Abstract
Background
Declines in muscle mass and function are inevitable developments of the advanced aging process. Corresponding dimensions of longitudinal changes in at-risk populations are still scarce although clinically relevant. The present study monitored changes in morphologic and functional sarcopenia criteria related to sarcopenia in older men with low muscle mass over a period of 24 months.
Objectives
The main objective of the present study was to determine whether changes in muscle mass and function were comparable across the body. Our hypothesis was that both (1) fat free mass (FFM) and (2) function decline at a significantly higher rate in the lower versus the upper extremities.
Design
We conducted an observational study of 24 months.
Setting
Community dwelling men living in the area of Northern Bavaria were initially included in the Franconian Sarcopenic Obesity (FranSO) study by the Institute of Medical Physics University of Erlangen-Nürnberg, Germany.
Participants
One hundred and seventy-seven (177) men (77.5±4.5 years) within the lowest skeletal muscle mass index (SMI) quartile of the FranSO study were included in the present 24 month analysis.
Measurements
Fat free mass (direct-segmental, multi-frequency Bio-Impedance-Analysis (DSM-BIA)), handgrip strength (hand-dynamometer) and 10-m habitual gait velocity (photo sensors) were assessed at baseline and 24-month follow-up.
Results
Lower extremity fat free mass (LEFFM: −2.0±2.4%), handgrip strength (−12.8±11.0%) and gait velocity (−3.5±9.0%) declined significantly (p<.001) during the follow-up period, while upper extremity FFM was maintained unchanged (UEFFM: 0.1±3.1%). Changes in LEFFM were significantly higher (p<.001) compared with UEFFM, however contrary to our expectation the decline in handgrip strength representing upper extremity muscle function was 3.7-fold higher (p<.001) than the decline in gait velocity.
Conclusion
Medical experts involved in diagnosis, monitoring and management of sarcopenia should consider that parameters constituting morphologic and functional sarcopenia criteria feature different rates of decline during the aging process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Declines in muscle mass and function are inevitable developments during the aging process in later life stages. However, what is the “normal age-appropriate” decline of muscle mass and function, i.e. muscles’ ability to produce force and motion in older adults? Several cross- and longitudinal studies focus on this issue (review in (1–3)). Relying on longitudinal studies that determined changes in fat free mass (FFM)(4–6), reductions of 0.5–0.8% p.a. for Caucasian men 70 years+ were reported. In parallel, the same studies observed a 3–5-fold higher decline in muscle strength compared with FFM (4–7). However, there is some evidence that both, changes in muscle mass and function are not uniform across the body (review in (1, 2)). Most but not all of the few studies that address this issue usually observed higher reductions of muscle mass and function in the lower compared with the upper limbs (8–13). Considering the highly relevant effect of lower limb mass and performance on mobility limitations, falls, disability, morbidity and mortality in older people (14, 15), it is important to determine whether and to which degree, non-uniform, age-dependent changes in upper vs. lower extremity muscle mass and function occurred in populations with or with increased risk for sarcopenia.
Thus, the aim of the present study was to determine longitudinal changes in muscle parameters in older men with low muscle mass over a period of 24 months. Our hypotheses were that both (1) fat free mass and (2) muscle-function decline at a significantly higher rate in the lower versus the upper extremities.
Methods
The present analysis is part of the Franconian Osteoporosis and Sarcopenia (FrOST) project and focuses on community-dwelling (cdw) men with low skeletal muscle mass of the Franconian Sarcopenic Obesity study (FranSO)(16). The Institute of Medical Physics, University of Erlangen-Nürnberg (FAU), Germany, initiated the project, which was approved by the University Ethics Committee of the FAU (Ethikantrag 67_15b and 4464b). After detailed information, all study participants gave written informed consent.
Participants
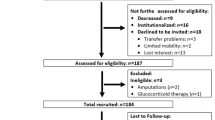
Briefly, in January/February 2018, i.e. two years after the baseline assessment (16), men initially 70 years or older and within the lowest skeletal muscle mass index quartile (SMI: ≤7.78 kg/m2) of the FranSO-study (n=242) were contacted by personalized letters and subsequent phone calls. After applying inclusion criteria for the 2-year follow-up (FU) assessment, i.e. (1) community-dwelling (cdw) status (2) no amputations of limbs or cardiac pacemaker implants (3) no implementation of glucocorticoid therapy >7.5 mg/d (4) any cognitive impairments that confounded the assessments, 177 men were eligible and willing to participate. Fig. 1 gives reasons for loss to follow-up. Of relevance, we were unable to determine the status of 12 men, although citizen registers and obituary columns were carefully checked and neighbors were contacted. Table 1 gives the structured baseline characteristics of the 242 subjects contacted.
Outcomes
Primary outcome
-
Changes of upper and lower extremity fat free mass during 24 months of observation.
Secondary outcome
-
Changes of functional muscle parameters related to recent sarcopenia operationalization’s (eg. (17)) during 24 months of observation:
-
habitual gait velocity (lower extremity function)
-
handgrip strength (upper extremity function)
-
Experimental outcomes
-
Changes of the Late Life Function and Disability Instrument (LLFDI) (18) during 24 months of observation:
-
basic and advanced lower extremity function
-
upper extremity function
-
Measurements
Baseline and follow-up assessments were conducted applying the same calibrated devices in exactly the same sequence and at the same time of the day.
Anthropometry
Body mass and composition were assessed after 8 h of largely physical inactivity and 15 min of rest in a supine position, applying multi-frequency, direct-segmental Bio-Impedance-Analysis (DSM-BIA; InBody 770, Seoul, Korea), using a standardized protocol. This included written instructions on nutrition 24 before testing. Skeletal muscle mass index (SMI) was calculated as ASMM (=fat free mass of the upper and lower extremities) divided by square body height (kg/m2). Intra Class Correlation coefficient for test-Retest reliability for the InBody DSM-BIA was 0.86 (95%-CI: 84–88;) in this male cohort aged 70 years and older (16).
Muscle function
The 10-m protocol recommended for research (19) was applied to determine habitual gait velocity (20). Participants started walking 3 m before the first photo sensor (HL 2–31, TagHeuer, La Chaux-de-Fonds, Switzerland) and stopped 2 m after the second photo sensor. Tests were performed wearing regular shoes without any specific walking aids. The standardized instruction was “walk at a velocity just as if you were walking along the street to go to the shops”.
Handgrip strength was measured, using a Jamar hand-held dynamometer (Sammons Preston Inc., Bollington, USA). Handgrip width was adjusted individually to participant’s hand size. Tests were performed in an upright position, arms down by the side (21). The standardized instruction was “squeeze as strongly as possible”. The mean value of three trials (rest: 30 s) of the dominant hand was used in the analysis.
Questionnaires
Apart from baseline characteristics (16), changes in medication, diseases and lifestyle including physical activity and exercise participation, number of falls, injurious falls and fractures were determined at two-year FU using a standardized questionnaire. To determine changes in perceived physical function we used the abridged version of the Late Life Function and Disability Instrument (LLFDI) provided by McAuley (18). This instrument is structured into three dimensions: basic (B-LEF) and advanced lower extremity function (A-LEF) and upper extremity functions (U-LEF), each addressed by five items.
Statistical analysis
After checking normal distribution by QQ plots and Shapiro-Wilks tests, data were given using mean values ± SD. Since the primary and secondary outcome variables were normally distributed, we used paired sample t-tests to determine changes from baseline to follow-up. However, the primary and secondary hypothesis, i.e. differences in percentage changes from baseline to follow-up between upper and lower extremity muscle mass or function were addressed by the more conservative Wilcoxon rank-test. We adjusted primary and secondary study endpoints for multiple testing by the Bonferroni procedure. We stated directed hypotheses, thus single-tailed tests were applied. Significance was accepted at p<0.05. The statistical procedures listed above were performed using SPSS Statistics (IBM, version 25).
Results
Table 1 shows baseline characteristics of men in the lowest quartile structured in “loss to follow-up” and “participants included”. Although not within the scope of this study, less surprisingly, men ineligible, unable, unwilling or not locatable were significantly older and demonstrated significantly more unfavorable anthropometric and functional features of sarcopenia (…and obesity).
Overall loss of FFM averaged 1.4±2.5%; SMI declined by 1.5±2.5% over the two-year period. Table 2 gives changes in primary and secondary outcomes. Addressing our primary hypothesis, the decline of lower extremity FFM was significantly (p<.001) more pronounced compared with upper extremity FFM. In contrast, gait velocity declines, representing lower extremity function, was significantly lower (p<.001) compared with the very high drop (−4.6±3.8 kg, p<.001) in upper extremity function (i.e. grip strength). Hence, we had to reject our hypotheses that (1) FFM and (2) muscle function decline at a significantly higher rate in the lower versus the upper extremities.
Interestingly, corresponding subscales of the LLFDI did not confirmed the latter differences. Based on favorable baseline values of 1.21±0.46 for U-LEF, 1.22±0.38 for B-LEF and 1.71±0.79 for A-LEF, longitudinal changes did not differ significantly (p<.520) between the three indices. Although all indices declined significantly (p=.001 to p=.007) by 0.09–0.10 score points, we did not consider these changes as clinically relevant.
Discussion
The present observational study provided evidence for nonuniform, age-dependent changes in upper vs. lower extremity muscle mass and -function in older men at risk for sarcopenia. Apart from cross-sectional studies (review in (1–3)), and the few observational studies (8, 11, 12), that however did not inference-statistically addressed this issue, the present study was the first longitudinal trial that clearly focus on differences between lower and upper extremity fat free mass in older adults. In general, we confirmed the finding of these studies, that indicated higher reductions of lower compared to upper muscle-or FFM in older men as assessed by Magnetic Resonance Imaging (MRI), Computed Tomography (CT) or Dual Energy x-Ray Absorptiometry (DXA). Of importance, the annual SMI loss of 0.75% observed in this study corresponds to longitudinal changes reported by other studies that determined changes of 0.4% to 0.9% p.a. of LBM/fat free mass, ASMM or SMI as assessed by BIA or DXA in men 70 years and older (4–6, 22, 23).
In parallel to muscle or free fat mass, only a limited amount of observational studies (e.g. (4, 10, 24, 25)) reported data of longitudinal changes in upper and lower extremity function (predominately strength parameters) in men 65 years+. In summary, the results were somewhat inconsistent, with higher (4, 24), or lower (25) declines in lower extremity strength; however none of these studies provided data on statistical differences. In diametral contrast to our study, Hicks et al. (24) reported a significant decline in gait velocity and maximum knee extension strength of ≈8–9% after 3 years of observation while handgrip strength and leg power increased significantly in this cohort of 451 men 65 years and older. However, the finding that age dependent reductions of muscle strength were also dependent on the specific strength testing protocol complicated a clear conclusion. This did not only refer to the type of muscle contraction (26), but also to different velocities during isokinetic testing (10, 27). Addressing the dimensions of these reduction, most longitudinal studies in older people reported lower annual changes of handgrip strength (2–4% p.a.; (e.g. (4, 14, 25, 28)) than the 6% p.a. decline in the present study. In contrast, the annual reduction in gait velocity (1.8% or 0.023 m/s) in our study is far from being alarming, considering that 0.12 m/s was calculated as a substantial meaningful change (ES: 0.5) of gait speed in cdw people 74±6 years (29).
Although the observation that muscle mass and function are only moderately related is not novel, our finding that local (upper extremity) muscle mass and corresponding (handgrip strength) function demonstrated converse developments is confusing. However, a simple explanation might be that due to its local origin, handgrip strength might not perfectly represent upper extremity strength generated by the large muscles of the upper arm. In parallel, there is some evidence that muscle size of the triceps surae, quadriceps and hamstrings as assessed by Magnetic Resonance Imaging (MRI) did not appear to be a relevant predictor for walking velocity (30).
However, what is the clinical significance of our finding? Firstly, when diagnosing and monitoring sarcopenia, medical experts involved in the corresponding process have to consider that morphologic and functional criteria feature different rates of decline during the aging process. Regular assessments of muscle mass are insufficient to exclude further functional deteriorations in at-risk populations. This refers not only to the different development of muscle mass and function (4–6), a finding confirmed by the present study, but also to the components of sarcopenia criteria (i.e. ASMM, grip strength, gait velocity). Our results further emphasize the relevance of exercise for older people. Unlike pharmaceutical agents that affect overall muscle mass at best, dedicated exercise programs generate very specific effects on muscle mass and function dependent on the protocol applied. In this context, we consider gait velocity and grip strength not as overall fitness parameters, but definite skills for managing daily life that must be specifically addressed.
Some study features, particularities and limitations might prevent an adequate comparison with other studies in this field. (1) We focus on the subgroup of cdw men within the lowest SMI quartile of the FranSO study. Quantified, SMI averaged below −1.3 SD T-Score (16) in all the participants included. However, handgrip strength and gait velocity performance were only slightly below age-related reference values (16). (2) Unlike other studies, we rely on DSM-BIA technology to determine body composition. However, with respect to annual FMM loss we confirmed data of studies that reported reductions of 0.5–0.8% p.a. for white men 70 years+, while applying other body composition assessment techniques (4–6). (3) There is a remarkable loss to follow-up, predominately due to men who died, were institutionalized or were unable to reach our lab (Tab. 1). Considering that these reasons might be significantly related to functional impairments, our results may underestimate the true decline of morphologic and functional decline of sarcopenia criteria in older people. This might affect generalizability of our results, however due to the aim of this ongoing project, we opt to focus on the target group of community dwelling men. (4) Due to the high sample size, statistically significance (p≤.05) should not be confounded with clinical relevance. (5) We placed very high emphasis on exact reproducibility of baseline and 2-year follow-up tests. Nevertheless, we have to acknowledge that baseline and FU-assessments tests were not always conducted by same research assistants. (6) It is difficult to estimate the external validity of our study with respect to its generalizability on other older cohorts. However, due to our approach of focusing on the lowest quartile of a representative sample of almost 1000 cdw men 70 years+, we think our results might be transferable to a considerable proportion of older men living in the community.
Funding: The study was funded by own resources of the Institute of Medical Physics, FAU-Erlangen-Nürnberg.
Conflict of Interest Disclosure Statement: Wolfgang Kemmler, Simon von Stengel und Daniel Schoene declare no conflicts of interest.
Author Contributions: Wolfgang Kemmler (WK), Simon von Stengel and Daniel Schoene designed the study, completed data analysis and/or interpretation and drafted the manuscript. WK accepts full responsibility for the integrity of the data sampling, analysis and interpretation.
References
Distefano G, Goodpaster BH. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb Perspect Med. 2018;8(3).
Mitchell WK, Williams J, Atherton P et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260.
Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19.
Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg, Sweden. Nutrition. 2009;25(6):613–9.
Goodpaster BH, Park SW, Harris TB et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64.
Koster A, Ding J, Stenholm S et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66(8):888–95.
Kemmler W, von Stengel S, Schoene D, Kohl M. Changes of Maximum Leg Strength Indices During Adulthood a Cross-Sectional Study With Non-athletic Men Aged 19–91. Front Physiol. 2018;9:1524.
Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol. 1983;38(6):673–7.
Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol (1985). 1991;71(2):644–50.
Frontera WR, Hughes VA, Fielding RA et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). 2000;88(4):1321–6.
Janssen I, Heymsfield SB, Wang HJ, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 years. J Appl Physiol. 2000;89:81–8.
Gallagher D, Visser M, De Meersman RE et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–39.
Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years.. Age Ageing 1994;23:371–7.
Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24(6):468–73.
Roshanravan B, Patel KV, Fried LF et al. Association of Muscle Endurance, Fatigability, and Strength With Functional Limitation and Mortality in the Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2017;72(2):284–91.
Kemmler W, Von Stengel S, Bebenek M, Sieber C, Freiberger E. Prevalence of Sarcopenic Obesity in Germany using Established Definitions. Baseline data of the FORMOsA Study. Osteo Int. 2016;27(1):275–81.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
McAuley E, Konopack JF, Motl RW, Rosengren K, Morris KS. Measuring disability and function in older women: psychometric properties of the late-life function and disability instrument. J Gerontol A Biol Sci Med Sci. 2005;60(7):901–9.
Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24–30.
Kressig RW, Beauchet O, European GNG. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin Exp Res. 2006;18(2):174–6.
Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg [Am]. 1984;9(2):222–6.
Newman AB, Lee JS, Visser M et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–8; quiz 915–6.
Shimokata H, Ando F, Yuki A, Otsuka R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: a 12-year longitudinal study. Geriatr Gerontol Int. 2014;14 Suppl 1:85–92.
Hicks GE, Shardell M, Alley DE et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67(1):66–73.
Lauretani F, Semba RD, Bandinelli S et al. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008;63(4):376–83.
Lindle RS, Metter EJ, Lynch NA et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol (1985). 1997;83(5):1581–7.
Akima H, Kano Y, Enomoto Y et al. Muscle function in 164 men and women aged 20–84 yr. Med Sci Sports Exerc. 2001;33(2):220–6.
Stenholm S, Harkanen T, Sainio P, Heliovaara M, Koskinen S. Long-term changes in handgrip strength in men and women—accounting the effect of right censoring due to death. J Gerontol A Biol Sci Med Sci. 2012;67(10):1068–74.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–9.
Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol. 2013;48(3):358–63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kemmler, W., Von Stengel, S. & Schoene, D. Longitudinal Changes in Muscle Mass and Function in Older Men at Increased Risk for Sarcopenia — The FrOST-Study. J Frailty Aging 8, 57–61 (2019). https://doi.org/10.14283/jfa.2019.9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2019.9