Abstract
Introduction
There is evidence that individual antioxidants may increase bone mineral density (BMD) in patients with low BMD. However, the association between overall dietary antioxidant intake and BMD is unclear. The objective of this study was to examine how overall dietary antioxidant intake is related to BMD.
Materials and methods
A total of 14,069 people participated in the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2010. Dietary Antioxidant Index (DAI) was calculated from the intake of vitamins A, C, E, zinc, selenium, and magnesium, which indicates a nutritional tool to assess the overall antioxidant properties of the diet. The correlation between the Composite Dietary Antioxidant Index (CDAI) and BMD was examined using multivariate logistic regression models. In addition to fitting smoothing curves, we fitted generalized additive models as well. Furthermore, to ensure data stability and avoid confounding factors, subgroup analysis was also conducted on gender and body mass index (BMI).
Results
A significant association was demonstrated by the study between CDAI and total spine BMD (β = 0.001, 95% CI 0–0.001, P = 0.00039). And just like that, CDAI was positively correlated with femoral neck (β = 0.003, 95% CI 0.003–0.004, P < 0.00001) and trochanter (β = 0.004, 95% CI 0.003–0.004, P < 0.00001). In the gender subgroup analysis, CDAI maintained a strong positive correlation with femoral neck and trochanter BMD in males and females. Nevertheless, the link with total spine BMD was only observed in males. In addition, in the subgroup analysis stratified by BMI, CDAI showed a significantly positive relation to BMD of the femoral neck and trochanter in each group. However, the significant relationship between CDAI and BMD of the total spine was only maintained when BMI was above 30 kg/m2.
Conclusion
This study found that CDAI correlated positively with femoral neck, trochanter, and total spine BMD. This suggests that intake of a diet rich in antioxidants can reduce the risk of low bone mass and osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common metabolic osteopathy, with characteristics of decreased bone mineral density (BMD), which can cause bone fragility and an increased risk of fracture [1, 2]. As the population ages, approximately 200 million individuals worldwide suffer from osteoporosis. It is estimated that 13.5 million Americans will have osteoporosis by 2030, an increase from 10.2 million in 2010 [3, 4]. In terms of finance, as the global population ages, the direct cost of osteoporosis is expected to reach around $25.3 billion per year by 2025 [5], imposing a heavy burden on the health care system and social economy [6]. Furthermore, osteoporosis has been globally recognized as a public health concern due to its rising morbidity, mortality, and healthcare costs.
Current osteoporosis treatment is aimed at correcting the imbalance between bone resorption and bone formation [7]. It has recently become clear that oxidative stress contributes significantly to bone resorption and osteogenesis imbalances [8]. As a result of oxidative stress, osteoblasts are more likely to die, undergo bone remodeling, and lose bone mass as a result of increased inflammation. Several studies have revealed that antioxidants reduce free radicals to prevent bone loss caused by oxidative stress [9]. Thus, dietary antioxidants probably contribute to osteoporosis prevention and treatment.
There has been a lot of research on osteoporosis and individual antioxidants in the past. Carotenoids, particularly lycopene, may protect older people's BMD from bone loss, according to Sahni et al. [10]. In addition, Hyun et al. also found that dietary zinc intake and plasma zinc were positively correlated with BMD in men [11]. Another meta-analysis observed a negative association between vitamin C intake and osteoporosis risk [12]. In addition to individual nutrition, food effects on health were also affected by the interaction between them. For this reason, we used the Compound Dietary Antioxidant Index (CDAI). Developed by Wright et al. to quantify dietary antioxidant capacity, CDAI measures the potential antioxidant capacity of a diet [13]. Current research has shown that the composite antioxidant index is negatively linked with cancer, cardiovascular disease, and diabetes [14,15,16]. However, there are few studies on the relationship between CDAI and BMD. In this study, we used data from the National Health and Nutrition Examination Survey (NHANES) 2005–2010 to investigate the relationship between CDAI and BMD.
Materials and methods
Study population
To obtain nationally representative health and nutrition data for the noninstitutionalized population, the NHANES employs a complex, multistage, and stratified sampling method. In addition, an ethics review board of the National Center for Health Statistics Research approved all NHANES protocols, and all participants provided informed consent [17].
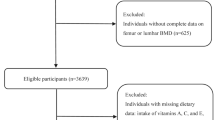
The NHANES dataset was utilized for this investigation from 2005 to 2010. A nutrition investigation, medical examination, and medical condition questionnaire were completed by all participants (n = 31,034). These criteria were used for exclusion: (1) missing BMD data (n = 14,344); (2) missing antioxidant data (n = 435); (3) patients with a history of cancer diseases, thyroid diseases or diabetes (2186); therefore, the final number of subjects included in this study was 14,069 (Fig. 1).
Calculation of the composite dietary antioxidant index
A 24-h dietary recall interview is offered to all NHANES participants. Using Survey Net, which was developed for computer-assisted food coding and data management, interview data files were electronically sent from the field and imported into the system. The NHANES website contains two types of dietary data: Individual Foods and Total Nutrient Intakes. Based on the Total Nutrient Intake Files, this study analyzed participants’ daily total nutrient and energy intakes. For vitamin A, NHANES provides information on vitamin A intake (mg) on its website. Six dietary antioxidant micronutrients were considered in calculating the DAI, including vitamins A, C, E, zinc, magnesium, and selenium.
In a previous report, CDAI development was described and validated [13, 18]. To estimate CDAI, we subtracted the mean and divided by the SD from each of the above antioxidant micronutrients. We then calculated the CDAI by adding up the standardized intakes of the micronutrients, as follows:
BMD measurement
Bone health is often assessed using BMD. As a standard method of measuring BMD and identifying osteoporosis risks, dual-energy X-ray absorptiometry (DXA) is conducted [19]. DXA with a fan-beam densitometer was used to measure BMD in this study (Hologic Inc., Bedford, MA, USA [20], which is an internationally accepted standard-of-care screening tool used to assess fragility-fracture risk. Certified and trained radiology technologists administered the DXA examinations. Participants aged 8 and older who qualified for the survey received DXA scans. A DXA examination was not available for pregnant women, patients with a recent history of radiographic contrast material, and patients weighing more than 300 pounds.
Baseline data collection
Data on demographics, diet, examination, laboratory, and questionnaires are the covariates. Demographics data covered age (years, range 8 to 85, average:30), gender (male and female), race, level of education (under high school graduate, high school, some college or over, missing), family income-poverty ratio (%) and marital status (separated/divorced/widowed/never married, married/living with partner, missing/refused/don’t know). Dietary data included Vitamin E (mg), Vitamin A RAE (mg), Vitamin C (mg), Magnesium (mg), Zinc (mg), Selenium (mg), Calcium (mg); Energy (kcal); Phosphorus (mg). Examination data included weight (kg), height (m), body mass index (BMI, kg/m2). And, laboratory data consisted of white blood cell (1000 cells/μL), lymphocyte (1000 cells/μL), segmented neutrophils (1000 cells/μL), C-reactive protein (mg/dL). Finally, questionnaire data covered alcohol consumption (yes/no), smoking behavior (now, former, never), arthritis (yes/no) and hypertension (yes/no). Smoking was diagnosed according to the following criteria: The current active smoker has smoked more than 100 cigarettes throughout life, and they smoke occasionally or every day now; Former smokers have smoked more than 100 cigarettes throughout their lives and have given up smoking; A non-smoker has smoked fewer than 100 cigarettes throughout their lifetime. On the other hand, the diagnostic criteria for the consumption of alcohol were as follows: drunk, had at least 12 alcoholic drinks of any type in your lifetime; never drunk, who does not meet the above criteria; missing drinking data [16].
Statistical analysis
According to the National Center for Health Statistics (NCHS) normative analysis criteria, the NHANES' complicated survey design factors—including weighting, clustering, and stratification—were taken into account. A percentage was used for categorical variables, while a mean and standard deviation were used for continuous variables. Univariate and multivariate linear regression models were used to evaluate the relationship between CDAI and BMD levels. To further investigate the correlation between independent variables and dependent variables, we carried out multiple regressions. In the multiple linear regression models, an unadjusted model (Model 1) was first established, followed by an adjusted model (Model 2) taking into account age, gender and race. Then, using variables like age, gender, race/ethnicity, education level, marital status, smoking status, drinking, white blood cells, lymphocyte number, segmented neutrophils, ratio of family income to poverty, C-reactive protein, arthritis, BMI, hypertension, calcium, energy intake, phosphorus, fully adjusted models (Model 3) were calculated. Previous studies have found a correlation between white blood cells, lymphocytes, neutrophils, and BMD, so we adjusted these factors in Model 3 [21, 22]. Furthermore, to increase confidence in the results, we stratified the data by gender (male and female) and BMI (≤ 18.5, 18.5–24.9, 25–29.9, ≥ 30). As a further method to deal with non-linearity, we also employed weighted generalized additive models. All analyses were conducted using R software (version 4.0.3; easily accessible at https://www.R-project.org) and Empower Stats 2.0 (available at http://www.empowerstats.com). We set the significance probability at P < 0.05.
Results
Baseline characteristics of the participants
Using CDAI quartiles as a classification method, the weighted characteristics of 14,069 participants were analyzed. This study's participants are grouped by CDAI quartile, so Table 1 provides some weighted baseline info. There were younger, non-Hispanic white people, more married, less smokers, and poorer households in the fourth quartile. More notably, significantly higher CDAI was associated with significantly higher femoral neck, trochanter, and total spine BMD (P < 0.001).
The relationship between CDAI and BMD
An analysis of the association between CDAI and BMD was conducted using a multiple linear regression model. Table 2 shows the results for Model 1 (unadjusted), Model 2, and Model 3 (fully adjusted model). A significant correlation was found in Table 2 between CDAI and BMD in Model 1 (β = 0.004 (0.003, 0.004) P < 0.00001). We detected this significant correlation in Model 3 (β = 0.003 (0.003, 0.004) P < 0.00001) after fully adjusting for covariates. In addition, we evaluated the association between CDAI and trochanter BMD (P < 0.00001) and total spine BMD (P = 0.00039) and discovered a strong positive association in the fully adjusted model. Furthermore, for the sensitivity analysis, CDAI was also treated as a categorical variable (quartiles). In all quartile categories, CDAI was correlated significantly with BMD, and BMD level increased as CDAI extent increased. It was found that the femoral neck BMD of the Q4 group was greater than that of the Q1 group by 0.034 g/cm2, the trochanter by 0.039 g/cm2, and the total spine BMD by 0.009 g/cm2. And the trend remained significant among different CDAI quartile groups (P for trend < 0.01).
Subgroup analyses stratified by gender and BMI
To further confirm the robustness of the results observed in Table 2 to potential confounders, we conducted a stratified analysis by subgroups defined by the major covariates known to influence CDAI. As shown in Table 3, in a subgroup analysis stratified by sex, CDAI in males (P < 0.00001) and females (P < 0.00001) were positively correlated with femoral neck and trochanter BMD in a fully adjusted model. Besides, in the gender subgroup analysis between CDAI and total spine BMD, there was a positive association between men [0.006 (0.005, 0.007)] and women [0.005 (0.004, 0.006)] when not adjusted, and this positive association remained significant in men after full adjustment.
Furthermore, in the BMI subgroup analysis, CDAI and femoral neck (P < 0.01) and trochanter BMD (P < 0.01) still showed significant correlation in the four BMI groups after completely adjusting for interference factors. Nevertheless, the relationship between total spine BMD (P = 0.008) and CDAI remained significantly positive only in BMI ≥ 30 group.
In addition, we used a generalized additive model and smooth curve fitting to define a relationship between CDAI and BMD. There was a positive correlation between their relationship in Figs. 2, 3 and 4.
The association between CDAI and total spine BMD. a Each black point represents a sample. Solid red line represents the sample trend. b Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. gender, age, race, education level, marital status, ratio of family income to poverty, white blood cell, lymphocyte number, Segmented neutrophils; C-reactive protein; arthritis; drinks; smoke; BMI; Hypertension; calcium; energy; phosphorus were adjusted
The association between CDAI and trochanter BMD. a Each black point represents a sample. Solid red line represents the sample trend. b Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. gender, age, race, education level, marital status, ratio of family income to poverty, white blood cell, lymphocyte number, Segmented neutrophils; C-reactive protein; arthritis; drinks; smoke; BMI; Hypertension; calcium; energy; phosphorus were adjusted
The association between CDAI and femoral neck BMD. a Each black point represents a sample. Solid red line represents the sample trend. b Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. gender, age, race, education level, marital status, ratio of family income to poverty, white blood cell, lymphocyte number, Segmented neutrophils; C-reactive protein; arthritis; drinks; smoke; BMI; Hypertension; calcium; energy; phosphorus were adjusted
Discussion
To our knowledge, this is the first study to explore the relationship between CDAI and BMD based on the NHANES database. After adjusting for all potential covariates, it was found that CDAI was positively correlated with BMD, suggesting that CDAI is a protective factor for bone mass development. The increasing trend in CDAI was associated with higher BMD levels. Additionally, those in the highest CDAI quartile had greater BMD than those in the lowest quartile.
Most of the studies observed the relationship between individual antioxidants and BMD. The link between certain antioxidant micronutrients and BMD has been examined in earlier research. For instance, a cross-sectional study found that selenium intake was associated with BMD in postmenopausal women, and women with higher selenium levels were less likely to develop osteoporosis [23]. Furthermore, increased dietary consumption of vitamin C was related to higher lumbar and femoral neck BMD, according to a comprehensive review and meta-analysis of observational studies [12]. In addition, a protective effect of carotenoids on BMD in older individuals has also been observed. Carotenoids, especially lycopene, may have a protective effect against bone loss in older adults [10]. Previous cohort studies have shown that both higher blood alpha-tocopherol levels and higher dietary alpha-tocopherol intake are related to higher BMD and reduced osteoporosis and fracture risk, including hip fractures [24,25,26]. A correlation between antioxidants and BMD might be due to an oxidative stress mechanism.
The science of human nutrition has moved over the last several decades from emphasizing particular nutrients to stressing overall dietary quality. Studies that concentrate on the health consequences of a particular nutrient intake have a fundamental flaw in that they neglect the possibility of interactions between other dietary intakes. Because the foods people eat contain a variety of antioxidants, recent studies focused on the total dietary antioxidant index, which is a more comprehensive and accurate approach. In a recent study, the relationship between BMD and dietary intake of antioxidant vitamins and carotenoids was demonstrated [27]. The authors of the study emphasized the need to study combinations of antioxidants, rather than a single intake of antioxidants. In view of the above, we further considered examining the association between the antioxidant dietary quality index and BMD. The results showed that the dietary antioxidant mass fraction was significantly associated with BMD among all study participants. Our findings support the hypothesis that a high intake of antioxidants may be beneficial for bone health. According to our findings, we observed that higher overall intakes of the antioxidant vitamins A, C, E, zinc, selenium, and magnesium, corresponding to higher BMD.
Recent studies have discovered that oxidative stress is a pathogenic factor for many disease states. There is a strong link between oxidative stress and osteoporosis, according to some studies [28]. Oxidative stress affects bone metabolism from bone resorption and bone formation and then affects BMD change. Oxidative stress affects osteoblast activity and osteoblast number, which accelerates bone loss. In addition, the inactivation of β-catenin will lead to delayed bone mineralization. Thus, the Wnt/β-catenin signaling pathway is essential for bone formation and maintenance [29]. In addition, oxidative stress is related to the activation of NADPH oxidase and decreased levels of antioxidant enzymes and glutathione, which can enhance bone resorption [30], and other studies have shown that reactive oxygen species can regulate bone resorption by promoting osteoclast formation and increasing osteoclast activity [31]. Furthermore, the body's antioxidant system protects the body from free radical damage and can prevent the occurrence of oxidative stress. For the loss of bone mass caused by oxidative stress, the intake of antioxidants can be used to protect bone health and prevent osteoporosis [32]. Similarly, dietary antioxidants, such as vitamin E (also known as alpha-tocopherol) and C, beta-carotene and lycopene, are also significant in this context [33], so we explore the relationship between CDAI and BMD, increasing the corresponding supporting evidence.
We conducted subgroup analyses by gender to examine how sex affects bone metabolism. It has been observed that CDAI and BMD are significantly correlated in both genders. The association remained significant after adjusting for multiple confounding factors, suggesting that the relationship was independent of sex. Furthermore, in a subgroup analysis stratified by BMI, we found that CDAI was significantly associated with femoral neck, trochanter, and total spine BMD in all four BMI groups in Model 1. However, after the complete adjustment of confounding factors, the correlation between CDAI and femoral neck and trochanter BMD remained positive, and compared to those with low BMI, those with high BMI had higher BMD. It is worth noting that CDAI and total spine BMD were only found in the BMI ≥ 30 kg/m2 group in Model 3. There was no association between the other BMI groups. Similarly, a cohort study of 845 people reported higher BMD among older subjects in patients with high BMI. This was in addition to the protective effect of high BMI on femoral neck BMD [34]. Similarly, Saarelainen and colleagues found that low BMI increases the risk of osteoporosis and fractures [35].
In our understanding, this is the first study involving a relatively large sample size that examines the association between CDAI and BMD. Food items were evaluated for their overall antioxidant capacity rather than just on single antioxidants, and the accuracy of the assessments was increased. This study has several limitations, including the following: (1) the cross-sectional design of the original survey made it impossible to establish a causal relationship between CDAI and BMD, (2) it is possible that residual confounding exists, (3) food intake can be inaccurately assessed since participants’ memories were used to record food intake for one day.
In conclusion, in this cross-sectional study, we discovered that CDAI were positively correlated with BMD in the general American population, suggesting that people can reduce the risk of low BMD and further prevent the development of osteoporosis by taking antioxidant supplements. However, further prospective and mechanistic studies are needed to confirm these findings.
Data availability
The survey data were publicly available on the internet for data users and researchers throughout the world http://www.cdc.gov/nchs/nhanes/.
References
Kanis JA, Mccloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis Int 24:23–57
Looker AC, Sarafrazi IN, Fan B, Shepherd JA (2017) Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporosis Int 28:1979–1988
Guo J, Huang Y, Bian S, Zhao C, Jin Y et al (2018) Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in US adults, NHANES 2005–2010. Environ Pollut 240:209–218
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29:2520–2526
Dempster DW (2011) Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care 17:S164–S169
Khosla S, Hofbauer LC (2017) Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endo 5:898–907
Appelman-Dijkstra NM, Papapoulos SE (2015) Modulating bone resorption and bone formation in opposite directions in the treatment of postmenopausal osteoporosis. Drugs 75:1049–1058
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33:359–370
Hu D, Cheng L, Jiang W (2018) Fruit and vegetable consumption and the risk of postmenopausal osteoporosis: a meta-analysis of observational studies. Food Funct 9:2607–2616
Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL (2009) Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: the framingham osteoporosis study. Am J Clin Nutr 89:416–424
Hyun TH, Barrett-Connor E, Milne DB (2004) Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo study. Am J Clin Nutr 80:715–721
Welch AA, Jennings A, Kelaiditi E, Skinner J, Steves CJ (2020) Cross-sectional associations between dietary antioxidant vitamins C, E and carotenoid intakes and sarcopenic indices in women aged 18–79 years. Calcif Tissue Int 106:331–342
Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D (2004) Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol 160:68–76
Yu YC, Paragomi P, Wang R, Jin A, Schoen RE, Sheng LT, Pan A, Koh WP, Yuan JM, Luu HN (2022) Composite dietary antioxidant index and the risk of colorectal cancer: findings from the Singapore Chinese health study. Int J Cancer 150:1599–1608
Wang W, Wang X, Cao S, Duan Y, Xu C, Gan D, He W (2022) Dietary antioxidant indices in relation to all-cause and cause-specific mortality among adults with diabetes: a prospective cohort study. Front Nutr 9:849727
Wang L, Yi Z (2022) Association of the composite dietary antioxidant index with all-cause and cardiovascular mortality: a prospective cohort study. Front Cardiovasc Med 9:993930
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J (2013) National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1:1–37
Luu HN, Wen W, Li H, Dai Q, Yang G, Cai Q, Xiang YB, Gao YT, Zheng W, Shu XO (2015) Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Sign 22:951–959
Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB et al (2018) Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. Jama J Am Med Assoc 319:2521–2531
Cai S, Fan J, Zhu L, Ye J, Rao X, Fan C, Zhong Y, Li Y (2020) Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: a population-based cohort study. Bone 141:115597
Li Y, Hao W, Guan J, Li B, Meng L et al (2022) Relationship between indices of circulating blood cells and bone homeostasis in osteoporosis. Front Endocrinol 13:965290
Valderrabano RJ, Lui LY, Lee J, Cummings SR, Orwoll ES, Hoffman AR, Wu JY (2017) Bone density loss is associated with blood cell counts. J Bone Miner Res 32:212–220
Grili P, Vidigal CV, Da CG, Albergaria BH, Marques-Rocha JL, Pereira T, Guandalini VR (2022) Dietary consumption of selenium inversely associated with osteoporosis in postmenopausal women. Front Nutr 9:997414
Mulligan AA, Hayhoe R, Luben RN, Welch AA (2021) Positive associations of dietary intake and plasma concentrations of vitamin E with skeletal muscle mass, heel bone ultrasound attenuation and fracture risk in the EPIC-Norfolk cohort. Antioxidants Basel 10:159
Chin KY, Ima-Nirwana S (2014) The effects of alpha-tocopherol on bone: a double-edged sword? Nutrients 6:1424–1441
Michaelsson K, Wolk A, Byberg L, Arnlov J, Melhus H (2014) Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am J Clin Nutr 99:107–114
Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Shimokata H, Yano M (2011) Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteoporosis Int 22:143–152
Kimball JS, Johnson JP, Carlson DA (2021) Oxidative stress and osteoporosis. J Bone Jt Surg Am 103:1451–1461
James AW (2013) Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013:684736
Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, Serviddio G (2013) Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol 1:340–346
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15:468–477
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA et al (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Bonaccorsi G, Piva I, Greco P, Cervellati C (2018) Oxidative stress as a possible pathogenic cofactor of post-menopausal osteoporosis: Existing evidence in support of the axis oestrogen deficiency-redox imbalance-bone loss. Indian J Med Res 147:341–351
Barrera G, Bunout D, Gattas V, de la Maza MP, Leiva L, Hirsch S (2004) A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects. Nutrition 20:769–771
Saarelainen J, Kiviniemi V, Kroger H, Tuppurainen M, Niskanen L, Jurvelin J, Honkanen R (2012) Body mass index and bone loss among postmenopausal women: the 10-year follow-up of the OSTPRE cohort. J Bone Miner Metab 30:208–216
Acknowledgements
We thanked the staff and the participants of the NHANES study for their valuable contributions.
Funding
This work was supported by the Elderly Health Research Project of Jiangsu Commission of Health (LKZ2022008), the Natural Science Foundation of Nanjing University Of Chinese Medicine (XZR2021060), and the Foundation of The Second Affiliated Hospital of Nanjing University of Chinese Medicine (SEZ202003).
Author information
Authors and Affiliations
Contributions
SC and JJ collected data. XW and XL organized the study and performed the statistical analysis. HH and ZL drafted the manuscript, to which all authors contributed, and approved the final version for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All analyses were based on data of the National Health and Nutrition Examination Survey (NHANES). The study was approved by the ethics review board of the National Center for Health Statistics. The detailed information is located on the NHANES website. Written informed consent was obtained from each participant before their inclusion in the NHANES database. Detailed information on the ethics application and written informed consent are provided on the NHANES website.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Han, H., Chen, S., Wang, X. et al. Association of the composite dietary antioxidant index with bone mineral density in the United States general population: data from NHANES 2005–2010. J Bone Miner Metab 41, 631–641 (2023). https://doi.org/10.1007/s00774-023-01438-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01438-7