Abstract
Summary
A cross-sectional analysis of 1,662 community dwelling elderly Japanese men suggested that habitual natto intake was significantly associated with higher bone mineral density (BMD). When adjustment was made for undercarboxylated osteocalcin levels, this association was insignificant, showing the natto–bone association to be primarily mediated by vitamin K.
Introduction
Low vitamin K intake is associated with an increased risk of hip fracture, but reports have been inconsistent on its effect on BMD. Our first aim was to examine the association between BMD and intake of fermented soybeans, natto, which contain vitamin K1 (20 μg/pack) and K2 (380 μg/pack). Our second aim was to examine the association between undercarboxylated osteocalcin (ucOC), a biomarker of vitamin K intake, and BMD to evaluate the role of vitamin K in this association.
Methods
Of the Japanese men aged ≥65 years who participated in the baseline survey of the Fujiwara-kyo Osteoporosis Risk in Men study, 1,662 men without diseases or medications known to affect bone metabolism were examined for associations between self-reported natto intake or serum ucOC levels with lumbar spine or hip BMD.
Results
The subjects with greater intake of natto showed significantly lower level of serum ucOC. Analysis after adjustment for confounding variables showed an association of greater intake of natto with both significantly higher BMD and lower risk of low BMD (T-score < −1 SD) at the total hip and femoral neck. This association became insignificant after further adjustment for ucOC level.
Conclusion
Habitual intake of natto was associated with a beneficial effect on bone health in elderly men, and this association is primarily due to vitamin K content of natto, although the lack of information on dietary nutrient intake, including vitamin K1 and K2, prevented us from further examining the association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin K plays an important role in bone metabolism and is expected to have beneficial impact on bone health [1]. Vitamin K naturally exists in two major forms: vitamins K1 and K2. Vitamin K1 is widely distributed in green and leafy vegetables, while vitamin K2 is produced by bacteria during fermentation or is contained in animal-derived foods. The predominant dietary form of vitamin K in the USA, Europe, and most parts of the world is vitamin K1. However, the major form is vitamin K2 in Japan, especially menaquinone-7 (MK-7), which is a component of the fermented soybean product referred to as “natto.”
Many epidemiologic studies have been conducted to evaluate the association between vitamin K intake and bone health, mostly in subjects from the USA or Europe. Feskanich et al. first reported that low intake of vitamin K1 was associated with increased risk of hip fracture in the Nurses’ Health Study [2]. This finding was supported by the Framingham Osteoporosis Study where a protective effect of dietary vitamin K1 for hip fracture was observed in men as well as in women, but unexpectedly no effect was seen on bone mineral density (BMD) [3]. Booth et al. further examined the data from the Framingham Offspring Study and reported that low dietary vitamin K1 intake was associated with low BMD at the spine and hip only in women [4], while low plasma vitamin K1 level was related to low BMD at the femoral neck only in men in a subgroup of the same cohort [5]. The reason for these inconsistencies remains unclear, but they could be due to inaccurate estimation of the intake of green vegetables (the primary source of vitamin K1) obtained from food-frequency questionnaire data.
On the other hand, most studies that have evaluated the association between vitamin K2 and bone health have been conducted in Japan. This is because natto, a major source of vitamin K2 in Japan, is still consumed widely and frequently almost exclusively in Japan [6]. Natto is sold in a plastic pack that usually contains about 40 g of natto, i.e., the quantity considered to be suitable for a meal in Japan. One pack of natto contains about 20 μg of vitamin K1 and about 380 μg of vitamin K2 [6]. Kaneki et al. suggested a possible beneficial effect of natto intake on bone health by showing an inverse association between natto consumption and incidence rate of hip fracture in a prefecture-level correlation study in Japan [7]. This ecological finding led to exploration of the issue in epidemiologic studies. Using data from the Japanese Population-based Osteoporosis (JPOS) Cohort Study, Ikeda et al. found a significant positive association between natto intake and the rate of change in BMD at the hip in postmenopausal women [8]. Natto is usually sold throughout Japan in a plastic package containing approximately 40 g. This facilitates the accurate assessment of natto intake from self-reports in contrast to that of green vegetable intake and may account for the significant association obtained using JPOS study data.
Thus, proper evaluation of the association between vitamin K intake and BMD requires an accurate estimate of vitamin K intake for each individual. Objective biomarkers of vitamin K intake may satisfy this requirement. However, very few studies have used biomarkers for this purpose especially in men. Since vitamin K is a cofactor of γ-carboxylase (which converts glutamate residues to γ-carboxyglutamyl (Gla) residues in osteocalcin (OC), matrix Gla protein, and protein S) [9, 10], the amount of OC with uncarboxylated glutamate residues, i.e., under-γ-carboxylated OC (ucOC), is considered to be a sensitive marker of vitamin K status in the human body [11]. Validity of ucOC level as an indicator of vitamin K intake has been supported by several studies that showed significant inverse correlation between plasma vitamin K level and plasma ucOC level [12–14]. ucOC level may therefore be used to evaluate the association between vitamin K intake and BMD. The primary objective of the present study was to examine the association between natto intake and spine and hip BMD in healthy elderly Japanese men. The secondary objective was to examine the association between ucOC levels and spine and hip BMD to evaluate the role of vitamin K in this association.
Methods
Subjects
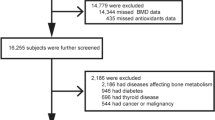
The Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study was conducted as part of a larger cohort study, the Fujiwara-kyo study (primary investigator: Norio Kurumatani, MD, PhD, Professor and Chairman, Department of Community Health and Epidemiology, Nara Medical University School of Medicine). Its aim was to provide a scientific basis for strategies used to prevent frailty, prolong healthy life expectancy, and maintain quality of life of elderly men and women in Japan. Details of the FORMEN study and Fujiwara-kyo study are described elsewhere [15]. The FORMEN study examined 2,174 male volunteers asked to participate in a baseline survey of the Fujiwara-kyo study conducted in 2007 and 2008. Participants were aged 65 years or older at baseline, living in their homes in the cities of Kashihara, Nara, Yamato-Koriyama, and Kashiba, and could walk without the assistance of another person. Of the 2,174 participants, 2,012 completed the study items for the FORMEN study. We excluded 350 men according to the exclusion criteria, including missing natto intake information (five men) and a history of illness or medication usage known to affect bone metabolism (parathyroid disease in 1 participant, connective tissue disease in 20, asthma in 24, ossification of posterior longitudinal ligament in 2, steroid therapy in 19, thyroid diseases with thyroid hormone therapy in 19, surgery for stomach cancer or ulcers in 83, diabetes mellitus with insulin therapy or HbA1c ≥6.5% in 170, prostate cancer with anti-androgen therapy in 37, and no information in 1; 29 men had multiple reasons for exclusion). The final sample consisted of 1,662 men. The study protocol was approved by the Medical Ethics Committee of Nara Medical University and the Ethics Committee of Kinki University School of Medicine. The participants provided written informed consent before enrolment in the study.
Bone mass measurement
BMD was measured by dual-energy X-ray absorptiometry (DXA) at the lumbar spine (L2–4) and the right hip in a posteroanterior projection (QDR4500A, Hologic Inc., Bedford, MA, USA). When subjects had a history of fractures or bone disease in the right hip, the subjects were scanned on the left side. The short-term precision as measured by the coefficients variation (CV) of the BMD measurements in vivo was 1.2%, 1.2%, and 1.6% for the lumbar spine, total hip, and femoral neck, respectively. BMD values at the spine with osteophytes of stage 4 (Nathan’s classification [16]) or excessive calcification due to osteoarthrosis were considered missing. According to the World Health Organization, osteopenia is defined as a T-score higher than −2.5 and lower than −1.0 [17]. Thus, we defined low BMD as ≥1 SD below the young adult mean (i.e., T-score < −1).
Bone turnover markers
Blood samples were collected following an overnight fast, and serum was obtained for several kinds of conventional biochemical tests planned in the Fujiwara-kyo study. The remaining serum was stored at −80°C until measurement of bone turnover markers. We measured levels of undercarboxylated osteocalcin (OC; ucOC) as a biomarker of vitamin K intake, OC as a marker of bone formation, and tartrate-resistant acid phosphatase isoenzyme 5b (TRACP-5b) as a marker of bone resorption [15]. Serum ucOC was measured by an electrochemiluminescence immunoassay. Serum OC was measured by a two-site immunoradiometric assay. Serum TRACP-5b was measured by a fragments absorbed immunocapture enzyme assay. The intraassay CV, interassay CV, and overall CV in the measurements for ucOC were 4.1%, 3.5%, and 5.4%, respectively, 4.9%, 3.7%, and 6.1% for OC, and 4.9%, 7.3%, and 8.8% for TRACP-5b.
Explanatory variables
Height (centimeters) and weight (kilograms) were measured using an automatic scale (Tanita TBF-215, Tanita Inc., Japan). Body mass index (BMI, kilograms per square meter) was calculated from these measurements.
Detailed interviews were conducted to confirm the information given on a questionnaire, including 250 items covering past medical history, medication history, smoking and drinking habits, intake of dairy products, intake of natto, and marital status. Natto is sold in a plastic pack that usually contains about 40 g of natto, i.e., the quantity considered to be suitable for a meal in Japan. One pack of natto contains about 20 μg of vitamin K1 and about 380 μg of vitamin K2 [6]. Participants were asked about the number of packs of natto consumed over a 1-week period and were classified into four groups (less than one pack/week, one pack/week, several packs/week, one pack/day and more). Energy expenditure index by daily physical activities was estimated using International Physical Activity Questionnaire [18] validated for the Japanese elderly [19]. These interviews were conducted by trained public health nurses or medical doctors.
Statistical analysis
SAS statistical software (version 9.1; SAS Institute, Cary, NC, USA) was used for all statistical analysis. We transformed the data of OC level into ranks for analysis since the OC data were not normally distributed, and the values are presented as medians. The geometric mean and SD are used for TRACP-5b and ucOC levels because they followed a logarithmic normal distribution. Analysis of variance (ANOVA) was used to evaluate the significance of the difference in mean BMD and other continuous variables among groups categorized according to natto intake. The chi-square statistic was used to compare the prevalence of lifestyle factors, such as smoking, drinking, milk intake, and history of illness. Analysis of covariance (ANCOVA) was used to evaluate the significance of the difference in mean BMD among groups based on natto intake or quartiles of serum ucOC concentration with adjustments for confounding factors. Adjusted mean BMD was obtained as the least square mean from the ANCOVA model with Tukey–Kramer adjustment for multiple comparisons. Multivariate logistic regression analysis was performed to assess the effect of natto intake on the risk of low BMD after adjustment for potential confounding factors. Also, to evaluate the association of serum ucOC concentration with low BMD, we used the multivariate logistic regression model. Next, we used R 2 as a generalized linear model and Akaike’s Information Criterion (AIC) for the logistic regression model to assess how well the adjusted model fit the data.
Results
Table 1 shows demographic, lifestyle, and clinical characteristics of participants classified by natto intake. There was no difference in age or BMI among the groups based on natto intake. Smoking, drinking, and milk intake were significantly associated with natto intake. Higher prevalence of diabetes mellitus was associated with greater natto intake.
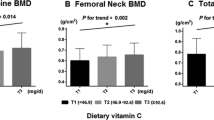
The relationship of levels of biochemical markers of bone turnover and BMD with natto intake is shown in Table 2. With increasing natto intake, a significant dose-dependent decrease in ucOC level and increase in total hip and femoral neck BMD were observed.
Table 3 shows mean values of BMD in the natto intake groups adjusted for age, BMI, milk intake, smoking, alcohol drinking, physical activity, and diabetes mellitus. There was a significant positive association between natto intake and mean values of total hip and femoral neck BMD. The significance of this association did not change when an additional adjustment for OC or TRACP-5b level was made, but it disappeared when the ucOC level was entered into the model for adjustment.
Table 4 shows the results of multiple logistic regression analysis. After adjustment for the same confounding factors used in the analysis shown in Table 3, a statistically significant odds ratio (OR) of low total hip or femoral neck BMD was observed. The OR remained significant when additional adjustments for OC or TRACP-5b levels were made, but it became insignificant when ucOC level was entered into the model for adjustment.
Table 5 shows the adjusted mean BMD and adjusted OR of low BMD in every quartile of serum ucOC concentration. The ucOC level showed a significant negative association with mean BMD and a significant positive association with OR of low BMD even after adjustment for covariates including natto intake.
Discussion
Role of vitamin K in natto’s effect on bone health
In this large-scale community-based single-center study of elderly Japanese men, subjects with a greater intake of natto had significantly higher BMD and a lower prevalence of low BMD at the total hip and femoral neck than subjects with lower intakes of natto, and this association was significantly attenuated when an additional adjustment was made for ucOC levels. Thus, the present study suggests that the natto–BMD association is primarily mediated by vitamin K contained in natto. To our knowledge, this is the first report to identify an association between natto intake and BMD in elderly men, and to examine the association through a biomarker of vitamin K status.
Although only a few studies have been published on the association between natto intake and BMD, there have been clinical trials on the effects of vitamin K2 supplementation on fracture risk or BMD. A systematic review and meta-analysis of randomized controlled trials (RCTs) by Cockayne et al. reported that vitamin K2 supplementation reduced the incidence of vertebral fracture by 60%, hip fracture by 77%, and non-vertebral fractures by 81% and reduced BMD loss by 0.27 SD [20]. However, the trials included in the meta-analysis were all conducted in postmenopausal Japanese women. More recent trials examining Caucasian women reported different results. An RCT examining early menopausal Norwegian women showed that MK-7, taken in the form of natto capsules, over a 1-year period, reduced serum levels of ucOC but did not influence bone loss rates [21]. Another study in postmenopausal American women also failed to find a significant beneficial effect of MK-4 on BMD or proximal femur geometric parameters [22].
For vitamin K1 supplementation, a double-blind controlled trial in Caucasians, Blacks, Hispanics, and Asians showed that the supplemented group had a significantly greater decrease in the proportion of non-carboxylated OC in the total OC than the control group, although there was no difference in BMD change between the groups [23]. Six months of vitamin K1 supplementation did not improve either the spine or femoral neck BMD in an RCT of healthy white women [24]. Observational studies on the association between self-reported dietary vitamin K1 intake and BMD have shown conflicting results. But the Framingham Offspring Study which used an objective measure of vitamin K intake as an exposure variable showed that vitamin K was associated with increased BMD in elderly men [5].
Most studies that reported a beneficial effect of vitamin K on BMD or fracture risk were conducted in Japan on Japanese women and assessed the effect of vitamin K2. In contrast, most studies conducted in Caucasians reported insignificant effects of vitamin K1 intake on BMD, and Gundberg commented that vitamin K supplementation was unlikely to prevent fracture [25]. Thus, ethnic differences between Caucasians and Japanese, including dietary culture and environmental and genetic factors, may exist with regard to the effects of vitamin K intake on BMD or fracture risk [26]. Currently, we have no convincing explanation for the apparent ethnic differences in the effectiveness of vitamin K on bone health, which should be studied further.
A review of RCTs reported that vitamin K1 or K2 supplementation reduced serum ucOC levels, regardless of dose and despite the absence of a significant change or only a modest increase in BMD, and that vitamins K1 and K2 supplementation improved bone strength in the femoral neck, improving femoral neck width and maintaining indices of compression, bending, and impact strength, and reducing the incidence of clinical fractures [12]. The risk reduction for fractures reported for MK-4 supplementation in Japanese subjects was much larger than expected from the change in BMD, indicating that vitamin K may have improved so-called bone quality, including bone geometric properties and/or material properties, rather than simply bone mass [12]. MK-4 supplementation improved hip bone geometry indices in postmenopausal women [27], and age-related changes in the section modulus and buckling ratio in the HSA indices in elderly Japanese women reportedly differ from those in Caucasian women [28]. However, further studies are necessary to explore and explain the apparent ethnic differences as well as to provide a mechanism for the fracture risk reduction by vitamin K.
In most of RCTs conducted in Japan, 45 mg/day of MK-4 was administered and resulted in relatively modest increase in BMD [12, 20]. Natto contains only a small amount of MK-4 (2 μg/100 g) but an extremely large amount of menaquinone-7 (MK-7) (939 μg/100 g) or 100 times the MK-7 content of various kinds of cheese [6, 29]. Nevertheless, the vitamin K content in a pack of natto is only a few percent of the pharmaceutical dose. MK-7 had a much longer half-life in serum than other forms of vitamin K (3 days vs. 1–2 h for vitamin K1) [29, 30]. Thus, people who habitually consume natto may maintain higher serum levels of MK-7 leading to reduction in bone loss over time.
Other mechanisms that may account for the effects of natto intake on bone metabolism
In addition to the above, natto may work via other mechanisms to regulate bone metabolism. As shown in Table 4, the OR of low BMD in the group with the greatest natto intake became statistically insignificant when adjusted for the ucOC level. However, the OR remained below 1. This may indicate that natto also protects bone via pathways independent of vitamin K. Natto contains large amounts of isoflavones in addition to vitamin K. Some studies have shown that soy isoflavone can effectively decrease bone resorption [31], and a high isoflavone-containing product may reduce spinal bone loss in postmenopausal women [32]. Natto is also relatively rich in calcium (90 mg per 100 g of natto). Calcium supplementation, alone or in combination with vitamin D, has been reported to reduce bone loss in a meta-analysis [33]. Thus, habitual natto intake may prevent bone loss by ensuring an adequate supply of isoflavones and calcium as well as vitamin K.
Association between natto intake and diabetes mellitus
In the present study, subjects with greater intakes of natto had significantly higher prevalences of diabetes mellitus than subjects with lower intakes. We also showed that natto intake was inversely associated with plasma ucOC level. Recently, it was reported that ucOC increased pancreatic β-cell proliferation and secretion of insulin, while enhancing insulin sensitivity and reducing the development of obesity and glucose intolerance in mice [34]. In previous reports, ucOC was inversely associated with fasting plasma glucose levels and fat mass in diabetic patients [35] and in mostly diabetic subjects [36]. However, a beneficial effect of vitamin K1 on glucose tolerance was reported in a subgroup analysis comprising male participants from a randomized controlled trial [37]. Provided that the hormonal functions of ucOC in mice are conserved in humans, natto intake may have an adverse effect on glucose metabolism.
Strengths and limitations of the study
The present study has some advantages over previous studies. The scale of this study was large enough to afford sufficient statistical power. Self-reports of natto intake from participants should be quite reliable because natto intake level was strongly correlated with ucOC levels, a biomarker for vitamin K intake.
However, the present study also has several limitations. First, participants were not randomly selected from the population, and patients with severe or symptomatic diseases may not have participated in the study. This sampling method may have biased the sample towards healthier individuals. Second, the present study was a baseline survey of a cohort, and cross-sectional analyses of these data cannot establish causality between natto or vitamin K intake and BMD. The effects of natto or vitamin K intake on change in BMD and on risk of osteoporotic fracture should be investigated in follow-up studies. Third, natto intake was significantly correlated with smoking, drinking, and milk intake, which were adjusted for in the multivariate analyses. R 2 values of the generalized linear model for BMD increased 10–30 times in all models, and AIC of the logistic regression model for low BMD decreased by approximately 50 by the adjustment, indicating that the adjusted models achieved a substantially better fit of the data. However, these adjustments may not eliminate the confounding effects, and other confounding factors that we did not consider or a healthier lifestyle related to natto intake that we did not assess may exist. Fourth, we obtained a significant natto–BMD association at the hip or femoral neck but not in the spine. Measurement of lumbar spine BMD using DXA cannot exclude aortic calcification and is affected by spondylosis deformans and osteoarthrosis [38]. We could not eliminate the effects of these extra-skeletal calcifications or vertebral deformities from the real association. Finally, information on dietary intake of energy, macro- or micronutrients, and vegetables was not available. We understand that this is a serious limitation of our study. If differences in these intakes among the natto intake groups existed, they may have confounded the association of interest. Using ucOC levels as a biomarker for vitamin K intake may have reduced any possible confounding effects due to energy or micro/macronutrients in the assessment of the natto–BMD association. However, ucOC levels do not represent intakes of vitamins K1 and K2 separately. Intake of vegetables that provide vitamin K1 may have confounded the association. However, a previous study found no difference in vegetable intakes between groups of Japanese with differing natto intakes [7]. Nevertheless, we accept that differences in vegetable intakes among the natto intake groups may have affected our results.
Conclusion
The FORMEN study showed that high natto intake was associated with lower ucOC and higher BMD. Habitual intake of natto was associated with a beneficial effect on bone health in elderly men, and this association is primarily due to the vitamin K content of natto.
References
Binkley NC, Suttie JW (1995) Vitamin K nutrition and osteoporosis. J Nutr 125:1812–1821
Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA (1999) Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr 69:74–79
Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PW, Ordovas J, Schaefer EJ, Dawson-Hughes B, Kiel DP (2000) Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr 71:1201–1208
Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Dawson-Hughes B, Wilson PW, Cupples LA, Kiel DP (2003) Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr 77:512–516
Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP (2004) Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab 89:4904–4909
Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S, Okano T (2007) Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol (Tokyo) 53:464–470
Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H (2001) Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 17:315–321
Ikeda Y, Iki M, Morita A, Kajita E, Kagamimori S, Kagawa Y, Yoneshima H (2006) Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J Nutr 136:1323–1328
Furie B, Bouchard BA, Furie BC (1999) Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood 93:1798–1808
Ferland G (1998) The vitamin K-dependent proteins: an update. Nutr Rev 56:223–230
Gundberg CM, Nieman SD, Abrams S, Rosen H (1998) Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 83:3258–3266
Iwamoto J, Sato Y, Takeda T, Matsumoto H (2009) High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: a review of the literature. Nutr Res 29:221–228
Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tanaka K, Okano T (2006) Vitamin K status of healthy Japanese women: age-related vitamin K requirement for gamma-carboxylation of osteocalcin. Am J Clin Nutr 83:380–386
Shearer MJ (1995) Vitamin K. Lancet 345:229–234
Iki M, Fujita Y, Tamaki J, Kouda K, Yura A, Kadowaki E, Sato Y, Moon JS, Okamoto N, Kurumatani N, Study Group for Functioning Capacity and Quality of Life in Elderly Japanese (Fujiwara-kyo Study Group) (2009) Design and baseline characteristics of a prospective cohort study for determinants of osteoporotic fracture in community-dwelling elderly Japanese men: the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. BMC Musculoskelet Disord 10:165
Nathan H (1962) Osteophytes of the vertebral column. J Bone Joint Surg 44-A:243–269
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395
Tomioka K, Hazaki K, Iwamoto J (2009) The cross-sectional study of daily step count, physical function and health-related Quality of Life for community-dwelling older adults. The 24th Research-Aid Report in medical and health science of Meiji Yasuda Life Foundation of Health and Welfare 24:1–11 (in Japanese)
Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ (2006) Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 166:1256–1261
Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fønnebø V (2010) Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int 21:1731–1740
Binkley N, Harke J, Krueger D, Engelke J, Vallarta-Ast N, Gemar D, Checovich M, Chappell R, Suttie J (2009) Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res 24:983–991
Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B (2008) Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 93:1217–1223
Volpe SL, Leung MM, Giordano H (2008) Vitamin K supplementation does not significantly impact bone mineral density and biochemical markers of bone in pre- and perimenopausal women. Nutr Res 28:577–582
Gundberg CM (2009) Vitamin K and bone: past, present, and future. J Bone Miner Res 24:980–982
Beavan SR, Prentice A, Stirling DM, Dibba B, Yan L, Harrington DJ, Shearer MJ (2005) Ethnic differences in osteocalcin gamma-carboxylation, plasma phylloquinone (vitamin K1) and apolipoprotein E genotype. Eur J Clin Nutr 59:72–81
Knapen MH, Schurgers LJ, Vermeer C (2007) Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 18:963–972
Iki M, Dongmei N, Tamaki J, Sato Y, Kagamimori S, Kagawa Y, Yoneshima H (in press) For the Japanese Population-based Osteoporosis (JPOS) Study Group (2010) Age-specific reference values of hip geometric indices from a representative sample of the Japanese female population: Japanese Population-based Osteoporosis (JPOS) Study. Osteoporos Int 30
Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C (2007) Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 109:3279–3283
Schurgers LJ, Vermeer C (2000) Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 30:298–307
Harkness LS, Fiedler K, Sehgal AR, Oravec D, Lerner E (2004) Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J Womens Health (Larchmt) 13:1000–1007
Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW Jr (1998) Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr 68:1375S–1379S
Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007) Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370:657–666
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T (2010) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. (in press)
Hwang YC, Jeong IK, Ahn KJ, Chung HY (2009) The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev 25:768–772
Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, Dawson-Hughes B, Dallal G, Booth SL (2008) Effect of vitamin K supplementation on insulin resistance in older men and women. Diab Care 31:2092–2096
Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK (1992) Age-related decrements in bone mineral density in women over 65. J Bone Miner Res 7:625–632
Acknowledgments
The Fujiwara-kyo Study Group, chaired by Norio Kurumatani with Nozomi Okamoto as a secretary general comprising Nobuko Amano, Yuki Fujita, Akihiro Harano, Kan Hazaki, Masayuki Iki, Junko Iwamoto, Akira Minematsu, Masayuki Morikawa, Keigo Saeki, Noriyuki Tanaka, Kimiko Tomioka, and Motokazu Yanagi, performed most non-skeletal measures in the present study and provided the data to the FORMEN study. The FORMEN study was supported by several grants as follows: Grants-in-Aid for Scientific Research (no. 20659103: 2008–2009, no. 21390210: 2009–2011, no. 20590661: 2008–2010) from the Japanese Society for the Promotion of Science, a Grant-in-Aid for Young Scientists (no. 20790451: 2008–2010) from the Japanese Ministry of Education, Culture, Sports, Science and Technology; a Grant-in-Aid for Study on Milk Nutrition (2008) from the Japan Dairy Association; a grant (2007) from the Foundation for Total Health Promotion; a St. Luke's Life Science Institute Grant-in-Aid for Epidemiological Research (2008); and a grant (2008) from the Physical Fitness Research Institute, MEIJIYASUDA Life Foundation of Health and Welfare. The authors acknowledge Eisai Co. Ltd. (Tokyo, Japan) and Sanko Junyaku Co. Ltd. (Tokyo, Japan) for their cooperation in measuring ucOC in sera, Toyukai Medical Corporation (Tokyo, Japan) and Toyo Medic Inc., (Osaka, Japan) for DXA scanning, and SRL Inc. (Tokyo, Japan) for their technical assistance in laboratory measurements.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, Y., Iki, M., Tamaki, J. et al. Association between vitamin K intake from fermented soybeans, natto, and bone mineral density in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Osteoporos Int 23, 705–714 (2012). https://doi.org/10.1007/s00198-011-1594-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1594-1