Abstract
Summary
In a cohort study, bone mineral density (BMD) around uncemented femoral components after total hip arthroplasty (THA) was evaluated. The results suggest that there are no clinically relevant changes in overall periprosthetic BMD in the second decade. However, continuous remodeling with limited proximal bone loss (stress-shielding) occurs, predominantly in female patients.
Introduction
Progressive periprosthetic bone loss and stress-shielding are a major concern in THA. Little is known about the extent and pattern of periprosthetic bone remodeling around uncemented stems in the second decade.
Methods
In a cohort study, periprosthetic BMD was measured in 131 patients with 146 uncemented CLS stems using dual-energy X-ray absorptiometry (DXA) at a mean of 12 years postoperatively (t1). Patients were followed clinically and radiographically, and a second DXA was performed at a mean of 17 years postoperatively (t2) using the identical protocol.
Results
We obtained a complete set of two consecutive DXA measurements for 88 hips (78 patients, 35 male, 43 female). On radiographic evaluation at t1 and t2, regular bone ongrowth was present in all cases and no signs of radiographic loosening, severe bone loss or diaphyseal cortical hypertrophy were detected. There was no clinically relevant change in overall periprosthetic BMD (netavg) between t1 and t2 for both male and female patients. We analyzed the differences in BMD in the periprosthetic regions of interest (ROIs) according to Gruen and found a slight decrease in periprosthetic BMD in ROI 7 in male patients and in ROIs 1, 4, 6 and 7 in female patients.
Conclusions
The study suggests that there are no clinically relevant changes in overall periprosthetic BMD around stable, straight uncemented stems between 12 and 17 years postoperatively. However, continuous remodeling with limited proximal bone loss occurs, predominantly in female patients. After secondary osteointegration of this implant, stress-shielding remains minimal in the second decade.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Straight, uncemented titanium stems have recently demonstrated good and excellent long-term survival rates at 15–20 years after surgery, comparable to the reported outcomes of well-cemented femoral stems [1–3]. One major concern with regard to long-term stem performance is progressive periprosthetic bone loss that may reduce implant stability, leading to subsidence and aseptic loosening [4]. Stress-mediated bone resorption (i.e., stress-shielding) at the metaphyseal region of the proximal femur as a result of distal load transfer may affect the clinical outcome of total hip arthroplasty (THA) and will make revision surgery difficult [5]. There is also concern that an increased risk for late periprosthetic fractures is present in cases of substantial bone loss around the implant [6]. Although the consequences of stress-shielding for implant survival are controversely discussed, the preservation of femoral and acetabular bone stock after THA is a desirable goal, and it seems advantageous to monitor changes in bone mineral density (BMD) and the underlying remodeling pattern in the second decade after implantation.
In the literature, data on changes in BMD after 10 years is only available for selected stems and small patient numbers [7–10]. To our knowledge, there is no study that investigates bony remodeling in periprosthetic BMD within the second decade after THA comparing potential differences between male and female patients.
The aim of this investigation was to determine the extent and the pattern of periprosthetic bone remodeling following uncemented THA around a straight, double-tapered, grit-blasted titanium stem (CLS, Zimmer, Warsaw, IN, USA). We performed a cohort study at a mean follow-up of 12 (t1) and 17 (t2) years postoperatively using dual-energy X-ray absorptiometry (DXA) to prove or reject the presence of relevant bone loss or remodeling in males and females in the second decade. We also assessed radiographic changes on plain radiographs and determined clinical outcomes using the Harris hip score (HHS) and Devane activity score.
Methods
Cohort study
Between January 1985 and December 1989, 326 consecutive patients (166 male, 160 female, 354 hips) underwent primary THA at our institution. In all patients, an uncemented, straight, titanium stem (CLS Spotorno®, Zimmer, Warsaw, USA, Fig. 1) with a centrum collum diaphysis (CCD) angle of 145° was implanted using the press-fit technique described by Spotorno et al. [11]. The rectangular CLS stem is wedge-shaped and tapered in all three planes with proximal, anterior and posterior located ribs/flutes. Secondary osseointegration is achieved by osseous ongrowth to the grit-blasted implant surface. The implant is made of Ti6Al7Nb alloy with a microporous surface treatment (R a = 4.4 μm). Three hundred forty-nine hips (99%) underwent acetabular reconstruction using smooth, uncemented, threaded cups: 222 hips (63%) received a threaded, spherical, uncemented Mecron cup (Mecron, Berlin, Germany) and 127 hips (36%) received a threaded, conical, uncemented Weill ring (Zimmer Warsaw, USA). In five hips (1%), a cemented cup (Braun/Aesculap, Tuttlingen, Germany) was used. A polyethylene-bearing surface and a 32 mm Biolox ceramic head (Ceramtec, Plochingen, Germany) were implanted in all cases. The operative procedure, the postoperative protocol, clinical and radiographic long-term results and stem survival rates of this cohort have been previously reported [1, 12].
To determine changes in periprosthetic BMD in the second decade, we performed baseline measurements at a mean of 12 years postoperatively. From the initial cohort of 354 hips (100%), 59 hips (17%) deceased, 25 hips (7%) underwent femoral revision and 8 hips (2%) were lost to follow-up. For 116 (33%) hips, patients refused to participate in the study for DXA measurements.
A total of 131 patients (146 hips, 41%) gave informed consent and underwent a DXA examination at a mean follow-up of 12 years postoperatively (t1, range 10–15 years). Patients were prospectively monitored and a second DXA was performed on 78 patients (88 hips, 25%) at a mean follow-up of 17 years (t2, range 15–20 years) using the identical protocol. From the original cohort of 146 hips (t1), 17 hips (12%) were deceased and 14 hips (9.5%) were not able to attend the second DXA examination due to various health conditions. Eight hips (5.5%) were excluded because of a late periprosthetic fracture with consecutive revision surgery. For 19 hips (13%), patients either did not give consent for a second DXA or were lost to follow-up.
The demographic data and the preoperative diagnoses leading to primary THA at t1 and t2 are given in Table 1. Patients with consumptive or metabolic disease, hormone substitution or medication affecting bone metabolism were excluded from the study to generate normative values for regular implant osteointegration.
All patients gave informed consent prior to inclusion and the study was approved by our university institutional review board (reference 346/2004) and by the Federal Office for Radiation Protection/Germany (reference Z 5-22462/2-2004-056).
Clinical and radiographic follow-up
Clinical follow-up was performed using the HHS [13]. Patient activity at final follow-up (t2) was assessed according to Devane et al. [14, 15].
At t1 and t2, low-centered pelvic radiographs and lateral radiographs of the hip were taken and independently examined by two experienced orthopaedic surgeons to determine stem subsidence, radiolucent lines, cortical hypertrophy, osteolysis, stress-shielding and femoral loosening. Stem subsidence was defined as continuous migration of >5 mm on anteroposterior radiographs comparing the appearance on the first postoperative radiograph to the radiographs taken at t1 and t2. Radiolucent lines and areas of osteolysis were described according to the zones established by Gruen et al. [16]. Bone hypertrophy was defined as thickening of the distal periprosthetic diaphyseal bone of >2 mm on the most recent anteroposterior radiographs (t2) compared with the measurement on radiographs taken at t1. Osteolysis was defined as areas of localized bone resorption or endosteal erosion [17]. Stress-shielding was determined according the criteria of Engh et al. [18]. We considered only second-, third- and fourth-degree stress-shielding with resorption of cortical bone medially, anteriorly or laterally to be stress-shielding. The femoral stem was regarded as loose if a radiolucent line of >2 mm in width was present around the entire implant or if a change in varus/valgus alignment of >2° occurred on serial radiographs.
DXA analysis
Femoral BMD (g/cm²) was measured with DXA using the QDR 2000® (Hologic, Waltham, USA) with an identical and examination protocol at t1 and t2 following the manufacturer’s guidelines. BMD was determined with the manufacturer’s metal and soft tissue removal software (version 7.2, Hologic). At t1 and t2, the identical scanner and software version were used and the examination was performed by the same technologist. Comparison mode was used to ensure reproducible placement of the periprosthetic regions of interest (ROIs) at t2 compared to the baseline measurements performed at t1. Measurement precision was assessed using a spine phantom prior to all DXA examinations (Spine Phantom #1179; Hologic). Coefficient of variation over the 5-year follow-up period was 1.53%.
The patient’s leg was fixed in 12° internal rotation and 5° flexion using a positioning device to achieve reproducible projection and to minimize malrotation errors [19, 20]. Periprosthetic ROIs were defined around the stem according to the zones described by Gruen et al. [16] (Fig. 2). ROIs 1–3 were placed lateral and ROIs 5–7 were placed medial, dividing the stem into thirds. Because of the variability of the level of the femoral neck, the proximal medial ROI 7 was defined individually in length. ROI 4 was positioned at the tip of the stem with a standard length of 15 pixels and with a distance of 2 pixels (P) to the external rim of the femoral cortical bone. For each ROI, BMD was expressed in g/cm². Overall periprosthetic BMD was summarized in the ROIs (1–7) net average (netavg) in g/cm².
Mapping of the periprosthetic regions of interest (ROI) around the stem according to Gruen et al. [16]
Statistics
Distributions of clinical data in groups of patients with and without follow-up measurement at t2 were compared by the Mann–Whitney U-test in case of continuous data and by Fisher’s exact test in case of categorical data using only one observation per patient. For descriptive analysis, mean absolute BMD values (g/cm2) were calculated at t1 and t2 for overall BMD (netavg) and BMD in the periprosthetic ROIs 1–7 directly from the measurements. Changes between the BMD measurements at t1 and t2 are expressed as difference of either absolute values or relative values (%) with the t1 measurement being the reference value.
Since DXA measurements were performed bilaterally in n = 10 patients, mixed linear models were used to estimate mean changes (absolute and relative) of BMD values with 95% confidence intervals (CI) and to test mean changes of absolute values against zero. An absolute change in BMD of 0.05 g/cm² was defined as clinically relevant. Results with p values < 0.05 were considered statistically significant. However, due to multiple testing and estimation, only the results concerning changes in mean netavg values, which was defined as primary endpoint, can be interpreted in a confirmatory sense. Statistical analyses were performed using the Statistical Analysis System Software, Version 9.1 for Windows (SAS Institute, Cary, USA).
Results
Demographics
A complete set of data was obtained for 88 hips (78 patients, 35 male, 43 female). Comparing the DXA cohort at t1 (146 hips) to the patients of the initial cohort who were not included in the study (208 hips) showed no significant differences with regard to preoperative diagnosis (p = 0.08) and body mass index (BMI, p = 0.14). However, the DXA cohort was significantly younger at the time of surgery with a median age of 55 years (range 15–74) compared to 58 years (range 13–81) in patients of the initial cohort (p = 0.0007). Comparing the DXA cohort with complete measurements both at t1 and t2 (n = 88 hips) to the patients with measurement only at t1 (n = 58 hips), no significant differences with regard to BMI (p = 0.85), age at time of surgery (p = 0.07) and preoperative diagnosis (p = 0.48) were observed (Table 1).
Clinical findings
Mean HHSs at t1 and t2 are given in Table 2. Assessment of patient activity at final follow-up according to Devane et al. [14, 15] indicated that the patient cohort was active, in general, with a mean score of 3.0 for male and 2.93 for female patients. In the male group, activity levels were grade 1 (1 patient), grade 2 (5 patients), grade 3 (22 patients) and grade 4 (7 patients). In the female group, activity levels were grade 1 (0 patients), grade 2 (6 patients), grade 3 (34 patients) and grade 4 (3 patients), respectively. There was no significant difference between males and females with regard to mean activity levels (p = 0.60).
Radiographic findings
On radiographic evaluation at t1 and t2, regular femoral bone ongrowth was seen in all cases, and there were no signs of radiographic loosening. No radiographic signs of stress-shielding in the proximal femur, no cortical hypertrophy and no signs of progressive osteolysis were detected. Comparing the first postoperative radiographs to the radiographs taken at t1 and t2, no stem subsidence (>5 mm) was observed.
Overall BMD (netavg)
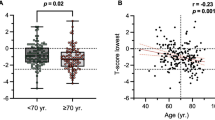
Over the 5-year follow-up period, BMD netavg values for the entire cohort of 88 hips remained at a stable level (−0.017 g/cm²/−1.4%, Fig. 3). Absolute netavg BMD was higher in male patients than in female patients both at t1 and t2. There was a slight decrease in absolute netavg BMD between t1 and t2, with −0.024 g/cm2 for female patients and −0.010 g/cm2 for male patients. In relative terms, netavg BMD decreased slightly to 97.6% (t2) in female patients, whereas in male patients, no change in relative netavg BMD was seen (t2, 99.8%). No significant difference in netavg BMD changes between male and female patients was observed (p = 0.40). Netavg BMD values and estimated netavg BMD changes between t1 and t2 are given in Table 3 (male patients) and Table 4 (female patients).
BMD in ROIs 1–7
In the entire cohort, absolute periprosthetic BMD with regard to the zones according to Gruen was lower in the proximal regions (ROIs 1 and 7) and higher in the distal regions (ROIs 3, 4 and 5) compared to the intermediate regions (ROIs 2 and 6). This finding was consistent for both male and female patients.
In male patients, a nonsignificant increase in BMD was observed in ROI 2 (+0.027 g/cm) and ROI 4 (+0.012 g/cm2), and there was a statistically significant decrease in BMD in the calcar region (ROI 7) with −0.042 g/cm2 (p = 0.02).
In female patients, statistically significant changes in BMD were seen in ROI 1 (−0.037 g/cm2; p = 0.001), ROI 4 (−0.029 g/cm2; p = 0.001), ROI 6 (−0.073 g/cm2; p = 0.001) and ROI 7 (−0.061 g/cm2; p = 0.02).
Relative changes in periprosthetic BMD are demonstrated in Fig. 3. The lowest relative BMD values at t2 were observed in the calcar region (ROI 7) for both males (t2, 95.7%) and females (t2, 93.4%). BMD values and estimated BMD changes in ROIs 1–7 between t1 and t2 are given in Table 3 (male patients) and Table 4 (female patients).
Discussion
Bone resorption at the proximal femur is commonly seen after THA, especially after implantation of uncemented components [8, 19, 21–24]. The etiology of periprosthetic bone remodeling is multifactorial. It is observed in all stem designs, and the amount of bony remodeling is dependent on patient- and implant-related factors [25–27].
The most rapid changes in periprosthetic BMD occur early within the first 3–12 months after implantation [28, 29]. Greatest bone loss is detected in the metaphyseal region due to altered load transmission with a more distal stress transfer after implantation of the femoral component. Whereas some authors suggest that stress-shielding will stabilize after the initial remodeling process and a plateau phase in well-fixed stems is reached at 12–36 months postoperatively [24, 30], others report progressive bone loss around the stem up to 42% [9] in the calcar region and 36% [8] in the greater trochanter. Karachalios et al. [31] performed a prospective randomized study on four different stem designs and reported a slow progressive recovery of initial bone loss up to 10 years postoperatively in all groups, postulating that the phenomenon of stress-shielding is overestimated in the literature.
Little is known about the changes in periprosthetic BMD in the second decade. There is concern that continuous femoral periprosthetic bone loss may predict adverse clinical outcomes such as periprosthetic fractures and late aseptic loosening. Moreover, revision arthroplasty can be more difficult when a poor bone stock is present. Thus, substantial data about the changes in periprosthetic BMD is crucial for the assessment of long-term stem performance [28].
The important question to be addressed in this study is whether bone loss continues in the second decade around well-fixed uncemented femoral implants. In the performed radiographic evaluation, all patients included in the study showed a stable stem with good osteointegration and no radiographic signs of stress-shielding or distal cortical hypertrophy, so the results can be regarded as normative for the CLS stem. Our data demonstrate that overall BMD (netavg) remained stable during the observation period between 12 and 17 years postoperatively. A statistically significant decline in netavg BMD (p = 0.04) was observed in female patients; however, the decrease cannot be considered as clinically relevant as the defined value of −0.05 g/cm2 was not within the given 95% CI. No significant difference in netavg BMD was seen in male patients.
With regard to the defined periprosthetic ROIs, we observed a limited remodeling process with slight bone loss in the calcar region in males (ROI 7) and in the proximal (ROIs 1, 6, 7) and distal (ROI 4) regions in females. However, these changes are of limited clinical relevance, given that a precision error of 5% can occur using DXA [22]. According to Hannan et al. [32], a yearly reduction in BMD of about 1% can be attributed to normal aging. Considering this, the estimated maximum decline to 93.3% of the baseline value seen in the calcar region (ROI 7) in females has to be interpreted accordingly.
The following limitations of the study should be considered: From the initial DXA cohort, only 60% of patients were available for follow-up measurement at t2. However, there was no evidence that suggests a difference in age at time of surgery, BMI and preoperative diagnoses comparing the collective at t1 and t2. Patient demographics showed a typical distribution of diagnoses leading to THA. The low HHS at follow-up can be attributed to the relatively high number of patients who had undergone acetabular revision for failed acetabular components [33], in most cases, with major reconstruction (cages), and the natural decline of hip scores with age.
Moreover, we did not use the contralateral hip as control to demonstrate the effect of aging, as a not negligible number of patients received a contralateral THA in the observation period, limiting the size of the final control group. Secondly, BMD in the contralateral hip has to be interpreted with caution, as up to 20% difference in BMD in the diseased side may preexist [32, 34]. A further limitation of the present study is the lack of postoperative baseline BMD measurements. However, previously reported results for the CLS stem [7] demonstrate that, after the initial remodeling process, a plateau phase is reached and no significant further bone loss in netavg BMD occurs up to 156 months postsurgery. The present study confirms this observation for the second decade and shows that at this stage after THA BMD changes remain small and are largely age- and sex-related.
Despite the lack of radiographic evidence, a slow remodeling process with slight decline in BMD in the proximal regions and stable conditions in the distal regions seems to occur during the follow-up period. This confirms previous observations that proximal loading cannot be normalized with the use of certain stem designs that aim for a proximal intertrochanteric fixation [10]. Moreover, this finding is consistent with the hypothesis based on previous studies suggesting that bone loss will be greatest in areas in which physiologic loading is reduced by the implant. We suggest that for the CLS stem the fixation occurs more likely in the meta-diaphyseal region, which is somewhat contradictory to the proximal load transfer advocated by the designers of the implant [11]. Besides altered load transfer associated with implant design, patient-related factors that may affect periprosthetic bone loss have to be considered. The observation that slight bone loss was not strictly limited to the proximal regions but also occurred in zones that are typically differently affected by stress-shielding (i.e., ROIs 4 and 7) may be attributed to the natural decline of BMD with age. Moreover, postmenopausal alteration of bone regulation has to be regarded as a potential factor influencing periprosthetic bone loss and may explain why females were more strongly affected by bone loss than males during the study period.
The results of our study demonstrate that there are no clinically relevant changes in overall periprosthetic BMD around stable straight uncemented CLS stems between 12 and 17 years postoperatively. However, continuous bone remodeling with limited proximal bone loss occurs, predominantly in female patients. Once secondary osteointegration of this uncemented, straight, grit-blasted stem has occurred, stress-shielding remains minimal in the second decade.
References
Aldinger PR, Jung AW, Breusch SJ, Ewerbeck V, Parsch D (2009) Survival of the cementless Spotorno stem in the second decade. Clin Orthop Relat Res 467:2297–2304
Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI (2007) Medium- and long-term performance of 11.516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg Br 89:1574–1580
Makela KT, Eskelinen A, Pulkkinen P, Paavolainen P, Remes V (2008) Total hip arthroplasty for primary osteoarthritis in patients fifty-five years of age or older. An analysis of the Finnish arthroplasty registry. J Bone Joint Surg Am 90:2160–2170
Wilkinson JM, Hamer AJ, Rogers A, Stockley I, Eastell R (2003) Bone mineral density and biochemical markers of bone turnover in aseptic loosening after total hip arthroplasty. J Orthop Res 21:691–696
Engh CA Jr, Young AM, Engh CA Sr, Hopper RH Jr (2003) Clinical consequences of stress shielding after porous-coated total hip arthroplasty. Clin Orthop Relat Res 157–163
Lindahl H (2007) Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury 38:651–654
Aldinger PR, Sabo D, Pritsch M, Thomsen M, Mau H, Ewerbeck V, Breusch SJ (2003) Pattern of periprosthetic bone remodeling around stable uncemented tapered hip stems: a prospective 84-month follow-up study and a median 156-month cross-sectional study with DXA. Calcif Tissue Int 73:115–121
Boden HS, Skoldenberg OG, Salemyr MO, Lundberg HJ, Adolphson PY (2006) Continuous bone loss around a tapered uncemented femoral stem: a long-term evaluation with DEXA. Acta Orthop 77:877–885
Panisello JJ, Herrero L, Canales V, Herrera A, Martinez AA, Mateo J (2009) Long-term remodeling in proximal femur around a hydroxyapatite-coated anatomic stem: ten years densitometric follow-up. J Arthroplasty 24:56–64
Stiehl JB (2009) Long-term periprosthetic remodeling in THA shows structural preservation. Clin Orthop Relat Res 467:2356–2361
Spotorno L, Romagnoli S, Ivaldo N, Grappiolo G, Bibbiani E, Blaha DJ, Guen TA (1993) The CLS system. Theoretical concept and results. Acta Orthop Belg 59(Suppl 1):144–148
Aldinger PR, Breusch SJ, Lukoschek M, Mau H, Ewerbeck V, Thomsen M (2003) A ten- to 15-year follow-up of the cementless spotorno stem. J Bone Joint Surg Br 85:209–214
Harris WH (1969) Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 51:737–755
Devane PA, Robinson EJ, Bourne RB, Rorabeck CH, Nayak NN, Horne JG (1997) Measurement of polyethylene wear in acetabular components inserted with and without cement. A randomized trial. J Bone Joint Surg Am 79:682–689
Devane PA, Horne JG, Martin K, Coldham G, Krause B (1997) Three-dimensional polyethylene wear of a press-fit titanium prosthesis. Factors influencing generation of polyethylene debris. J Arthroplasty 12:256–266
Gruen TA, McNeice GM, Amstutz HC (1979) “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res 17–27
Willert HG, Bertram H, Buchhorn GH (1990) Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res 95–107
Engh CA, Bobyn JD, Glassman AH (1987) Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br 69:45–55
Kiratli BJ, Checovich MM, McBeath AA, Wilson MA, Heiner JP (1996) Measurement of bone mineral density by dual-energy X-ray absorptiometry in patients with the Wisconsin hip, an uncemented femoral stem. J Arthroplasty 11:184–193
Mortimer ES, Rosenthall L, Paterson I, Bobyn JD (1996) Effect of rotation on periprosthetic bone mineral measurements in a hip phantom. Clin Orthop Relat Res 269–274
Ang KC, De Das S, Goh JC, Low SL, Bose K (1997) Periprosthetic bone remodelling after cementless total hip replacement. A prospective comparison of two different implant designs. J Bone Joint Surg Br 79:675–679
Kiratli BJ, Heiner JP, McBeath AA, Wilson MA (1992) Determination of bone mineral density by dual X-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res 10:836–844
Trevisan C, Bigoni M, Randelli G, Marinoni EC, Peretti G, Ortolani S (1997) Periprosthetic bone density around fully hydroxyapatite coated femoral stem. Clin Orthop Relat Res 109–117
Venesmaa PK, Kroger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM (2001) Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry—a 3-year follow-up study. J Bone Miner Res 16:1056–1061
Rahmy AI, Gosens T, Blake GM, Tonino A, Fogelman I (2004) Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int 15:281–289
Gibbons CE, Davies AJ, Amis AA, Olearnik H, Parker BC, Scott JE (2001) Periprosthetic bone mineral density changes with femoral components of differing design philosophy. Int Orthop 25:89–92
Sychterz CJ, Engh CA (1996) The influence of clinical factors on periprosthetic bone remodeling. Clin Orthop Relat Res 285–292
Kroger H, Venesmaa P, Jurvelin J, Miettinen H, Suomalainen O, Alhava E (1998) Bone density at the proximal femur after total hip arthroplasty. Clin Orthop Relat Res 66–74
Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S (1997) Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop Relat Res 121–131
Kroger H, Miettinen H, Arnala I, Koski E, Rushton N, Suomalainen O (1996) Evaluation of periprosthetic bone using dual-energy X-ray absorptiometry: precision of the method and effect of operation on bone mineral density. J Bone Miner Res 11:1526–1530
Karachalios T, Tsatsaronis C, Efraimis G, Papadelis P, Lyritis G, Diakoumopoulos G (2004) The long-term clinical relevance of calcar atrophy caused by stress shielding in total hip arthroplasty: a 10-year, prospective, randomized study. J Arthroplasty 19:469–475
Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP (2000) Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15:710–720
Simank HG, Brocai DR, Reiser D, Thomsen M, Sabo D, Lukoschek M (1997) Middle-term results of threaded acetabular cups. High failure rates five years after surgery. J Bone Joint Surg Br 79:366–370
Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK, Cummings SR (1995) Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum 38:907–916
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merle, C., Streit, M.R., Volz, C. et al. Bone remodeling around stable uncemented titanium stems during the second decade after total hip arthroplasty: a DXA study at 12 and 17 years. Osteoporos Int 22, 2879–2886 (2011). https://doi.org/10.1007/s00198-010-1483-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1483-z