Abstract
Summary
Low bone mineral density (BMD) was diagnosed in 24% of childhood cancer survivors (CCS), whereas very low BMD was relatively uncommon at 8%. We suggest that low BMD in CCS may become alleviated over time. Stem cell transplantation, radiotherapy, and underweight were the strongest independent predictors of decreased BMD.
Purpose
Childhood cancer survivors (CCS) are at risk of premature bone loss, although published studies are inconsistent. The objective of this study was to evaluate the prevalence and pattern of low bone mineral density (BMD) in short- and long-term CCS, and to determine clinical factors affecting skeleton after anticancer treatment.
Methods
This retrospective study was conducted in a cohort of 326 children and young adult CCS (147 females) who completed anticancer treatment. BMD was determined by dual-energy X-ray absorptiometry (DXA). Low BMD was defined as a Z-score ≤ − 1.0, and the very low BMD as a Z-score ≤ − 2.0. Additionally, the changes in BMD over time were studied in 123 CCS who had been re-examined by DXA during follow-up.
Results
Median age at diagnosis was 7.27 years (range, 4.4–10.6); median time between end of treatment and DXA was 6.12 (range, 4.0–22.0). Low BMD was found in 24% of CCS, while very low BMD was relatively uncommon (8%). Based on multivariate analysis, the following were significantly associated with low BMD at the follow-up: hematopoietic stem cell transplantation (OR 3.13, 95% CI 1.02–9.63), head and neck radiotherapy (OR 2.54, 95% CI 1.32–4.90), and body weight below the standard reference (OR 3.57, 95% CI 1.24–10.23). The time-related trajectory showed an improvement (BMDLS) or stabilization (BMDTB) in Z-scores values.
Conclusion
These data based on serial DXA measurements, encompassing a long-lasting observation period, show that CCS may not be at risk of premature bone loss in young adulthood. However, it is unknown how the scenario for skeletal mass is until the CCS will achieve older or postmenopausal age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past five decades, changes in the diagnostic approach resulting from the implementation of advanced diagnostic methods, increasingly better standards in healthcare system, and new treatment strategies, have caused a significant increase in the number of subjects who had experienced cancer in childhood. Currently, the 5-year event-free survival rate (EFS) among childhood cancer survivors (CCS) has reached up to 80%. The remarkable improvement is attributed to the use of advanced cancer treatment including multi-agent chemotherapy, radiotherapy, immunotherapy, surgery, and excellent supportive care. At the same time, some available large-scale studies have emphasized that more than two-thirds of survivors suffered from at least one chronic condition, and nearly 30% developed severe or life-threatening sequelae. Most late effects appeared with age, often decades after the end of therapy [1].
Metabolic disturbances are among the most frequently reported complications in CCS, resulting mainly from the treatment used, and may trigger off individual negative health effects and may also confer essential public health issues. The most common abnormalities include hypothalamic/pituitary dysfunction, thyroid disorders, obesity and metabolic syndrome, infertility, and bone mineral deficits or premature bone loss. In the present study, we have focused on bone status in CCS [2].
In healthy people, the peak bone mass (PBM) acquisition is gained during puberty and early adulthood, and several confounding factors may lead to suboptimal bone mass and decreased quality. Alterations in bone metabolism related to anticancer treatment may interfere with bone mass accrual and achieving peak bone mass, which is normally reached between the ages of 20 and 30 years. This, in turn, is crucial for maintaining bone health in adulthood and, at least partly, preventing fragility fractures in old age [3].
Cancer itself and its treatment can produce latent or subclinical changes in the bone tissue that may become clinically significant with age and lead to osteoporosis and severe fragility fractures. Of all the anticancer drugs administered, according to some but not all studies, the best-established deteriorating effect on bone mineral density (BMD) is triggered by glucocorticoids (GCS) and methotrexate (MTX). Furthermore, alkylating agents may cause bone loss or inadequate skeletal accrual, but in most cases, it is secondary to gonadal impairment, whereas the risk is associated with cumulative dose [4]. Apart from chemotherapy, irradiation of central nervous system (CNS) is the best-proven factor disturbing the normal development of the skeleton during growth. Radiation therapy (RT) to the neuroendocrine axis is a frequent and well-established causal pathway of growth hormone (GH) deficiency. Patients who had received RT to the brain with a dose above 18 Gy were reported particularly vulnerable ones. The pituitary gland itself appears to be damaged (overt gonadotrophin deficiency) if a cranial dose above 40 Gy has been given used. Survivors who underwent hematopoietic blood stem cell transplantation (HSCT) are at a particular higher risk of low bone mass as a result of more aggressive treatment (high-dose alkylating agents, mega chemotherapy, and total body irradiation). It is estimated that a decrease in BMD of about one-tenth significantly exacerbates fracture risk in patients after HSCT [5].
There is a limited number of published studies conducted on large cohorts of long-term CSS. One of the reports showed that as many as 45% of adult survivors had low bone mass [6]. However, available data are inconsistent and limited by small sample size, short observation period, or not taking into analysis potential confounding factors. Moreover, changes in BMD during the treatment have already been well understood, but still little is known about age-related long-term variations in bone mass following childhood cancer, i.e., BMD trends over time after treatment completion.
The aim of the study was to evaluate the prevalence and pattern of low and very low BMD in short-term and long-term CCS, and to determine specific clinical factors affecting skeleton in children and young adults after anti-cancer treatment.
Material and methods
Study population
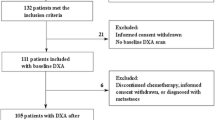
This study was conducted in a cohort of 326 Caucasian children and young adult CCS. We retrospectively reviewed the records of childhood cancer survivors who had been consecutively diagnosed between 1987 and 2015 in the Department of Pediatric Oncology and Hematology of the Medical University of Bialystok, Poland. All children were treated according to international protocols approved by the Polish Pediatric Leukemia/Lymphoma Group and Solid Tumor Group. Throughout the 30-year-long period of data collection, the study protocols were consistent with ethical guidelines. Informed consent was obtained from patients or their parents/guardians who had not reached adulthood. The study was approved by the Ethical Committee of the Medical University of Bialystok in accordance with the Declaration of Helsinki. Participants who met the following criteria were included in the study: (1) diagnosed with cancer under 18 years of age, (2) availability of complete DXA scan performed after cessation of treatment, and (3) had no history of conditions which may have affected bone mineral density and content (i.e., apparent endocrine or renal disorders). Brain tumor and bone tumor survivors, and patients with relapse were excluded from the analysis. The characteristics of the study population are shown in Table 1.
For all subjects, comprehensive clinical history including demographic information, comorbidities, smoking behavior, and data associated with previous anticancer treatment, such as cumulative dose of chemotherapeutic drugs and GCS, radiotherapy, and hematopoietic stem cell transplantation, were derived from medical records. The total cumulative GCS dosage (prednisone and/or dexamethasone) was calculated with equivalent prednisone dosage [7]. All patients underwent a physical examination. Anthropometric traits were collected using standard procedures and were rigorously recorded: body weight was measured on an electronic scale (Seca, Germany) and height was measured using Martin anthropometer. Body mass index (BMI) was calculated using the formula: weight [kg] / height2 [m2]. Due to the fact that many changes in cancer treatment protocols have been implemented within the last three decades, we have not analyzed the amendments, but only individual variables (specified in tables) which may have disturbed bone mineral density.
For statistical purposes, where separate analysis of particular types of tumors was not possible, the group of participants was stratified into subsets according to their diagnosis (leukemia vs. lymphoma vs. solid tumor). The time period after the end of treatment—short-term and long-term CCS (< 5 vs. > 5 years) and the calendar age during the study (< 18 vs. > 18 years) were evaluated. In addition, the changes in BMD (ΔBMD) over time were studied in 123 childhood cancer survivors who had received DXA scan at least twice during follow-up.
Bone mineral density assessment
Bone mineral density (BMD) was evaluated by dual-energy X-ray absorptiometry (DXA) (DPX-L, GE-Healthcare Lunar, Madison, WI). The crude values of bone mineral density in the total body (BMDTB, g/cm2) and anteroposterior lumbar spine L1–L4 (BMDLS, g/cm2) were compared to age- and sex-matched reference, i.e., adjusted for normative data provided by the manufacturer. All parameters were expressed as Z-scores reflecting the number of standard deviations that an individual BMD value differs from the mean BMD of a healthy reference population matched by age and gender. The estimated reproducibility error in vivo (coefficient of variation) was 2.9%, based on a duplicate lumbar spine DXA examinations performed in pediatric subjects. DXA scans were performed mainly after treatment, as part of a cross-sectional study within several research projects, as well as a standard procedure in case of justified and suspected skeletal complications, bone pain or mineral disorders. In accordance with the official position of the International Society for Clinical Densitometry (ISCD), the Z-scores were used for further analyses and stratification. For the longitudinal assessment and comparisons, both the alterations in Z-scores and changes in crude BMD values—incorporating least significant change—were investigated. Due to the fact that just few patients met the strict criteria of low BMD proposed by ISCD, and trustworthy analysis would not be possible, we decided to use a different cutoff point in order to show moderate BMD deficits, in line with prior studies [8,9,10,11]. Thus, low BMD was defined as a Z-score ≤ − 1.0, and the very low BMD as a Z-score ≤ − 2.0. The rationale for this approach is that those with lower BMD are presumably to remain in the lower normal range [11], leading to a lower peak bone mass [12], and possibly predisposing to an increased risk of fracture later in life [8]. Every subject had both the BMDTB and BMDLS measurements done. If two post-treatment DXA scans were available, the most recent one was included into analysis of the total group (a longer period elapsed from the end of therapy). In order to avoid the confounding effect of height on areal BMD, we decided to adjust BMDTB and BMDLS for body height Z-scores.
Statistical analysis
Statistical analysis was performed using Stata/IC 12.1 version (StatCorp, College Station, Texas, USA). All continuous variables were tested for normal distribution using the Shapiro-Wilk test. The data were expressed as means ± standard deviation (SD), or median and quartiles when appropriate. In the univariate analysis, Fisher exact test and χ2 test were used, whereas continuous variables were compared with the Wilcoxon rank-sum test or Student t test. Univariate and multivariate logistic regression models were used to examine the association between dependent variables (BMDTB and BMDLS) and independent variables postulated as having a potentially negative impact on bone density. The number of variables was adapted to the size of the group. Confounding factors associated with an increased risk of low bone mineral density in the univariate analyses at p ≤ 0.2 were included in multivariable analyses. The strength of the association between the independent variable and BMD was reported using the odds ratio (OR) and 95% confidence interval (CI). OR it is clinically significant when 95% confidence interval does not include one. The models describing BMD included adjustment for age and body height. The analysis of variance and post hoc test (Tukey’s test) were used to compare bone mineral density according to diagnosis. A Wilcoxon signed-rank test and Lowess regression algorithm were used to assess the mean differences in BMD Z-score between the DXA scans in the same participants after the end of treatment. The level of statistical significance was set to 0.05.
Results
Characteristics of the study population
Among 773 childhood cancer survivors who visited oncology outpatient’s clinic between 1990 and 2016 for late effects, 326 have performed DXA scan at least one time after the end of treatment. The participants were 54.9% female and all white ethnicity. The median age at diagnosis was 7.27 years for a total group (range, 3 months–17.9 years) and did not differ according to sex. The median age at the DXA scan and the follow-up time from the end of treatment to the study was 16 years (range, 3–27 years) and 6.12 years (range, 3 months–22 years), respectively. Of the 326 patients, 222 (68%) had DXA scan performed at least 5 years after the cessation of treatment (range, 5–22 years) and 104 (32%) up to 5 years after treatment (range, 3 months–4.98 years). The characteristics of the study group are shown in Table 1.
Frequency of low and very low BMD
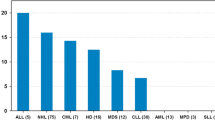
Low bone mineral density (Z-score ≤ − 1) of total body and lumbar spine was found in 24% and 20% of study participants. The overall prevalence with a BMD Z-score ≤ − 2 was 8%, and the prevalence with a BMDTB and BMDLS Z-score between < − 1 and > − 2 was 15% and 12%, respectively. Therefore, 76% and 80% of patients had normal BMDTB and BMDLS (Z-score > − 1). Among the neuroblastoma patients, low BMDLS was found in 46%, but the size of the subgroup was relatively small. Eight out of thirteen NBL patients had abdominal radiotherapy and two of them had a decreased BMD. The high occurrence of low BMD in soft tissue sarcoma (STS) and Langerhans cell histiocytosis (LHC) were also found in patients (31% vs. 71%, respectively). There were no differences in the mean Z-score BMD value and the number of patients with low BMD in the analysis conducted between the groups of diagnoses (leukemia vs. lymphoma vs. solid tumor) and according to gender.
Factors associated with low BMD
Univariate analysis showed that low BMDLS was significantly associated with the use of radiotherapy to the head and neck in childhood (OR 1.95; 95% CI 0.08–0.65). Radiation doses > 24 Gy were associated with the occurrence of low BMDTB (OR 5.18; 95% CI 1.12–23.29). The underweight patients had three times increased odds of low BMDTB (OR 3.16; 95% CI 1.17–8.55) and low BMDLS (OR 3.16; 95% CI 1.17–8.55) than the patients who had normal body mass index. Other risk factors previously described as having a potential negative impact on bones were not related to low BMD (Table 2). The multiple regression models confirmed the negative influence of radiotherapy to the head and neck on BMDLS and association between low BMD and underweight subjects as in univariate analysis. The stem cell transplantation procedure was an independent variable significantly affecting the occurrence of low BMDTB (OR 3.13; 95% CI 1.02–9.63). Other potential confounding variables included in the models were not significantly associated with bone deficits (Table 3).
Changes in BMD over time
Among 326 CCS, we identified 123 patients who had at least two DXA scan during follow-up visits. The mean time between the second (DXA2) and first (DXA1) densitometry was 5.54 years (mean age, 17.11 ± 3.67 vs. 11.57 ± 4.03 years, respectively). There were no significant differences in BMDTB and BMDLS Z-scores between DXA1 and DXA2 (− 0.176 vs. − 0.262, p = 0.293 and − 0.277 vs. − 0.180, p = 0.842), respectively. We did not notice BMD Z-scores worsened over the time period between the first and subsequent visit (the number of patients with decreased BMDTB Z-score < − 2 was 18 vs. 6, and that with Z-score < − 1 and ≥ − 2 was 23 vs. 19; with decreased BMDLS Z-score < − 2 was 9 vs. 6 and Z-score < − 1 but > − 2 was 28 vs. 14 patients). None of the study participants was treated for osteoporosis between sequential DXA measurements. The BMDTB and BMDLS did not differ significantly between leukemia, Hodgkin lymphoma, or solid tumor groups, and were not different in either of genders. The analysis of changes in BMD showed an improvement (BMDLS) or alleviation and stabilization (BMDTB) in Z-score values over time. The relationship between age at DXA2 and ΔBMDTB and ΔBMDLS Z-scores is shown in the scatter plots with Lowess curves in Fig. 1a and b.
Bone status in pediatric CCS (< 18 years)
Of all study participants, there were 196 (60%) children in total. The median age at DXA scan was 13.16 (range, 3–17.9 years), while the median time after the end of treatment was 6.05 years (range, 0.03–15.7 years). Low BMDTB was independently associated with the following: radiotherapy used with doses above 24 Gy in comparison with those who received lower doses (OR 10.76; 95% CI 0.196–0.702), time from treatment completion (OR 0.89; 95% CI 0.803–0.994), and BMI Z-score (OR 0.49; 95% CI 0.370–0.664). The multivariable logistic regression model taking into account more than one independent variable showed a significant negative effect of radiotherapy to the head and neck area (OR 10.76; 95% CI 0.196–0.702) and increased odds of bone deficits in survivors with lower BMI Z-score. Bone density did not differ significantly depending on the stage in the Tanner scale.
Bone status in adult CCS (> 18 years)
Separate analyses (univariate and multivariate) in a subset of 130 (40%) participants who had reached adulthood had been conducted. The median age at a follow-up visit and time after completion treatment were 20 years (range, 18–27 years) and 6.4 years (range, 0.3–22 years) respectively. In univariate regression model, age at diagnosis (OR 0.89; 95% CI 0.819–0.973), age at the end of treatment (OR 0.89; 95% CI 0.815–0.977), and age at study (OR 0.68; 95% CI 0.510–0.911) were significantly associated with the occurrence of low BMDTB. The multivariate models have only shown a significant association between age at study and low BMDTB (OR 0.59; 95% CI 0.387–0.898). Low BMDLS in the univariable model was associated with age at study (OR 0.4; 95% CI 0.296–0.771), whereas in multivariable analysis younger age at study and use of radiotherapy to the head and neck in childhood increased the odds of developing low BMDLS. When we plotted the change in BMD (ΔBMD) by age at second DXA (Lowess curves), it turned out that the peak bone mass (PBM) for both, BMDTB and BMDLS, has been reached between 26 and 27 years of age (Fig. 1a and b).
Short-term and long-term CCS
The age limit between short-term and long-term survivors was set at 5 years from the end of treatment. Thus, the two groups were separated (up to 5 and above 5 years) in order to analyze the bone mineral density depending on the time of treatment completion (Table 4). Univariate analysis in short-term CCS showed significant association between BMDTB and methotrexate used (OR 3.1; 95% CI 1.27–7.56), age at diagnosis (OR 0.84; 95% CI 0.77–0.92), age at DXA exam (OR 0.84; 95% CI 0.39–0.82), BMI Z-score (OR 0.18; 95% CI 0.04–0.82), Tanner stage (OR 0.69; 95% CI 0.54–0.88), and between BMDLS and weight Z-score (OR 0.73; 95% CI 0.54–0.99). In long-term survivors’ independent variables predicting low bone mass were: for BMDTB were hematopoietic stem cell transplantation with total body irradiation (OR 10.2; 95% CI 1.91–54.3), BMI Z-score (OR 6.0; 95% CI 1.67–21.7); for BMDLS HSCT with TBI (OR 6.3; 95% CI 1.36–29.5), BMI Z-score (OR 0.1; 95% CI 0.01–0.67). There was also a significant difference in the values of BMDTB Z-scores between two groups (< 5 years vs. > 5 years: mean − 0.44 ± 2.06 vs. − 0.14 ± 1.94, p = 0.043).
Discussion
In this retrospective cohort study conducted on 326 childhood cancer survivors, we found that 24% of subjects had low bone mineral density defined as Z-score < − 1, whereas 8% of them had very low BMD (Z-score < − 2). The strongest factors associated with the occurrence of low BMD were body weight below the standard reference at the follow-up visit, radiation to the head and neck region (> 24 Gy), and total body irradiation. Our results are consistent with some, but not all, previous reports, and provide further evidence that treatment-related factors affect BMD in CCS to some extent [8, 13,14,15,16].
Depending on the authors, the frequency of low bone mass ranged from 5 to 25% in long-term CCS, which has been now confirmed by our research. However, recently published studies with one of the longest observation periods since the discontinuation of treatment (17 years) identified low BMD in 45% of adult CCS [6]. The discrepancy may be due to a shorter observation period in most studies, and indicates that bone deficits may occur in later decades of life.
A number of research on CCS is mainly focused on survivors of acute leukemia and lymphoma, while data from studies addressing other types of cancer are very limited [11, 17,18,19,20]. In our work, we extended the data by describing the prevalence of low BMD in some less frequent types of cancer. Interestingly, the high occurrence of low BMD in neuroblastoma, soft tissue sarcoma, and Langerhans cell histiocytosis were found in patients; however, these groups were underrepresented for meaningful analysis. High doses and multimodal therapy in NBL, the use of alkaloid agents (including those causing proximal tubular acidosis, e.g., ifosfamide) and radiotherapy in STS, as well as direct bone involvement and chronic inflammation in LCH can altogether lead to bone loss or inadequate skeletal accrual in CCS. Hence, further studies on larger patient cohorts are necessary.
Among all factors, central nervous system irradiation is the best-proven factor disturbing the normal development of the skeleton in children and young adult survivors [21]. Despite the fact that patients treated for brain tumors were excluded from our analysis, head and neck radiotherapy used in other types of cancers was also found to significantly increase the occurrence of low BMD. The vast majority of those patients were treated for acute lymphoblastic leukemia or non-Hodgkin lymphoma, and they had pre-empty cranial radiation. A dose greater than 24 Gy in our study was an independent factor associated with low BMD, supporting evidence published elsewhere [22, 23].
There is little data in the literature on bone density in patients undergoing hematopoietic stem cell transplantation [24, 25]. They tend to have more serious skeletal and mineral complications due to a more aggressive treatment, and presumably HSCT itself. Our results support the hypothesis that HSCT significantly exacerbates skeletal impairment resulting in low BMD among CCS [4, 26, 27]. Noticeably, HSCT is very often preceded by TBI, which is commonly used as part of the conditioning regimen. In order to obtain reliable analysis, those patients who have undergone HSCT should be analyzed separately depending on whether TBI was used or not. In our study, TBI increased significantly the odds of low BMD in long-term CCS survivors, which is in accordance with previous studies [28], though data are very limited.
Furthermore, methotrexate is known to have a negative effect on bone formation through cytotoxic effects on osteoblasts and nephrons [29, 30]. However, the association between treatment with MTX and low BMD is still unclear due to conflicting data on this field [4]. Our univariate analysis found methotrexate used to be associated with low BMD in a subgroup of patients who were examined up to 5 years after the end of treatment, but not those over 5 years. This finding may indicate a long-term recovery from chemotherapy-induced effect. Nevertheless, we could not support evidence that a higher cumulative dose of MTX was associated with a lower BMD. It is also doubtful whether methotrexate and GCS have a synergistic effect on bone loss. None of our analyses confirmed the negative effects of such a combined therapy on BMD. Many studies have highlighted the negative effect of GCS on bone mass, by inhibiting osteoblast function and increasing bone resorption. In this study, the vast majority of analyzed subjects had received GCS, including dexamethasone which is postulated to have the most negative effect on bone. Although we sought an effect of this potentially harmful treatment modality on the skeleton in studied CCS, we found no association between low BMD and past history of long-term GCS treatment in.
Published data indicate that male survivors appeared to be more susceptible to treatment effects on BMD than female survivors, which might reflect higher vulnerability to chemotherapy-related hypogonadism during puberty [23]. Moreover, males achieve peak bone mass a few years later than females, which also may explain the greater susceptibility of the skeleton to treatment-induced damage. We did not confirm the association between low BMD and sex in this study.
In addition, we examined changes in BMD over time in 123 CCS. In the subgroup of participants with two DXA scans performed over time, 66% of those, who had had BMD Z-score below − 2 at the first measurement, improved their BMD up to ≥ − 2, whereas 50% of those, who had previously had BMD Z-score between < − 1 and ≥ − 2, showed a time-related shift of their BMD assignment to a higher range (i.e., above > − 1). This supports the view that BMD trajectory may normalize with age, and catch-up with same-age healthy population. Moreover, Lowess regression plots showed the improvement of BMD until the age of about 26–27, while in healthy individuals the peak bone mass is usually attained in their early-thirties. Importantly, our results suggest that CCS may achieve peak bone mass later in life compared with general population. This has been reported in previous studies [13, 31]. However, the possibility of selection bias, resulting from the partial analysis of some CCS population only, should be taken into consideration. Furthermore, one should be very cautious in generalizing the results to the whole population of survivors due to the probability of regression toward the mean effect.
We found significantly higher BMD Z-scores in the long-term CCS compared to those who had an observation time shorter than 5 years. Furthermore, among short-term survivors (follow-up time < 5 years), it was found that the odds of low BMD decreased with age of diagnosis, but this link in a group of long-term survivors (follow-up time > 5 years) disappeared. Presumably, a longer time interval after the end of the treatment may be beneficial for bone remodeling despite their delayed repair.
There are several limitations in this study. As there were some missing data at the moment of diagnosis (i.e., initial BMD may have been either within or beyond the normal reference), the association or cause-effect between pre-treatment BMD and subsequent treatment-related changes in BMD may remain unexplained. Moreover, the results of the retrospective single-center cohort study should be interpreted in a context of possible bias which may occur; these include the following: (1) not all types of cancers were included (brain and bone tumors were not analyzed), (2) the analysis over time was based on only part of the subjects (survivors with two DXA measurement after treatment), and (3) not all confounding factors were taken into consideration because no sufficient data were available on physical activity, diet, vitamin D and calcium intake, family history, fractures, and genetics [32,33,34,35,36,37,38]. We decided not to extrapolate our results to the older populations in whom the risk of bone loss is similar as in general population. Finally, there are some methodological issues to be discussed as our approach may be affected by the predefined cutoff of low BMD being at Z-scores less than − 1, instead of ≤ − 2 (as recommended by ISCD) [39].
The strengths of our research include the reasonably large cohort, a long follow-up time, no ethnic diversity, and the inclusion of several types of pediatric cancers. An additional advantage of our study is the approach using BMD Z-scores adjusted for age and height, which enables a better comparison of the results with the most available reports. Moreover, this is one of the few studies assessing changes in BMD over time in CCS [13, 31, 40]. Our results, consistently with those previously published, may play a role in a better understanding of the factors associated with a decreased BMD among CCS, and may therefore be another contribution to develop guidelines for this specific population.
In recent years, great effort has been made to determine which CCS are at risk of low BMD and to establish which particular treatment-related factors have the most deleterious effect on bones. This is particularly important in the context of recent studies showing that reduced BMD is associated with a higher fracture risk in CCS [8]. As the population of survivors is becoming increasingly numerous, there is a need to provide a simple, useful tool for routine medical practice, helping to detect CCS most at risk of bone loss. Van Atteveld et al. have attempted to develop and validate prediction models for low BMD (Z-score ≤ − 1) and very low BMD (Z-score ≤ − 2) among adult CCS. They examined two large cohort—2032 survivors from St Jude Lifetime Cohort (SJLIFE, development) and 403 survivors treated at Erasmus Medical Centre (validation). The models included male sex, height, weight, age at DXA scan, current smoking status, and irradiation (cranial and abdominal). The risk calculator is available online [41]. The authors recommend their models as a reasonable tool for personalized diagnostics which is likely to improve identification of young adult CCS who might benefit from DXA exam. We share the opinion that such a tool will be very helpful in everyday practice, but at the same time we are aware that the impact of factors that have not been well researched so far (i.e., lifestyle, fracture risk, medical interventions at improving low BMD) may significantly influence the risk assessment [42]. Nevertheless, there are still no large observational or randomized studies taking into account all the above-mentioned variables in one research [29]. It is also worth noting that the use of new densitometric measurements, such as the total body less head (TBLH), as well as the application of new imaging techniques may allow faster progress in this field [43].
Little recommendations are available for long-term follow-up (LFTU) of bone condition among CCS [5]. Most of them highlighted the detrimental influence of cranial radiotherapy, previous treatment with GCS or methotrexate, and hematopoietic stem cell transplantation. However, these recommendations are not superb and need to be updated in the context of a growing number of recent studies. In this respect, evidence-based LFTU surveillance guidelines should provide an optimal tool to carry out a targeted screening to early detection of decreased bone mass among CCS, which is likely to lead to fewer fractures in the future. Finally, it should be stressed that the most crucial moment for bone development takes place in childhood, especially during puberty [44, 45]. Therefore, most of the effective interventions should take place at the time when bone mass is accrued. Future studies are needed to investigate lifestyle, physical activity, diet, long-term fracture, and genetics in this growing population.
Conclusions
In summary, we showed that low BMD occurred in 24% of childhood cancer survivors, while very low BMD was relatively uncommon at 8%. We confirm that low BMD in CCS may become alleviated over time. Hematopoietic stem cell transplantation, radiotherapy to the head and neck, and body weight below the standard reference at the follow-up visit were the strongest independent predictors of decreased BMD in this study. In CCS, the risk of low skeletal mass in young adulthood is regarded similar to that in general non-cancer population, at least based on the BMD assessment. These data, encompassing a long-lasting observation period and based on serial DXA measurements, provide some optimistic scenario showing that neither female nor male CCS are at a higher risk of premature bone loss in young adulthood. Though it is not yet clear how the trajectory of skeletal mass is until the CCS will achieve older age and/or postmenopause. Thus, conclusions from this study should be drawn with caution as bone mass and quality remains under several multifactorial environmental effects. Our data may contribute to the development of future surveillance guidelines for CCS, dealing with long-term effects of anti-cancer therapy on bone.
References
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572–1582. https://doi.org/10.1056/NEJMsa060185
Chemaitilly W, Cohen LE, Mostoufi-Moab S, Patterson BC, Simmons JH, Meacham LR, van Santen HM, Sklar CA (2018) Endocrine late effects in childhood cancer survivors. J Clin Oncol 36:2153–2159. https://doi.org/10.1200/JCO.2017.76.3268
Gordon CM, Zemel BS, Wren TAL, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK (2017) The determinants of peak bone mass. J Pediatr 180:261–269. https://doi.org/10.1016/j.jpeds.2016.09.056
Siegel DA, Claridy M, Mertens A, George E, Vangile K, Simoneaux SF, Meacham LR, Wasilewski-Masker K (2017) Risk factors and surveillance for reduced bone mineral density in pediatric cancer survivors. Pediatr Blood Cancer 64:e26488. https://doi.org/10.1002/pbc.26488
Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR (2008) Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics 121:e705–e713. https://doi.org/10.1542/peds.2007-1396
den Hoed MAH, Klap BC, te Winkel ML, Pieters R, van Waas M, Neggers SJCMM, Boot AM, Blijdorp K, van Dorp W, Pluijm SMF, van den Heuvel-Eibrink MM (2015) Bone mineral density after childhood cancer in 346 long-term adult survivors of childhood cancer. Osteoporos Int 26:521–529. https://doi.org/10.1007/s00198-014-2878-z
Lim JS, Kim DH, Lee JA, Kim DH, Cho J, Cho WH, Lee SY, Jeon DG (2013) Young age at diagnosis, male sex, and decreased lean mass are risk factors of osteoporosis in long-term survivors of osteosarcoma. Pediatr Hematol Oncol 35:54–60. https://doi.org/10.1097/MPH.0b013e318275193b
Bloomhardt HM, Sint K, Ross WL, Rotatori J, Ness K, Robinson C, Carpenter TO, Chow EJ, Kadan-Lottick NS (2020) Severity of reduced bone mineral density and risk of fractures in long-term survivors of childhood leukemia and lymphoma undergoing guideline-recommended surveillance for bone health. Cancer 126:202–210. https://doi.org/10.1002/cncr.32512
van Atteveld JE, Pluijm SMF, Ness KK, Hudson MM, Chemaitilly W, Kaste SC, Robison LL, Neggers SJCMM, Yasui Y, van den Heuvel-Eibrink MM, Wilson CL (2019) Prediction of low and very low bone mineral density among adult survivors of childhood cancer. J Clin Oncol 37:2217–2225. https://doi.org/10.1200/JCO.18.01917
Latoch E, Muszyńska-Rosłan K, Panas A, Panasiuk A, Rutkowska-Żelazowska B, Konstantynowicz J, Krawczuk-Rybak M (2015) Bone mineral density, thyroid function, and gonadal status in young adult survivors of childhood cancer. Contemp Oncol (Pozn) 2:142–147. https://doi.org/10.5114/wo.2014.44123
Kaste SC, Metzger ML, Minhas A, Xiong Z, Rai SN, Ness KK, Hudson MM (2009) Pediatric Hodgkin lymphoma survivors at negligible risk for significant bone mineral density deficits. Pediatr Blood Cancer 52:516–521. https://doi.org/10.1002/pbc.21908
Baxter-Jones ADG, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA (2011) Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J Bone Miner Res 26:1729–1739. https://doi.org/10.1002/jbmr.412
Gurney JG, Kaste SC, Liu W, Srivastava DK, Chemaitilly W, Ness KK, Lanctot JQ, Ojha RP, Nottage KA, Wilson CL, Li Z, Robison LL, Hudson MM (2014) Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: Results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer 61:1270–1276. https://doi.org/10.1002/pbc.25010
Muszyńska-Rosłan K, Latoch E, Konstantynowicz J, Panasiuk A, Stewart A, Krawczuk-Rybak M (2014) Bone mineral density in pediatric survivors of Hodgkin and non-Hodgkin lymphomas. Adv Med Sci 59:200–205. https://doi.org/10.1016/j.advms.2014.02.004
Muszyńska-Rosłan K, Panasiuk A, Latoch E, Krawczuk-Rybak M, Konstantynowicz J (2012) Little evidence of low bone mass in acute lymphoblastic leukemia survivors. J Clin Densitom 15:108–115. https://doi.org/10.1016/j.jocd.2011.07.004
Polgreen LE, Petryk A, Dietz AC, Sinaiko AR, Leisenring W, Goodman P et al (2012) Modifiable risk factors associated with bone deficits in childhood cancer survivors. BMC Pediatr 12:40 12(1). https://doi.org/10.1186/1471-2431-12-40
Utriainen P, Vatanen A, Toiviainen-Salo S, Saarinen-Pihkala U, Mäkitie O, Jahnukainen K (2017) Skeletal outcome in long-term survivors of childhood high-risk neuroblastoma treated with high-dose therapy and autologous stem cell rescue. Bone Marrow Transplant 52:711–716. https://doi.org/10.1038/bmt.2016.345
Makras P, Terpos E, Kanakis G, Papatheodorou A, Anastasilakis AD, Kokkoris P, Kaltsas GA (2012) Reduced bone mineral density in adult patients with Langerhans cell histiocytosis. Pediatr Blood Cancer 58:819–822. https://doi.org/10.1002/pbc.23166
Schündeln MM, Hauffa PK, Bauer JJ, Temming P, Sauerwein W, Biewald E, Bornfeld N, Hauffa BP, Grasemann C (2015) Pediatric survivors of retinoblastoma are at risk for altered bone metabolism after chemotherapy treatment early in life. Pediat Hematol Oncol 32:455–466. https://doi.org/10.3109/08880018.2015.1048912
Muszyńska-Rosłan K, Konstantynowicz J, Panasiuk A, Krawczuk-Rybak M (2009) Is the treatment for childhood solid tumors associated with lower bone mass than that for leukemia and Hodgkin disease? Pediatr Hematol Oncol 26:36–47. https://doi.org/10.1080/08880010802625472
Remes TM, Arikoski PM, Lähteenmäki PM, Arola MO, Pokka TM-L, Riikonen VP, Sirkiä KH, Rantala HMJ, Harila-Saari AH, Ojaniemi MK (2018) Bone mineral density is compromised in very long-term survivors of irradiated childhood brain tumor. Acta Oncol 57:665–674. https://doi.org/10.1080/0284186X.2018.1431401
Kaste SC, Jones-Wallace D, Rose SR, Boyett JM, Lustig RH, Rivera GK, Pui CH, Hudson MM (2001) Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia 15:728–734. https://doi.org/10.1038/sj.leu.2402078
Isaksson S, Bogefors K, Åkesson K, Øra I, Egund L, Bobjer J, Leijonhufvud I, Giwercman A (2020) Low bone mineral density is associated with hypogonadism and cranial irradiation in male childhood cancer survivors. Osteoporos Int. 31:1261–1272. https://doi.org/10.1007/s00198-020-05285-4
Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel B, Shults J, Leonard MB (2012) Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res 27:760–769. https://doi.org/10.1002/jbmr.1499
Mostoufi-Moab S, Ward LM (2019) Skeletal morbidity in children and adolescents during and following cancer therapy. Horm Res Paediatr 91:137–151. https://doi.org/10.1159/000494809
Wei C, Candler T, Davis N, Elson R, Crabtree N, Stevens M, Crowne E (2019) Bone mineral density corrected for size in childhood leukemia survivors treated with haematopoietic stem cell transplantation and total body irradiation. Horm Res Paediatr 89:246–254. https://doi.org/10.1159/000487996
Mostoufi-Moab S, Brodsky J, Isaacoff EJ, Tsampalieros A, Ginsberg JP, Zemel B, Shults J, Leonard MB (2012) Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab 97:3584–3592. https://doi.org/10.1210/jc.2012-2393
Buxbaum NP, Robinson C, Sinaii N, Ling A, Curtis LM, Pavletic SZ, Baird K, Lodish MB (2018) Impaired bone mineral density in pediatric patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant 24:1415–1423. https://doi.org/10.1016/j.bbmt.2018.02.019
Marcucci G, Beltrami G, Tamburini A, Body JJ, Confavreux CB, Hadji P, Holzer G, Kendler D, Napoli N, Pierroz DD, Rizzoli R, Brandi ML (2019) Bone health in childhood cancer: review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncology 30:908–920. https://doi.org/10.1093/annonc/mdz120
Chemaitilly W, Sklar CA (2010) Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer 17:R141–R159. https://doi.org/10.1677/ERC-10-0002
Pluijm S, den Hoed M, van den Heuvel-Eibrink M (2015) Catch-up of bone mineral density among long-term survivors of childhood cancer? Letter to the editor: Response to the article of Gurney et al. 2014. Pediatr Blood Cancer 62:369–370. https://doi.org/10.1002/pbc.25324
den Hoed MAH, Pluijm SMF, Stolk L, Uitterlinden AG, Pieters R, van den Heuvel-Eibrink MM (2016) Genetic variation and bone mineral density in long-term adult survivors of childhood cancer. Pediatr Blood Cancer 63:2212–2220. https://doi.org/10.1002/pbc.25324
Choudhary A, Chou J, Heller G, Sklar C (2013) Prevalence of vitamin D insufficiency in survivors of childhood cancer. Pediatr Blood Cancer 60:1237–1239. https://doi.org/10.1002/pbc.24403
Kaste SC, Qi A, Smith K, Surprise H, Lovorn E, Boyett J, Ferry RJ Jr, Relling MV, Shurtleff SA, Pui CH, Carbone L, Hudson MM, Ness KK (2014) Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer 61:885–893. https://doi.org/10.1002/pbc.24882
Sloof N, Hendershot E, Griffin M, Anderson L, Marjerrison S (2019) The Impact of Physical Activity on the Health of Young Adult Survivors of Childhood Cancer: An Exploratory Analysis. J Adolesc Young Adult Oncol 8:602–609. https://doi.org/10.1089/jayao.2019.0001
te Winkel ML, Pieters R, Hop WCJ, Roos JC, Bökkerink JPM, Leeuw JA, Bruin MCA, Kollen WJW, Veerman AJP, de Groot-Kruseman HA, van der Sluis IM, van den Heuvel-Eibrink MM (2014) Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the DCOG-ALL9 protocol. Bone 59:223–228. https://doi.org/10.1016/j.bone.2013.11.017
Zhang FF, Ojha RP, Krull KR, Gibson TM, Lu L, Lanctot J et al (2016) Adult survivors of childhood cancer have poor adherence to dietary Guidelines. J Nutr 146:2497–2505. https://doi.org/10.1002/ijc.32963
Zürcher SJ, Jung R, Monnerat S, Schindera C, Eser P, Meier C, Rueegg CS, von der Weid NX, Kriemler S (2020) High impact physical activity and bone health of lower extremities in childhood cancer survivors: a cross-sectional study of SURfit. Int J Cancer. 147:1845–1854. https://doi.org/10.1002/ijc.32963
Guss CE, McAllister A, Gordon CM (2020) DXA in children and adolescents. J Clin Densitom 25:S1094-6950(19)30233-1. https://doi.org/10.1016/j.jocd.2020.01.006
Inaba H, Cao X, Han AQ, Panetta JC, Ness KK, Metzger ML, Rubnitz JE, Ribeiro RC, Sandlund JT, Jeha S, Cheng C, Pui CH, Relling MV, Kaste SC (2018) Bone mineral density in children with acute lymphoblastic leukemia. Cancer 124:1025–1035. https://doi.org/10.1002/cncr.31184
Risk Calculator of low and very low bone mineral density among individual childhood cancer survivors. https://riskcalculator-bonemineraldensity-childhoodcancer.azurewebsites.net 2020 [Accessed 30 April 2020]
Skinner R (2019) Optimizing Detection of Low Bone Mineral Density in Childhood Cancer Survivors. J Clin Oncol 37:2193–2195. https://doi.org/10.1200/JCO.19.01669
Di Iorgi N, Maruca K, Patti G, Mora S (2018) Update on bone density measurements and their interpretation in children and adolescents. Best Pract Res Clin Endocrinol Metab 32:477–498. https://doi.org/10.1016/j.beem.2018.06.002
Wilson CL, Ness KK (2013) Bone mineral density deficits and fractures in survivors of childhood cancer. Curr Osteoporos Rep 11:329–337. https://doi.org/10.1007/s11914-013-0165-0
Mogil RJ, Kaste SC, Ferry RJ, Hudson MM, Mulrooney DA, Howell CR et al (2016) Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors. JAMA Oncol 2:908–914. https://doi.org/10.1001/jamaoncol.2015.6557
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 31.6 kb)
Rights and permissions
About this article
Cite this article
Latoch, E., Konstantynowicz, J., Krawczuk-Rybak, M. et al. A long-term trajectory of bone mineral density in childhood cancer survivors after discontinuation of treatment: retrospective cohort study. Arch Osteoporos 16, 45 (2021). https://doi.org/10.1007/s11657-020-00863-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-020-00863-9