. LBM was assessed by dual-energy X-ray absorptiometry (DXA). LBM T-scores were calculated on the basis of LBM in the young participants. Muscle function in the lower extremities was measured using a leg extension power (LEP) rig in the ageing participants.
Results
Total lean body mass (TLB) was (mean (SD)) 64.7 kg (6.8) in the young and 60.4 kg (6.4) in the ageing men (p < 0.001). Lower extremity lean mass (LELB) was 22.0 kg (2.6) in the young and 19.2 kg (2.4) in the ageing men (p < 0.001). In the ageing men, TLB and LELB T-scores were −0.64 (0.94) and −1.09 (0.94). A total of 4.8 and 8.5 % had a TLB or LELB T-score of less than −2 and a LEP in the lowest quartile.
Conclusions
This study provides population-based reference data on LBM in men, and these data may be used clinically for the diagnosis of sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous important health-related outcomes may be negatively affected by the reduction in skeletal muscle mass and function with ageing [1–3]. The age-related change in muscle mass and function is generally referred to as sarcopenia [4]. Previously, sarcopenia was described on the basis of measurement of muscle mass by dual-energy X-ray absorptiometry (DXA) [5–7]. Even though muscle mass and strength are both associated with age, muscle function deteriorates to a larger extent than muscle mass [8, 9]. Allowing the use of different methods of diagnosing sarcopenia, the European Working Group on Sarcopenia in Older People (EWGSOP) recently defined sarcopenia as low muscle mass and reduced muscle function [10]. Using the bottom thirds of lean body mass (total lean body mass (TLB)) measured by DXA to identify individuals with low muscle mass, the prevalence of sarcopenia based on the EWGSOP definition was 6.8 and 4.6 % in two British cohorts of men aged 73 and 67 years, respectively [11]. By comparison, 14.5 % men aged 65–74 years in a French study were sarcopenic based on normative data from 107 men aged 18–40 years from New Mexico in the USA [12]. These differences in the prevalence of sarcopenia may be explained by dissimilar definitions of low muscle function and mass, in part due to absence of established reference data [11], as well as different diagnostic criteria [13]. Furthermore, the prevalence of sarcopenia is highly influenced by the age of the study population [5, 14]. Normative reference data retrieved from population-based studies of young healthy individuals are required in order to designate a threshold below which the level of muscle mass may be considered deficient. Therefore, the main objective of the present study was to provide reference data on peak lean body mass in a population-based sample of men aged 20–29 years. Moreover, we aimed to assess the importance of obesity defined as a body mass index (BMI) of more than 30 kg/m2 and comorbidities on lean body mass in a population-based sample of men aged 60–74 years. Leg extension power (LEP), which has been related to mobility [15], falls [16], activities of daily living [17] and all-cause mortality in patients with peripheral arterial disease [18], was measured in the ageing participants of this study, and measures of lean mass and LEP were used to calculate the prevalence of sarcopenia.
Subjects and methods
Study populations
The Odense Androgen Study is an observational, single-centre study on endocrine status and body composition in two population-based samples of young and ageing Danish men, respectively. Detailed information on the procedures used for the recruitment of the study populations has been described elsewhere [19, 20]. The Danish Civil Registration System (CRS), which comprises a unique identification number of each Danish citizen, was used for the recruitment of both young and the ageing participants in 2003–2004 and 2004–2005, respectively.
Young study population
A random sample of 3000 men, aged 20–29 years, received a mailed questionnaire on demographics, physical activity, medical history, medication and lifestyle factors. A total of 73 % responded to the mail, and among these, 783 men agreed to participate in clinical investigations. Due to rearrangements of the original appointments, three individuals were included prior to their 20th birthday, and two individuals were included after their 30th birthday. Using data on age, socioeconomics and health, those included in the study were found to be comparable to the background age- and sex-matched population.
Ageing study population
A random sample of 4975 men aged 60–74 years were retrieved from the CRS, and each of these received a mailed questionnaire analogous to that used in the young study population. Eighty-five per cent responded to the questionnaire. An age-stratified random sample of 1845 of these respondents was asked to participate in further investigations, and a total of 946 accepted to partake in further investigations. Phone interviews were performed in 846; 697 men accepted additional clinical investigations, and 600 men were consecutively invited for clinical investigations. The prevalence of comorbidities was comparable to the background population with the exception of pulmonary diseases, which were less common in the ageing study participants [20]. All participants were Caucasians.
Stratification of the ageing study population
A healthy subpopulation without small testicles (total testes volume <9 ml, n = 41), excessive consumption of alcohol (>42 units per week. n = 14), treatment with systemic corticosteroids (n = 3), use of anabolic drugs (n = 5) or chronic diseases or current non-steroid or anabolic medications (n = 384) was identified among the senior participants [21]. Furthermore, the ageing study population was stratified according to their BMI. Individuals with a BMI exceeding 30 kg/m2 were categorized as obese (n = 126).
The local ethics committee approved both studies, and all participants received written and oral information before written consent. The study was performed in accordance with the Helsinki II Declaration and registered in ClinicalTrials.gov as NCT00155961 and NTC0015016, respectively.
Body composition
Body weight was measured to the nearest 0.1 kg (SECA, Hamburg, Germany), height was determined to the nearest 0.1 cm (Harpenden stadiometer, Crymych, UK), and BMI was calculated as weight divided by height squared (kg/m2). Lean mass was measured by use of dual-energy X-ray absorptiometry (DXA, H4500, Hologic Inc., Waltham, MA, USA) in all participants with the exception of those with metal implants or other features that would render the DXA scan analysis impossible. Total lean body mass (TLB) and lower extremity lean mass (LELB) were assessed in order to investigate if these measurements explained a similar proportion of the variation in physical performance of the leg.
Physical performance
A leg extensor power rig (Medical Engineering Unit, University of Nottingham, Nottingham, UK) was used to measure leg extension power (LEP), which is defined as the ability of the leg extensor muscles to perform work over periods of half a second or less [22], in those aged 60 years or more. The power rig has previously been shown to provide reproducible measures of LEP in ageing men [22, 23]. The participants sat in a chair with one foot on a lever, which was attached to a flywheel, and the force was measured every time the participant depressed the lever. Ten tests of the non-dominant leg were performed in each individual, and the three best attempts were used in the analyses. Seven participants were not tested due to knee pain or other motion-limiting disabilities.
Smoking and alcohol
Smoking was classified as current smoker or non-smoker (including ex-smoker). Intake of alcohol was classified as units per week.
Statistics
Data are shown as mean (±standard deviation) or median [25–75 percentiles] according to distribution. The young and senior participants including those ageing individuals, who were considered healthy, i.e. reported no concurrent diagnoses or medication, or were obese, were compared using Student’s t test or non-parametric tests as appropriate.
Measures of LBM obtained in the young participants were used as a reference in order to calculate T-scores of TLB and LELB in the entire group of senior participants as well as the healthy senior or obese subgroups. Subsequently, the prevalence of T-scores of one, two or less below the mean in the young individuals was calculated in the senior population as well as subgroups. The distributions of T-scores were assessed using chi-square test.
Statistical analyses were performed using Stata, release 11 (Stata Corp, College Station, TX, USA).
Results
General characteristics
Information regarding anthropometrics including physical examination, concomitant diseases and medications was available in 783 of the young and 593 of the ageing participants. Median ages of the two study populations were 25.7 [23.1–27.8] and 68.6 years [65.0–72.1], respectively. Height and weight differed significantly between the young and senior participants (181.7 (6.7) vs. 174.3 cm (6.7) and 81.8 (12.1) vs. 83.7 kg (12.5), respectively, p < 0.001), and BMI was lower in the younger study population (24.4 (3.4) vs. 27.6 kg/m2 (4.0), p < 0.001). In total, 189 of the ageing participants had testis volume of 9 ml or greater and did not report chronic disease, concomitant medication including corticosteroids and anabolic drugs, or excessive alcohol intake.
Weight was lower among the 194 healthy ageing men compared to the remaining ageing population (80.7 (11.0) vs. 85.1 kg (12.9), p < 0.001). There were no differences in height in the subgroup analyses (Table 1).
The frequency of smoking was similarly common between both study populations (26.1 vs. 24.4 %, NS), whereas alcohol intake was lower in the younger study population (eight units per week [5–15] vs. 10 units per week [6–19], p < 0.05).
Lean body mass
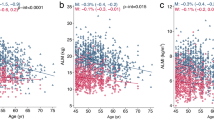
Measurements of TLB and LELB mass were available in 779 (99.5 %) of the young participants and 569 (96.0 %) of the ageing participants. The distributions of TLB and LELB according to age are presented as scatter and locally weighted scatterplot smoothing (LOWESS) plots in Fig. 1. Neither TLB nor LELB was associated with age in the young participants (R = 0.15 (−0.02; 032), p = 0.08 and R = 0.03 (−0.03; 0.10), p > 0.1). In the ageing participants, age TLB and LELB were both inversely associated with age (R = −0.21 (−0.34; −0.08) and R = −0.08 (−0.13; −0.04), both p = 0.001). Both TLB and LELB were higher in the young study population compared to the ageing (64.7 (6.8) vs. 60.4 kg (6.4) and 22.0 (2.6) vs. 19.2 kg (2.4), p < 0.001). No difference in TLB or LELB between healthy ageing participants and the remaining ageing population was observed (Table 1). In contrast, TLB and LELB were higher in the obese than those in the lean participants (66.1 (5.4) vs. 58.8 kg (5.6) and 21.0 (2.3) vs. 18.7 kg (2.2), p < 0.001). Relative to total body weight, TLB and LELB were lower in obese ageing participants (Table 1).
TLB and LELB T-scores in the ageing participants were −0.64 (0.93) and −1.088 (0.94), respectively. TLB T-scores were below 0, −1, −2 and −3 in 76.3, 34.4, 7.7 and 0.7 % of the senior study population, whereas the prevalence of LELB T-scores below 0–1, −2 or −3 was 67.5, 28.3, 15.8 and 2.6 % of the senior participants, respectively (Table 2). While mean TLB T-scores were similar in healthy and the other ageing participants (Table 2), both TLB and LELB T-scores were higher among the obese individuals (0.24 (0.79) vs. −0.87 (0.83) and −0.37 (0.89) vs. −1.28 (0.86), both p < 0.001).
LEP in ageing men
Due to physical disabilities, measures of muscle power were available in 587 of the 593 participants (99.0 %). LEP was higher in healthy ageing men compared to that in the other ageing men (205 (57) vs. 187 W (57), p < 0.001) and higher in obese than that in lean ageing participants (204 (61) vs. 190 W (57), p < 0.05).
The relationships between LEP and age are presented as a scatter and LOWESS plot in Fig. 2. LEP appeared to decline continuously with age. Furthermore, the association between LEP and lean body mass was continuous without any indication of a potential cut-off value that could be used for the identification of individuals with a low muscle power based on lean mass measured by DXA. The relationships between lean mass T-scores and LEP are presented in Table 3. In all, 4.8 % of those with a LEP in the lowest quartile had a TLB T-score of less than −2, and 6.4 % of those with a LEP in the lowest two quartiles had a TLB T-score of less than −2. Among those with a LEP in the lowest quartile, 8.5 % had a LELB of less than −2, whereas 10.4 % of those with a LEP in the lowest two quartiles had a LELB T-score of less than −2 (Table 3).
Discussion
Normative data on lean mass
The primary aim of this study was to provide normative data on lean mass in men. We did not observe any age-related changes in lean mass in the young participants, suggesting that men aged 20–30 years have reached peak lean mass or may continue to gain lean mass in the fourth decade of life. Compared to previous reports on lean mass assessed by DXA such as the study by Rosetta (n = 107) [3] and the European multicentre MYOAGE (n = 86) studies [13], our study included a significantly larger, uniform and population-based group of individuals at time of maximal levels of muscle mass. Thus, the present study provides normative data for peak total body and LELB measured by DXA in young men, which may be used for the diagnosis of sarcopenia in ageing men.
Prevalence of sarcopenia based on lean mass and LEP
We applied the estimates of normal lean mass to the population-based cohort of ageing men aged 60–75 years and found that 7.7 and 15.6 % of these men had a TLB or LELB T-score of less than −2. The European Working Group on Sarcopenia in Older People (EWGSOP) recommends that sarcopenia is diagnosed on the basis of low muscle mass as well as impaired physical performance or reduced muscle strength or physical performance [10]. Low muscle power defined as a LEP in the lowest quartile and total body or LELB T-score of −2 or less was observed in 4.8 and 8.5 % of the ageing participants, respectively. The prevalence of sarcopenia in men participating in the Hertfordshire Sarcopenia Study (mean age 73 years) and the Hertfordshire Cohort Study (mean age 67 years) was 6.8 and 4.6 %, respectively [11], which appears to be in line with our results, possibly due to similar age of the participants. By contrast, the prevalence of sarcopenia among Italian men aged more than 80 years was 17.4 % [24]. Based on the EWGSOP criteria, 14.5 % of French men aged 65–74 years and 45.5 % of men aged more than 85 years were sarcopenic, whereas 13.8 % of Korean men aged more than 65 years had sarcopenia [24]. Differences in the prevalence of sarcopenia between our study and some of the previous investigations may be explained by dissimilar measurements of muscle body mass and reference data, varying methods used for the assessment of muscle strength or physical performance and, importantly, differences in the age of the study populations. In addition, selection bias could influence the estimates of sarcopenia.
Lean mass in ageing men
As shown by Bijlsma et al. [13], the relationships between tests of physical function vary with the criteria used to express the level of muscle mass. In the present study, absolute measures of total and LELB were used in order to provide unbiased data on whole body lean mass as well as lean mass at the site where muscle performance was investigated, but relative lean mass could be calculated on the bases of the results. Because vertebral fractures were previously reported in 6.3 % of the ageing participants in this study [25], we did not report lean mass relative to body height. Fractures as well as secular trends may explain the observed differences in height between the study populations, which may influence the comparison of measures of lean mass if normalized on the basis of height. We observed an inverse association between muscle mass assessed as TLB or LELB and age, which is in line with previous studies [5, 7]. Lean mass was higher in obese individuals, but the relative level was lower than in their lean counterparts.
Muscle power
While muscle mass was the same in healthy and the remaining ageing participants, muscle power was higher in healthy individuals, strengthening the notion that muscle mass does not appropriately identify those with reduced muscle strength. In this study, leg extensor power was used to assess muscle function. LEP has been defined as the capacity of leg extensor muscles to perform work over half a second or less [22]. We observed an inverse association between LEP and age in men, which is in line with several other studies [22, 26]. There was no suggestion of a lean mass level below which LEP was substantially lower. These observations offer no specific thresholds for lean mass or muscle power that are suitable for the diagnosis of sarcopenia, but longitudinal studies may help in providing information on suitable cut-offs for LEP and lean mass that identify individuals at particular risk of consequences of sarcopenia. Recently, Studenski et al. [27] provided cut points for low hand-grip strength and appendicular lean mass adjusted for BMI that were based on impaired mobility, i.e. gait speed, in ageing individuals rather than on a comparison with young, healthy individuals. Although different, both methodologies may prove useful in identification of individuals with sarcopenia or impaired physical performance.
Strengths and limitations
Our study has a number of limitations. We used a power rig to measure muscle power, but we did not perform formal tests of physical performance. Assessment of grip strength, chair stand and gait speed or similar tests of physical performance may provide information beyond that gained from investigations of muscle power. Although the direction of the effect is unknown, this may have influenced our estimate of the prevalence of sarcopenia. Also, the prevalence of sarcopenia may be different, if the prevalence was derived using the EWGSOP flow algorithm. Comparative data on LEP was unavailable because of lack of access to the power rig at time of inclusion of the young participants. Furthermore, this study only comprises male Caucasians, and reference data from study populations with women and with different genetic and environmental background are required. The major strengths of this study are the population-based design and the size of the study populations, which includes both young and ageing men. Furthermore, we assessed lean mass in the young men prior to the time of major decrease in IGF-1 and at the time of maximum levels of testosterone.
In conclusion, we provide population-based reference data for lower extremity and total body lean mass at time of peak levels of hormones known to be anabolic to muscle mass. We estimate that the prevalence of sarcopenia in a population-based sample of men aged 60–74 years is 4.8–8.5 % depending of the site used for the measurement of lean mass.
References
Delmonico MJ, Harris TB, Lee JS et al (2007) Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 55(5):769–774
Roubenoff R (2001) Origins and clinical relevance of sarcopenia. Can J Appl Physiol 26(1):78–89
Gallagher D, Visser M, De Meersman RE et al (1997) Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol 83(1):229–239
Wickham C, Cooper C, Margetts BM, Barker DJ (1989) Muscle strength, activity, housing and the risk of falls in elderly people. Age Ageing 18(1):47–51
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50(5):889–896
Melton LJ 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL (2000) Epidemiology of sarcopenia. J Am Geriatr Soc 48(6):625–630
Ferrucci L, Guralnik JM, Buchner D et al (1997) Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the women’s health and aging study. J Gerontol A Biol Sci Med Sci 52(5):M275–M285
Goodpaster BH, Park SW, Harris TB et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10):1059–1064
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39(4):412–423
Patel HP, Syddall HE, Jameson K et al (2013) Prevalence of sarcopenia in community-dwelling older people in the UK using the European working group on sarcopenia in older people (EWGSOP) definition: findings from the Hertfordshire cohort study (HCS). Age Ageing 42(3):378–384
Cherin P, Voronska E, Fraoucene N, de Jaeger C (2014) Prevalence of sarcopenia among healthy ambulatory subjects: the sarcopenia begins from 45 years. Aging Clin Exp Res 26(2):137–146. doi:10.1007/s40520-013-0132-8.
Bijlsma AY, Meskers CG, van den Eshof N et al (2014) Diagnostic criteria for sarcopenia and physical performance. Age (Dordr) 36(1):275–285
Morley JE (2008) Sarcopenia: diagnosis and treatment. J Nutr Health Aging 12(7):452–456
Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L (2003) A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci 58(8):728–733
Skelton DA, Kennedy J, Rutherford OM (2002) Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing 31(2):119–125
Kuo HK, Leveille SG, Yen CJ et al (2006) Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the national health and nutrition examination survey (NHANES), 1999–2002. Am J Phys Med Rehabil 85(8):650–658
McDermott MM, Liu K, Tian L et al (2012) Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol 59(13):1159–1167
Nielsen TL, Wraae K, Brixen K, Hermann AP, Andersen M, Hagen C (2006) Prevalence of overweight, obesity and physical inactivity in 20- to 29-year-old, Danish men. Relation to sociodemography, physical dysfunction and low socioeconomic status: the Odense androgen study. Int J Obes (Lond) 30(5):805–815
Frost M, Wraae K, Gudex C et al (2012) Chronic diseases in elderly men: underreporting and underdiagnosis. Age Ageing 41(2):177–183. doi:10.1093/ageing/afr153.
Frost M, Wraae K, Nielsen T et al (2013) Similar reference intervals for total testosterone in healthy young and elderly men: results from the Odense Androgen Study. Clin Endocrinol (Oxf) 78(5):743–751. doi:10.1111/cen.12042.
Bassey EJ, Short AH (1990) A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol 60(5):385–390
Blackwell T, Cawthon PM, Marshall LM, Brand R (2009) Consistency of leg extension power assessments in older men: the osteoporotic fractures in Men (MrOS) study. Am J Phys Med Rehabil 88(11):934–940
Volpato S, Bianchi L, Cherubini A et al (2014) Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci 69(4):438–446. doi:10.1093/gerona/glt149.
Frost M, Wraae K, Abrahamsen B et al (2012) Osteoporosis and vertebral fractures in men aged 60–74 years. Age Ageing 41(2):171–177
Aadahl M, Beyer N, Linneberg A, Thuesen BH, Jorgensen T (2011) Grip strength and lower limb extension power in 19–72-year-old Danish men and women: the Health2006 study. BMJ Open 1(2):e000192
Studenski SA, Peters KW, Alley DE et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69(5):547–558
Funding sources
Financial support was received from the Novo Nordisk Foundation, World Anti-Doping Agency, the Danish Ministry of Culture, the Clinical Research Institute at the University of Southern Denmark, Novo Nordisk Scandinavia and Pfizer Denmark.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frost, M., Nielsen, T.L., Brixen, K. et al. Peak muscle mass in young men and sarcopenia in the ageing male. Osteoporos Int 26, 749–756 (2015). https://doi.org/10.1007/s00198-014-2960-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2960-6