Abstract
Although periprosthetic changes after THA have been well documented in short-term studies of less than 5 years, little is known about long-term changes. Long-term mineral changes must be evaluated against an unaffected limb control and for regional differences about a prosthesis. This study evaluated long-term periprosthetic remodeling using dual-energy xray absorptiometry in a prospective study of patients who had noncemented THAs with a modular titanium alloy proximal-loading prosthesis. In 15 randomly selected patients, bone mineral content was measured within 15 months of surgery and then at late mean followup of 13 years. In the affected femur, there was a major decrease in periprosthetic bone mineral content in Zones 1, 2, 6, and 7 (Gruen et al.) over the course of the study. The overall decrease in Zone 7 was 39% in bone mineral content. Estimates made after controlling for the contralateral unaffected femur indicate a major loss only in Zone 7 and preservation of mineral content in Zones 3, 4, and 5 of the proximal femur. The data suggest bone remodeling maintains the overall structural integrity of the upper femoral shaft.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uncemented stems of modern design have high survival rates (89% to 97%) at longer than 15 years of followup [7, 20, 25]. Enduring clinical performance and persistent osseointegration have encouraged the use of this concept, especially in younger patients. However, proximal bone resorption resulting from distal stress transfer (ie, stress shielding) has been identified, particularly in devices that seek initial rigid distal fixation in the proximal femoral shaft [5, 6, 12]. The greatest loss of bone mineral density occurs in the first 3 to 6 months after primary surgery (ie, 10% to 20%) [18]. Despite the long-term survival of these implants, some authors nonetheless have expressed concern there will be further gradual long-term decline in periprosthetic bone density attributed to stress shielding in excess of the normal aging process [2, 10, 14, 15, 23]. They have articulated particular concern with cases of cemented and fully porous-coated fixation where substantial bone loss around the entire prosthesis has occurred.
Numerous investigators have used dual-energy xray absorptiometry (DEXA) to evaluate periprosthetic bone mineral content (BMC) and density after THA. This method has a precision with an overall coefficient of variation of 3% to 5% from several validation studies [2, 3, 13, 24]. However, several studies have identified difficulty in reproducibly defining regional zones such as the medial femoral neck [12, 13, 16]. In one study, the coefficient of variation was reported to be at least 9% in this area [13].
Most DEXA studies have lacked followup longer than 5 years, and many have used the opposite normal as the control at the time of the investigation. Parallel changes will occur in the normal control with time, which decreases the sensitivity of the measure unless the control is measured at all of the time comparisons. A second problem has been the reliance on DEXA bone mineral density, which measures the BMC of a two-dimensional projection of density and not that of the actual three-dimensional volume. In several studies, area alterations of the medial femoral cortex radiographic area (Zone 7 of Gruen et al.) with proximal calcar rounding off have been reported as occurring after femoral prosthetic placement [5, 12, 15]. This areal change will alter the bone density measurements.
A satisfactory study therefore requires an extended time to compare densities with proper controls (typically the unaffected contralateral side) observed during the same period. Loosening and osteolysis must be excluded because these factors reduce the amount of bone measured. The study must include only one implant device because there may be major differences with differing designs. Finally, based on the findings of Bloebaum et al. [1], bone mineral changes should best be assessed by the measure of DEXA BMC. This is because BMC is least affected by changes in bone area or image projection error.
The primary purpose of this study, therefore, was to determine if long-term periprosthetic bone remodeling about the entire uncemented femoral stem placement would exceed that found in the normal control limb. The secondary purpose was to confirm whether the long-term changes would be greatest in Zones 1 and 7 as consistently shown by prior DEXA studies.
Materials and Methods
I prospectively followed 10 women and five men, from an original group of 27 patients, having THA between December 1993 and April 1995 with a modular titanium alloy press-fit implant. The average age of the group was 61 ± 7.9 years and the average bone morphologic index was 28 ± 5 (Table 1). Inclusion criteria included late radiographic control indicating a solidly fixated femoral stem with no apparent periprosthetic osteolysis or bone changes. All patients were asymptomatic in the affected hip at the time of final followup. Two distinct populations were defined from the overall group differing by the time of the primary DEXA evaluation. In Group 1 (six of 15 patients), DEXA had been performed during the first postoperative week of the index procedure (Table 1). Group 2 (nine of 15 patients) had the original DEXA performed 3 to 15 months postoperatively (average, 6.2 months). For late followup, five of 15 patients had not undergone THA of the contralateral side: three in Group 1 and two in Group 2. This group provided a contralateral control group to assess long-term changes in the normal proximal femur. The minimum followup was 12.3 years (mean, 13 years; range, 12.3–13.3 years). The study had prior approval of the hospital ethics committee.
All operations were performed by one surgeon (JBS). The implant had an hydroxyapatite coating on the proximal metaphyseal segment (Infinity®; Wright Medical Technology, Memphis, TN). The device was inserted with line-to-line reaming of the proximal femoral canal followed by broaching with a modular rasp that rotated to seek the best fit and fill of the proximal femoral metaphyseal section. The approach was posterior-lateral and a nonmodular porous press-fit acetabular component (Whiteside; Wright Medical Technology) was used in all patients.
The BMC was determined by using DEXA scanning (Lunar Prodigy Advance; GE Medical Systems, Madison, WI) using a specific software protocol that measures the seven zones of Gruen et al. [8] after placement of the hip prosthesis in an automated fashion. The software had been upgraded to a newer version with the current DEXA radiographic hardware, but the manufacturer confirmed the similarity of systems. The original software protocol had an overlay template that allowed for determination of the contralateral normal side with the same implant being superimposed on the normal proximal femur. This template overlay was not available in the software upgrade used in the final studies. Data recorded included the seven zones applied over the normal bone without the overlay.
The DEXA procedure was performed following the manufacturer’s guidelines, which includes careful positioning of the templated zones to the specific prosthetic dimensions in the patient. In addition, leg positioners allow the technician to place the leg in a reproducible neutral position with the knee patellae horizontal to the table. For this longitudinal study, all procedures were performed by the same radiology technician (MM). Each patient underwent two studies, including the initial study postoperatively and the study performed at final followup.
The basic analysis approach was repeated-measures modeling. The change in BMC between two times was determined by fitting a general linear model with zone and time-within-zone as predictors. The correlation between the 14 values measured for the same patient (seven zones at both times) was modeled by the direct product of two unstructured covariance matrices. This covariance structure assumes the correlation between the two times is the same for all zones; however, the among-zone and among-time correlations are estimated from the data, and equality of variances is not assumed. The “sandwich” estimator of variance was used. This approach can be viewed as a generalization of the paired t test that adjusts for correlation between the zones. Residual plots were examined visually, and no substantial violations of the assumptions were found. When analyzing the changes in the legs with THA, additional terms allowing the change to vary by group (Group 1, Group 2) were introduced; however, no improvement was found (p = 0.8), and thus only the results of the combined analysis are reported.
We evaluated the change in the BMC in the legs with THA while adjusting for the change in the control leg by fitting a general linear model with leg type (control, THA), zone, time, and their interactions; the three-way interaction term measures the difference in the effect of time between the two leg types for each zone. The correlation between the 28 measurements per patient (two sides, seven zones, two times) was modeled as above: the direct product of an unstructured covariance matrix of all 14 measurements at one time and a covariance matrix for the correlation between the times. This study used all patients for whom long-term followup was available, and thus the sample size was set by circumstances, not by design. In such situations, prospective power calculations are not useful and were not performed. Instead, all effect estimates are presented with 95% confidence intervals, which provide an insight into the range of effect sizes that are not ruled out by the data. The analyses were performed with PROC MIXED in SAS® 9.1.3 (SAS Institute, Cary, NC).
Results
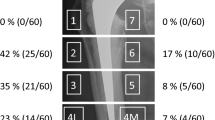
The area of the DEXA measure was substantially different with time in Zone 7 in the affected limbs. This would show the difficulty of comparing DEXA bone mineral density that assesses BMC divided by the radiographic area of bone. BMC would be the more sensitive measure. There are changes noted in all zones in the normal nonaffected limbs during the course of this study (Table 2). These changes were consistent from patient to patient in each zone measured (and underscore the importance in measuring the normal control with a long-term DEXA study) (Fig. 1).
A diagram illustrates the distribution of BMC changes noted in unaffected limbs after 13 years. (Modified and reprinted with permission and copyright 1979 of Lippincott Williams & Wilkins from Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components. Clin Orthop Relat Res. 1979;141:17–27.)
The amount of bone loss about the distal stem and greater trochanter was similar to that in the control femora. There was an increase in BMC in Zone 1 and a decrease in Zone 7 but no detectable differences in Zones 3, 4, and 5 (Table 3). Thus, in Zone 1, the BMC decreased less than expected based on what was seen in unaffected legs, whereas in Zone 7, BMC decreased more than expected. These findings refute the hypothesis that stress shielding will lead to generalized stress shielding about the entire prosthesis in excess of the normal aging process over a long period of time.
The DEXA findings regarding Zone 7 show this area is subject to substantial long-term stress shielding, which averaged 39% for each patient during the term of this study (Fig. 2). That finding remains consistent with the hypothesis based on prior studies suggesting periprosthetic bone loss will be greatest along the medial femoral neck, which is no longer loaded physiologically.
A diagram illustrates the distribution of BMC changes noted in affected limbs after 13 years. (Modified and reprinted with permission and copyright 1979 of Lippincott Williams & Wilkins from Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components. Clin Orthop Relat Res. 1979;141:17–27.)
Discussion
Periprosthetic femoral bone changes measured with DEXA occur early in the postoperative period with substantial involvement of Zones 1 and 7. Although some authors suggest stress shielding will stabilize after 2 years, others are concerned loss of BMC will be slowly progressive with time and exceed that found with the normal aging process. Therefore the primary purpose of this study was to determine if long-term periprosthetic changes were in fact greater than changes in the contralateral normal hip. The secondary purpose was to confirm whether the long-term changes would be greatest in Zones 1 and 7.
The most important limitation of this study is the small final cohort available for analysis. The majority of patients had eventual prosthetic placement of the unaffected contralateral limb, limiting the size of the final control group. It would be inappropriate to make generalizations about the particular implant used in this study or even to make greater conclusions regarding all uncemented femoral prostheses. However, the overall changes documented in this study reflect general trends that have been documented in the literature by shorter followup periods [2, 4, 9, 18, 19] (Table 4). Evaluating longitudinal changes in bone mineral density with DEXA can be problematic. Bone mineral density, as measured by this method, is defined by BMC divided by the area in the radiographic frontal plane of targeted bone. Minor changes in femoral rotation or patient position can lead to a 5% precision error possibly by altering the area of the medial femoral cortex [11–13, 16]. Other studies suggest important cross-sectional remodeling is occurring in femurs of patients who have undergone THA [17, 22] and sagittal plane areas shielded by the implant were not assessed by DEXA. Internal cortical thinning and lesser amounts of external cortical thickening occur that will affect bone mineral density. Bloebaum et al. [1] reported a consistent relationship between BMC values measured with DEXA and ash weight mineral content for given regions but not for bone mineral density. Additionally, BMC is not affected by small changes in projection [1, 13]. For these reasons, I do not report the bone mineral density or zonal areas measured. Finally, without serial measurements it is not possible to determine when these changes occurred and whether they had stabilized, only that they had occurred at the time of the final scan.
Although periprosthetic bone changes have been consistently documented by most other DEXA studies, what has remained unknown is the long-term BMC about the entire prosthesis [2, 10, 11, 19]. The data show BMC does not diminish in the upper femoral shaft compared with the normal unaffected control and this is the most important finding. Bone loss in the lower Zones 3, 4, and 5 that occurs over a long period of time does not exceed that of the normal aging process. This in turn suggests loading of the existing bone stock is maintained.
Although I presumed greater losses would occur in Zones 1 and 7, the only substantial changes were seen in Zone 7 whereas Zone 1 actually increased in relation to the control. The overall bone loss in Zone 7 averaged 39% over 13 years and this would be comparable to reported loss (Table 4). Early authors have suggested stress shielding is the most important factor influencing long-term periprosthetic changes [5, 6, 10, 12–15]. This occurs very quickly and nominal differences were apparent within 6 months as noted by comparing Groups 1 and 2 in this study [2]. Long-term changes are likely from stress shielding, but their importance must be balanced against changes that normally would occur in this population of patients. Some authors suggest stress shielding may be minimized by a low-modulus, intimately fit, proximally fixed device that does not bypass the proximal medial regions with distal fixation [10, 18, 19]. That hope was not validated by the device used in this study. I also could find no evidence from any other DEXA study that proximal loading could be normalized by certain design features of a canal-filling stem. There is evidence, however, that the proximal canal that surrounds the distal portion of the stem is maintained or even enhanced with time: some studies showed cortical enlargement with limited quantitative loss of BMC in these regions [1, 11]. In the case of femoral revision arthroplasty, this preservation of bone stock could be valued as favorable [1, 17, 21].
Although the data reported here confirm those of earlier studies that show considerable bone remodeling and loss in the proximal medial femur after cementless THA, long-term BMC changes in the upper shaft of the proximal femur are negligible and comparable to those of the unaffected control limb. Proximal stress shielding appears to be the most important inherent characteristic of THA, but preservation of bone around the distal stem reflects the transfer of weightbearing stresses to this area, which could be regarded as a favorable outcome.
References
Bloebaum RD, Luau DW, Lester K, Rosenbaum TG. Dual-energy x-ray absorptiometry measurement and accuracy of bone mineral after unilateral total hip arthroplasty. J Arthroplasty. 2006;21:612–622.
Boden HS, Skoldenberg OG, Salemyr MO, Lundberg HJ, Adolphson PY. Continuous bone loss around a tapered uncemented femoral stem: a long-term evaluation with DEXA. Acta Orthop Scand. 2006;77:877–885.
Cohen B, Rushton N. Accuracy of DEXA measurement of bone mineral density after total hip arthroplasty. J Bone Joint Surg Br. 1995;77:479–483.
Dan D, Germann D, Burki H, Hausner P, Kappeler U, Meyer RP, Klaghofer R, Stoll T. Bone loss after total hip arthroplasty. Rheumatol Int. 2006;26:792–798.
Engh CA, McGovern TF, Bobyn JD, Harris WH. A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Joint Surg Am. 1992;74:1009–1020.
Engh CA Jr, Young AM, Engh CA Sr, Hopper RH. Clinical consequences of stress shielding after porous-coated total hip arthroplasty. Clin Orthop Relat Res. 2003;417:157–163.
Eskelinen A, Paavolainen P, Helenius I, Pulkkinen P, Remes V. Total hip arthroplasty for rheumatoid arthritis in younger patients: 2,557 replacements in the Finnish Arthroplasty Register followed for 0–24 years. Acta Orthop Scand. 2006;77:853–865.
Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components. Clin Orthop Relat Res. 1979;141:17–27.
Hananouchi T, Sugano N, Nishii T, Nakamura N, Miki H, Kakimoto A, Yamamura M, Yoshikawa H. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25:1062–1069.
Hughes SS, Furia JP, Smith P, Pellegrini VD. Atrophy of the proximal part of the femur after total hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77:231–239.
Karachalios T, Tsatsaronis C, Efraimis G, Papadelis P, Lyritis G, Diakoumopoulos G. The long-term clinical relevance of calcar atrophy caused by stress shielding in total hip arthroplasty. J Arthroplasty. 2004;19:469–475.
Kilgus DJ, Shimaoka EE, Tipton JS, Eberle RW. Dual-energy x-ray absorptiometry measurement of bone mineral density around porous-coated cementless femoral implants. J Bone Joint Surg Br. 1993;75:279–287.
Kiratli BJ, Heiner JP, McBeath AA, Wilson MA. Determination of bone mineral density by dual x-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res. 1992;10:836–844.
Maloney WJ, Sychterz C, Bragdon C, McGovern T, Jasty M, Engh CA, Harris WH. The Otto Aufranc Award. Skeletal response to well fixed femoral components inserted with and without cement. Clin Orthop Relat Res. 1996;333:15–26.
McCarthy CK, Steinberg GG, Agren M, Leahey D, Wyman E, Baran DT. Quantifying bone loss from the proximal femur after total hip arthroplasty. J Bone Joint Surg Br. 1991;73:774–778.
Mortimer ES, Rosenthall L, Paterson I, Bobyn JD. Effect of rotation on periprosthetic bone mineral measurements in a hip phantom. Clin Orthop Relat Res. 1996;324:269–274.
Muller S, Irgens F, Aamodt A. A quantitative and qualitative analysis of bone remodeling around custom uncemented femoral stems: a five-year DEXA follow-up. Clin Biomech. 2005;20:277–282.
Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S. Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop Relat Res. 1997;339:121–131.
Panisello JJ, Herrero L, Herrera A, Canales V, Martinez A, Cuenca J. Bone remodelling after total hip arthroplasty using an uncemented anatomic femoral stem: a three-year prospective study using bone densitometry. J Orthop Surg (Hong Kong). 2006;14:32–37.
Ragaratnam SS, Jack C, Tavakkolizadeh A, George MD, Fletcher RJ, Hankins M, Shepperd JA. Long-term results of a hydroxyapatite-coated femoral component total hip replacement: a 15- to 21-year follow-up study. J Bone Joint Surg Br. 2008;90:27–30.
Rosenbaum TG, Bloebaum RD, Ashrafi S, Lester DK. Ambulatory activities maintain cortical bone after total hip arthroplasty. Clin Orthop Relat Res. 2006;450:129–137.
Scott DF, Jaffe WL. Host-bone response to porous-coated cobalt-chrome and hydroxyapatite-coated titanium femoral components in hip arthroplasty. J Arthroplasty. 1969;11:429–437.
Sychterz CJ, Topoleski T, Sacco M, Engh CA. Effect of femoral stiffness on bone remodeling after uncemented arthroplasty. Clin Orthop Relat Res. 2001;389:218–227.
Williams-Russo P, Healey JH, Szatrowski TP, Schneider R, Paget S, Ales K, Schwartzberg P. Clinical reproducibility of dual energy x-ray absorptiometry. J Orthop Res. 1995;13:250–257.
Yoon TR, Rowe SM, Kim MS, Cho SG, Seon JK. Fifteen- to 20-year results of uncemented tapered fully porous-coated cobalt-chrome stems. Int Orthop. 2008;32:317–323.
Acknowledgments
I thank Mark Mathie, nuclear medicine technologist, Columbia St Mary’s Hospital, and Scot Jackson, MS, and Aniko Szabo, PhD, Division of Biostatistics, Medical College of Wisconsin, Milwaukee, WI, for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
The author has received funding from Columbia Hospital Foundation and Wright Medical Technology.
The author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Stiehl, J.B. Long-term Periprosthetic Remodeling in THA Shows Structural Preservation. Clin Orthop Relat Res 467, 2356–2361 (2009). https://doi.org/10.1007/s11999-009-0722-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-0722-0