Abstract
Summary
In an observational study population of 62,413 individuals (6,455 [10 %] with diabetes), diabetes was independently associated with major osteoporotic fractures (MOFs) but did not significantly modify the effect of FRAXTM risk factors or prior fracture site. However, the presence of diabetes exerted a much stronger effect on hip fracture risk in younger versus older individuals.
Introduction
Diabetes mellitus increases fracture risk independent of risk factors that comprise the WHO FRAXTM tool. We explored whether diabetes modifies the effect of FRAX clinical risk factors on MOF and hip fracture risk.
Methods
Using a registry of clinical dual-energy X-ray absorptiometry (DXA) results for Manitoba, Canada, we identified women and men aged 40 years and older undergoing baseline DXA in 1996–2011. Health services data were used to identify diabetes diagnosis, FRAX risk factors and incident fractures using previously validated algorithms. Prior fracture was stratified as clinical vertebral, hip, humerus, forearm, pelvis and ‘other’. Cox proportional hazards models were used to test for statistical interactions of diabetes with FRAX clinical risk factors and prior fracture site.
Results
During a mean follow-up of 6 years, there were 4,218 MOF and 1,108 hip fractures. Diabetes was a significant independent risk factor for MOF adjusted for FRAX risk factors including bone mineral density (BMD) (adjusted hazard ratio [aHR] 1.32 [95 % confidence interval (CI) 1.20–1.46]). No significant interactions of FRAX risk factors or prior fracture site with diabetes were identified in analyses of MOF. For predicting hip fractures, age significantly modified the effect of diabetes (aHR age <60, 4.67 [95 % CI 2.76–7.89], age 60–69, 2.68 [1.77–4.04], age 70–79, 1.57 [1.20–2.04], age >80, 1.42 [1. 10–1.99]; pinteraction <0.001).
Conclusions
Diabetes is an independent risk factor for MOFs and does not significantly modify the effect of FRAX risk factors or prior fracture site. However, diabetes exerts a much stronger effect on hip fracture risk in younger than older individuals which needs to be considered in hip fracture prediction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of osteoporosis, characterized by reduced bone strength and an increased risk for low-trauma fractures, increases dramatically with age [1]. Diabetes is also present more frequently in the elderly, and therefore, diabetes and osteoporosis often co-exist in older adults [2, 3]. Type 1 diabetes, and more recently type 2 diabetes, has been associated with increased fracture risk [4–6]. This increased risk of fractures in individuals with diabetes is evident even after adjustment for bone mineral density (BMD), despite the observation that BMD is higher in individuals with type 2 diabetes compared with non-diabetic individuals [7–10]. Since BMD is central to fracture prediction, a consequence of this paradox is a lack of suitable methods, including the WHO Fracture Risk Assessment tool (FRAXTM), to predict fracture risk in older adults with type 2 diabetes [11, 12]. Type 1 diabetes is considered among the causes of secondary osteoporosis in FRAX, but diabetes status is not a primary risk factor in the current formulation of FRAX and therefore does not affect fracture probability when BMD is included in the FRAX calculation [13].
Although a growing body of research suggests that diabetes is a clinical indicator of increased fracture risk independent of BMD and independent of the factors that comprise FRAX [11, 12], it remains uncertain how best to consider the effect of diabetes for better identifying the high-risk individuals most likely to benefit from treatment [14]. Therefore, we explored whether diabetes simply adds to, or modifies the effect of, FRAX clinical risk factors on the prediction of major osteoporotic fractures (MOF) and hip fractures.
Methods
Subjects and setting
Using a registry of all clinical dual-energy X-ray absorptiometry (DXA) results for Manitoba, Canada, we identified women and men aged 40 years and older undergoing baseline DXA in years 1996–2011. Age of 40 years was used as the inclusion cut-off since this is the minimum age considered by FRAX. In the Province of Manitoba, Canada, health services are provided to virtually all residents through a single public health care system. Bone density testing DXA has been managed as an integrated program since 1997 [15]. DXA testing criteria are broadly consistent with clinical guidelines and emphasize screening for women aged 65 years and older or targeted testing in men and younger women with additional risk factors (see www.gov.mb.ca/health/primarycare/providers/chronicdisease/bonedensity). The program maintains a database of all DXA results that can be linked with other population-based computerized health databases through an anonymous personal identifier. The DXA database, with a completeness and accuracy in excess of 99 %, has been previously described in detail [16]. For those individuals with more than one DXA examination, only the first record was included. The study was approved by the Health Research Ethics Board for the University of Manitoba, and data access was granted by the Health Information Privacy Committee.
DXA
Proximal femur DXA scans were performed and analyzed by technicians according to the manufacturer’s guidelines using either pencil-beam dual x-ray absorptiometry (DXA) (Lunar DPX; GE Lunar, Madison, WI, USA) if the measurement was taken before the year 2000 or fan-beam DXA (Lunar Prodigy; GE Lunar, Madison, WI, USA) after the year 2000. We cross-calibrated the instruments using 63 volunteers and did not identify any clinically important differences (<0.1 standard deviation (SD) femoral neck). Densitometers showed stable long-term performance (coefficient of variation [CV] <0.5 %) and good in vivo precision (CV 1.1 % for the total hip) [17]. Femoral neck (FN) T-scores (number of SDs above or below young adult mean BMD) were calculated based on reference data for US White females from the NHANES III survey [18].
Diabetes diagnosis
Subjects were categorized as to the presence or absence of diabetes using a previously validated method for identifying individuals with diabetes in population-based health services data [2]. Using data sources since 1987, diabetes was ascertained from the presence of two separate physician claims for diabetes within 2 years (coded using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) or a hospitalization with a diabetes diagnosis (coded using the ICD-9-CM prior to 2004 and ICD-10-CA thereafter). It is important to note that these administrative data sources cannot reliably distinguish between type 1 and type 2 diabetes in older adults.
Clinical risk factors
Weight and height were obtained by self-report at the time of the DXA examination before the year 2000, and after 2000, height was assessed with a wall-mounted stadiometer, and weight was assessed without shoes using a standard floor scale. Body mass index (BMI [in kg/m2]) was calculated as weight (in kg) divided by height (in m) squared. Current smoking and parental hip fracture were by self-report but were only available from March 2005 onwards. Prior fracture and other variables required for FRAX were assessed using hospital discharge abstracts and physician billing claims since 1987 as previously described [19]. We defined prior fragility fracture as fracture that occurred before the index BMD testing. Since the effect of prior fracture is modified by anatomical site, prior fracture was stratified as clinical vertebral, hip, humerus, forearm, pelvis and ‘other’ (excluding the head, neck, hands and feet) [20, 21]. Prolonged corticosteroid use (over 90 days dispensed in the year prior to DXA testing) was obtained from the provincial pharmacy system [22].
Fracture-related outcomes
Incident fractures that occurred after the index BMD measurement (observation period from the first recorded DXA examination to March 31 2011) were assessed through a combination of hospital discharge abstracts and physician billing claims [23]. Longitudinal health service records were assessed for the presence of hip, clinical vertebral, forearm and humerus fracture codes (collectively designated as major osteoporotic fractures) that were not associated with trauma codes [24]. Hip and forearm fractures were required to have a site-specific fracture reduction, fixation or casting code fracture to enhance specificity for an acute fracture event. To minimize potential misclassification of prior incident fractures, we required that there be no hospitalization or physician visit(s) with the same fracture type in the 6 months preceding an incident fracture diagnosis.
Statistical analysis
Demographic and clinical characteristics of individuals with and without diabetes at the time of the index date (baseline BMD measurement) are presented using means and SDs for continuous variables and frequencies and percentages for categorical variables. Group comparisons for continuous data were conducted with the Student’s t test and for categorical data using a χ 2 test of independence. Cox proportional hazards models were used to test for associations between individual covariates and incident MOFs and incident hip fractures. Models were initially stratified by diabetes status, with a final model that combined both groups to test for a statistically significant interaction (i.e. effect modification according to the presence or absence of diabetes) with individual clinical risk factors. Model 1 examined FRAX clinical risk factors including femoral neck BMD; model 2 examined FRAX clinical risk factors without BMD; model 3 examined prior fracture according to site (sex and age-adjusted). To estimate parameters for current smoking and parental hip fracture, separate models were run for the subgroup with index dates after March 2005. Due to multiple comparisons (up to 10 risk factors in model 1), a statistically significant interaction was defined using a conservative Bonferroni-adjusted p value of 0.01 (0.1 divided by 10). We also performed an omnibus test of all two-way interaction effects using the change in likelihood ratio statistics between a main effect only model and a model containing multiple two-way interaction terms [25]. The proportional hazards assumption was tested and no violations were detected. Statistical analyses were performed with Statistica (Version 10.0, StatSoft Inc, Tulsa, OK, USA).
Results
Study population
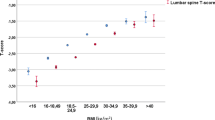
The study population included 62,413 individuals of whom 6,455 (10 %) had a diagnosis of diabetes. Self-reported ethnicity was predominantly White (97.8 %). Current smoking and parental hip fracture information was available in 27,401 individuals with a index date of BMD testing after March 2005 (3,239 with diabetes). Table 1 summarizes the baseline characteristics of the study population. Individuals with diabetes tended to be older (average age 66.5 ± 10.5 years vs 63.7 ± 11.3, p value <0.001) and less likely to be female (85.6 vs 92.0 %, p value <0.001). As expected, individuals with diabetes also had a significantly higher BMI (30.2 ± 6.2 kg/m2 vs 26.6 ± 5.2 kg/m2, p value <0.001) and greater femoral neck T-scores (−1.2 ± 1.1 vs −1.4 ± 1.2, p value <0.001) than those without diabetes.
Diabetes and major fractures
During a mean follow-up of 6 years, 4,218 individuals experienced one or more incident MOFs of which 492 (7.6 %) occurred in those with diabetes and 3,726 (6.7 %) in those without diabetes (p value 0.004). Table 2 summarizes hazard ratios (HRs) for incident MOFs from multivariable Cox proportional hazards regression analyses. With the exception of sex and parental hip fracture, all other variables showed significant associations with incident MOFs in one or more analyses. No statistically significant interactions (i.e. effect modification) of diabetes with any individual risk factors were identified (all Bonferroni-adjusted p values >0.01), and the omnibus tests for interactions were not statistically significant (all p values >0.1). There was a trend suggesting that higher BMI might be more protective in those without diabetes when adjusted for femoral neck T-score (model 1 p value interaction = 0.080), but this was not evident in without femoral neck T-score adjustment (model 2 p value interaction = 0.276). Diabetes was a significant independent risk factor for fracture after adjustment for all variables in model 1 (adjusted hazard ratio [aHR] 1.32 [95 % confidence interval (CI) 1.20–1.46]). Lower femoral neck BMD was strongly predictive of MOF (aHR per SD reduction 1.60 [95 % CI 1.44–1.79] with diabetes, 1.68 [95 % CI 1.61–1.75] without diabetes; p value for interaction 0.456). Results were similar in analyses without BMD (model 2) except that higher BMI showed a stronger protective effect (aHR per 5 kg/m2 0.79 [95 % CI 0.73–0.86] with diabetes, 0.83 [95 % CI 0.80–0.86] without diabetes; p interaction 0.276). Any prior fracture (excluding head, neck, hands and feet) was associated with an increased risk for subsequent MOF in models that adjusted for femoral neck BMD (aHR 1.72 [95 % CI 1.42–2.07] with diabetes, 1.68 [95 % CI 1.61–1.75] without diabetes; p value for interaction 0.588) as well as those that did not adjust for BMD (aHR 2.06 [95 % CI 1.71–2.47] with diabetes, 1.89 [95 % CI 1.76–2.02] without diabetes; p value interaction 0.387).
When prior fracture was stratified by anatomical site, vertebral fracture showed the strongest association with subsequent MOF in those with diabetes or without diabetes. Other fracture sites were also predictive of subsequent MOF (with the exception of a prior pelvis fracture in those with diabetes). There were no statistically significant interactions between diabetes diagnosis and site of prior fracture tested individually or with an omnibus test of all interactions (all p values >0.1).
Diabetes and hip fractures
Table 3 summarizes analyses for 1,108 incident hip fractures which were more common in those with diabetes (2.4 %) than those without diabetes (1.7 %, p value <0.001). Once again, with the exception of sex and parental hip fracture, all other variables showed significant associations with incident fractures in one or more analyses. Analysis by prior fracture site generally paralleled the results for incident MOF except that prior forearm fracture was not a significant risk factor for hip fracture in those with or without diabetes. There was a statistically significant interaction between diabetes status and age in all models, whereby age significantly modified the effect of diabetes hip fracture occurrence (Fig. 1). Although diabetes was independently associated with a significantly higher risk of hip fracture for all age subgroups, effect modification was much greater in the youngest subgroup with progressively less effect modification in older age subgroups (aHR age <60 years 4.67 [95 % CI 2.76–7.89], age 60–69 years 2.68 [1.77–4.04], age 70–79 years 1.57 [1.20–2.04], age ≥80 years 1.42 [1.01–1.99]; p value for interaction <0.001). Using a competing mortality framework [26] only slightly attenuated the effect modification for age (aHR age <60 years 3.84 [95 % CI 2.25–6.54], age 60–69 years 2.34 [1.55–3.53], age 70–79 years 1.32 [1.02–1.72], age ≥80 years 1.37 [0.97–1.93]; p value for interaction <0.001).
There was inconsistent evidence for significant interactions with other risk factors. The omnibus tests for interaction effects in hip fracture prediction (excluding age) were borderline (p value = 0.079 for model 2, other models' p value >0.1). There were trends suggesting larger effects in those without diabetes for rheumatoid arthritis (p value interaction = 0.061 for model 1, 0.038 for model 2) and high alcohol use (p value interaction = 0.035 for model 1, 0.036 for model 2), but this failed to reach the predefined cut-off for significance (Bonferroni-adjusted p value <0.01).
Discussion
Our study further supports that diabetes is a risk factor for both MOF and hip fracture, independent of age, sex, higher BMI, higher femoral neck BMD and other clinical risk factors. The absence of statistically significant interactions (effect modification) between diabetes status and risk factors for predicting MOF suggests a simple additive effect of diabetes to the MOF probability derived from FRAX clinical risk factors. This simplifies how this information can be incorporated into improved MOF prediction for individuals with diabetes. The only strong and consistent interaction that we observed was for hip fracture prediction where younger individuals showed a much larger diabetes-related risk than did older individuals, consistent with the previous report from Giangregorio et al. [11]. Indeed, the diabetes-related risk of hip fracture was threefold larger for those younger than 60 years compared with those aged 80 years and older and was only slightly attenuated when competing mortality was considered [26]. Additional studies are needed to confirm or exclude borderline interactions (e.g. rheumatoid arthritis and high alcohol use for hip fracture but not MOF prediction).
This study confirms and extends our previous report that was limited to incident MOF in women aged 50 years and older with diabetes compared with age-matched women without diabetes [27]. The current analysis includes men, has a wider age spectrum (40 years and above), was not matched on age thus permitting age-specific analyses, included more than twice the number of individuals with diabetes (6,455 vs 3,054), had a longer period of observation (6 vs 4 years) and had sufficient power to analyze incident hip fractures in addition to MOF.
Our findings have implications for fracture risk assessment in diabetes and for identifying high-risk individuals. Statistically significant interaction effects complicate the development of risk prediction models, and therefore, it is reassuring that the only effect modification associated with diabetes that we observed was for age and incident hip fractures. A meta-analysis found the risk of hip fracture to be higher among individuals with type 1 diabetes (relative risk (RR) = 6.3 [95 % CI 2.6–15.1]) than those with type 2 diabetes (RR = 1.7 [95 % CI 1.3–2.2]); type 2 diabetes showed a weaker association with fractures at non-hip sites [5, 6]. The relative proportion of type 1 diabetes decreases with age, and this may be contributing to the diminishing risk of diabetes for hip fracture with older age.
Limitations of the current study are acknowledged. Fracture risk may be different among individuals with type 1 versus type 2 diabetes, and we were not able to account for these differences in our databases. Given the age of our cohort, the great majority of the sample with diabetes would have type 2 diabetes; in the population-based Canadian Multicentre Osteoporosis Study (CaMos), 1.3 % of participants over the age of 50 years had type 1 diabetes, and 6.8 % had type 2 diabetes [8]. Furthermore, we do not have access to measures of glycemic control, duration of diabetes, episodes of hypoglycemia, or medications associated with falls that are more commonly used in people with diabetes such as anti-hypertensive agents. Whether changing diagnostic criteria for diabetes would affect our results is unknown. As well, the sample represents individuals referred for BMD testing and therefore is subject to selection bias. Despite the relatively large number of people with diagnosed diabetes available for this analysis (N = 6,455), when substratified by FRAX risk factors and incident fracture status, some cell sizes were small with limited power to detect or exclude interactions. Similar issues may affect other low-prevalence conditions not considered by FRAX.
In conclusion, diabetes is an independent risk factor for MOFs but did not significantly modify the effect of FRAX risk factors or prior fracture site. However, diabetes exerted a much stronger effect on hip fracture risk in younger versus older individuals. Methods need to be developed to incorporate these findings into risk prediction models in order to avoid systematically underestimating the risk of osteoporosis-related fracture in those with diabetes.
References
van Staa TP, Dennison EM, Leufkens HG et al (2001) Epidemiology of fractures in England and Wales. Bone 29:517–522
Blanchard JF, Ludwig S, Wajda A et al (1996) Incidence and prevalence of diabetes in Manitoba, 1986-1991. Diabetes Care 19:807–811
Wild S, Roglic G, Green A et al (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Strotmeyer ES, Cauley JA, Schwartz AV et al (2005) Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 165:1612–1617
Janghorbani M, Feskanich D, Willett WC et al (2006) Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes Care 29:1573–1578
Janghorbani M, Van Dam RM, Willett WC et al (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
De Liefde II, Van Der KM, de Laet CE et al (2005) Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 16:1713–1720
Hanley DA, Brown JP, Tenenhouse A et al (2003) Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-sectional results from the Canadian Multicentre Osteoporosis Study. J Bone Miner Res 18:784–790
Bonds DE, Larson JC, Schwartz AV et al (2006) Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab 91:3404–3410
Melton LJ III, Riggs BL, Leibson CL et al (2008) A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab 93:4804–4809
Giangregorio LM, Leslie WD, Lix LM et al (2012) FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 27:301–308
Schwartz AV, Vittinghoff E, Bauer DC et al (2011) Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 305:2184–2192
Kanis JA, Oden A, Johansson H et al (2009) FRAX and its applications to clinical practice. Bone 44:734–743
Leslie WD, Rubin MR, Schwartz AV et al (2012) Type 2 diabetes and bone. J Bone Miner Res 27:2231–2237
Leslie WD, Metge C (2003) Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom 6:275–282
Leslie WD, Caetano PA, MacWilliam LR et al (2005) Construction and validation of a population-based bone densitometry database. J Clin Densitom 8:25–30
Leslie WD (2006) The importance of spectrum bias on bone density monitoring in clinical practice. Bone 39:361–368
Looker AC, Wahner HW, Dunn WL et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Leslie WD, Lix LM, Johansson H et al (2010) Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res 25:2350–2358
Klotzbuecher CM, Ross PD, Landsman PB et al (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Morin SN, Lix LM, Leslie WD (2014) The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. J Bone Miner Res [Epub head of print]
WHO Collaborating Centre for Drug Statistics Methodology. (eds) (2005) Guidelines for ATC classification and DDD assignment. Oslo
Roos NP, Shapiro E (1999) Revisiting the Manitoba Centre for Health Policy and Evaluation and its population-based health information system. Med Care 37:JS10–JS14
Leslie WD, Tsang JF, Caetano PA et al (2007) Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab 92:77–81
Kleinbaum DG, Klein M. (eds) (2010) Logistic regression: a self-learning text (3rd edition). Springer (New York)
Leslie WD, Lix LM, Wu X (2013) Competing mortality and fracture risk assessment. Osteoporos Int 24:681–688
Fraser LA, Pritchard J, Ioannidis G et al (2011) Clinical risk factors for fracture in diabetes: a matched cohort analysis. J Clin Densitom 14:416–421
Acknowledgments
We are indebted to Manitoba Health for providing data (HIPC File No. 2011/2012—31). The results and conclusions are those of the authors, and no official endorsement by Manitoba Health is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Funding
None.
Conflicts of interest
SNM is chercheur-boursier des Fonds de Recherche du Québec en Santé. LML is supported by a Manitoba Health Research Chair. SRM holds the Endowed Chair in Patient Health Management (Faculties of Medicine and Dentistry and Pharmacy and Pharmaceutical Sciences, University of Alberta) and receives salary support as a Health Scholar of the Alberta Heritage Foundation for Medical Research and Alberta Innovates-Health Solutions. William Leslie is a speaker bureau (paid to facility) of Amgen, Eli Lilly, and Novartis and has research grants (paid to facility) from Amgen, Genzyme. Suzanne Morin is a consultant to Amgen, Eli Lilly, and Merck and a speaker bureau of Amgen and Eli Lilly and has a research grant from Amgen. Lisa M. Lix and Sumit R. Majumdar declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leslie, W.D., Morin, S.N., Lix, L.M. et al. Does diabetes modify the effect of FRAX risk factors for predicting major osteoporotic and hip fracture?. Osteoporos Int 25, 2817–2824 (2014). https://doi.org/10.1007/s00198-014-2822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2822-2