Abstract
Populations of East Asian countries have been known to have low calcium intakes and low serum 25(OH)D concentrations, suggesting that Ca and vitamin D (VitD)-deficiencies are commonly observed. These nutritional imbalances may lead to low peak bone mass (PBM). The low PBM seen in Ca/VitD-deficient individuals may lead to osteoporosis, as well as an increased risk of fracture. A survey was conducted in young Japanese women (n = 296, 21.2 ± 2.3 years old) on their Ca/VitD intakes and serum 25(OH)D levels, which demonstrated a significant positive correlation between VitD intake and serum 25(OH)D levels (R 2 = 0.020, P = 0.016), and the proportion with serum 25(OH)D over 20 ng/mL was significantly increased with VitD intake (P = 0.013). Serum 25(OH)D was negatively correlated to serum intact parathyroid hormone (R 2 = 0.053, P < 0.001). On receiver operating characteristic curve analysis, the VitD intake threshold for maintaining 25(OH)D levels at 20 ng/mL or higher was 11.6 μg/day or greater. It was suggested that the recommended VitD intake allowance, defined in the Adequate Intakes as 5.5 μg/day, may not be sufficient to maintain serum 25(OH)D levels for bone health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium (Ca) and vitamin D (VitD) insufficiencies remain a worldwide issue that is making itself felt particularly in East Asia [1], and they are known to adversely affect the acquisition of peak bone mass (PBM) and the maintenance of bone health [2]. Of note, serum 25(OH)D levels serve as the best single measure of VitD sufficiency, and focus on the level of 25(OH)D has led to various criteria/guidelines being published for VitD sufficiency/insufficiency [3,4,5,6,7].
Normal VitD activity is essential for maintenance of bone and mineral metabolism, and decreased VitD activity leads not only to an increased risk for bone fracture, but to various disorders of bone and mineral metabolism. It has been previously reported that intake of VitD contributes to the serum level of 25(OH)D in Japanese adolescents [8]. Moreover, the relationship between the serum level of 25(OH)D and quality of life (QOL) was investigated in Japanese elderly patients with osteoporosis [9, 10]. It was reported that the mean value of serum 25(OH)D was 23.7 ng/mL, and a low serum 25(OH)D level was a significant determinant of QOL. In addition, VitD insufficiency/deficiency has been reported to have a causal relationship with all-cause mortality [11]. Of all causes of decreased VitD activity, serum VitD insufficiency is considered the foremost, because it reflects decreased VitD supply to the body from its cutaneous synthesis and nutritional intake.
Against this background, the present study was conducted to determine the optimal VitD intake that allows serum 25(OH)D levels to be maintained to ensure bone health in young Japanese women who had just achieved PBM.
Materials and methods
This cross-sectional study drew on existing cohort data accumulated for a total of 296 healthy women comprised of nursing school students and nurses aged 19–29 years of age (mean age 21.2 ± 2.3 years). This study was conducted with the approval of the Institutional Review Board of Tokyo Women’s Medical University (TWMU) between December 2003 and January 2004. All subjects gave their written, informed consent to participate in the study prior to the conduct of the study.
All subjects were surveyed about their age, height, and body weight, and their body mass index (BMI) values were calculated. Their vertebral/femoral bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry (DXA) (Hologic, Waltham, MA, USA) at TWMU Hospital. Fasting blood samples were collected using venipuncture in the morning, centrifuged at 1940×g for 15 min at 4 °C, and the supernatant was stored at −35 °C until assayed. Serum 25(OH)D and intact parathyroid hormone (PTH) levels were determined by the Nichols Advantage Chemiluminescence protein-binding assay method [12]. In addition, concentrations of serum Ca, inorganic phosphorus (iP), albumin (Alb), and bone metabolic markers, including bone-specific alkaline phosphatase (BAP) MicroVue BAP EIA Kit (Quidel Corporation, San Diego, CA, USA) and cross-linked N-telopeptide of type I collagen (NTX), OSTEOMARK NTx Serum (Mochida Pharmaceutical Co., Ltd, Tokyo, Japan), were also measured.
All subjects were surveyed about their energy, protein, lipid, carbohydrate, VitD, P, Ca, and magnesium (Mg) intakes using a self-administered diet history questionnaire (DHQ) [13].
As background to the present study, it is to be noted that the estimated average requirement (EAR) for Ca and the adequate intake (AI) for VitD for women aged 19–29 years, who were the subjects, were defined as 550 and 5.5 μg/day, respectively, in the diet reference intakes (DRIs) 2015 in Japan [14]. Although the 25(OH)D level reflects the availability of VitD in the body, the 25(OH)D threshold as a measure of VitD sufficiency still needs to be determined. In this study, the 25(OH)D threshold was tentatively set at 20 ng/mL, as proposed by the Institute of Medicine (IOM) in 2011 [4] and the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society [15].
Statistical analysis
All continuous variables are expressed as mean ± standard deviation (SD). All statistical analyses were performed using the statistical software JMP 7.0J (SAS Institute Inc., Cary, NC, USA). The correlation of VitD intake or intact PTH and serum 25(OH)D levels was examined using Spearman’s rank correlation and the Cochran–Armitage trend test, and the VitD intake for maintaining the 25(OH)D threshold was calculated using receiver operating characteristic (ROC) curve analyses.
Results
The anthropometric and dietary characteristics of the subjects are summarized in Table 1. They had a mean calcium intake of 524.1 ± 248.5 mg/day, and 61.8% of the subjects had a calcium intake lower than the EAR (550 mg/day). They had a mean VitD intake of 12.4 ± 8.1 μg/day, and 82.4% of the subjects had a higher intake of VitD than the AI recommended in the DRIs (5.5 μg/day). Serum concentrations of 25(OH)D, PTH, calcium, phosphate, BAP, and NTX, as well as BMD values, of the subjects are summarized in Table 2. The subjects had a mean serum 25(OH)D concentration of 18.4 ± 4.9 ng/mL, with approximately 64% of the subjects found to have suboptimal 25(OH)D concentrations (< 20 ng/mL), and only 0.7% subjects (n = 2) were found to have serum 25(OH)D concentrations greater than 30 ng/mL. Thus, 99.3% of the subjects were found to have VitD insufficiency, with overt VitD deficiency (defined as serum 25(OH)D concentrations < 10 ng/mL) found in 3% of the subjects (n = 9).
The subjects with suboptimal serum VitD levels accounted for 61.9% of those with adequate VitD intake (≥ 5.5 μg/day), and those with adequate VitD intake accounted for a smaller proportion of those with suboptimal serum VitD levels than those with inadequate VitD intake (< 5.5 μg/day), but there was no significant difference (P = 0.078).
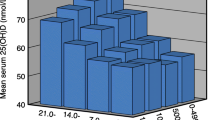
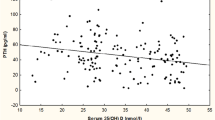
A significant correlation was found between VitD intake and serum 25(OH)D levels (Fig. 1). The proportion with a serum 25HOD over 20 ng/mL divided by the quartile of VitD intake is indicated in Fig. 2. The thresholds of VitD intake were 25th percentile = 6.8 μg/day, median = 10.5 μg/day, and 75th percentile = 16.3 μg/day. The Cochran–Armitage trend test was significant (P = 0.013). Again, on ROC curve analysis to determine the VitD intake threshold for maintaining serum 25(OH)D at a sufficient level (20 ng/mL or higher), 11.6 μg/day (AUC = 0.59) was found to be the threshold value (Fig. 3). A negative correlation was also observed between the levels of 25(OH)D and intact PTH (Fig. 4). Significant correlations were not observed between serum 25(OH)D and bone metabolic markers or BMD.
Discussion
As a phenomenon occurring highly frequently worldwide across all age brackets, VitD deficiency/insufficiency is becoming a major global health problem [16, 17]. Indeed, if defined as a VitD concentration less than 20 ng/mL, VitD deficiency was shown to affect about half of the Amsterdam elderly cohort [18]. Again, while the mean serum 25(OH)D concentration is reported to be 23.1 ng/mL even among US middle-aged women with access to VitD-fortified milk, 46% of these women were reported to be VitD-deficient (VitD < 20 ng/mL) [15, 19]. In Japan as well, the ROAD Study [20], a large-scale cohort study involving 595 men and 1088 women, reported that those with serum 25(OH)D concentrations 30 ng/mL or lower accounted for as many as 81.3% of the cohort. Similarly, Tamaki reported that only 10% of participants had blood 25OHD levels ≥ 30 ng/mL in community-dwelling women aged ≥ 50 years [21]. We also previously reported that VitD deficiency/insufficiency affected as many as 86.0% of elderly women with osteoporosis (mean age 76.5 years) [9].
In contrast, very few reports are available on serum 25(OH)D concentrations among younger age groups, with one small study of 38 subjects reporting that they had a mean serum 25(OH)D concentration of 13.6 ng/mL, and that VitD deficiency affected the majority [22]. The present study involved more than 300 young Japanese women and demonstrated that they had a mean serum 25(OH)D concentration of 18.4 ± 4.9 ng/mL, consistent with the earlier report in Japan that those with serum 25(OH)D concentrations less than 20 ng/mL accounted for as many as 64.2% of the entire study population. Again, the study also showed that the subjects had a mean VitD intake of 12.4 ± 8.1 μg/day, and 82.4% of the subjects had a VitD intake in excess of the AI in the DRIs 2015 (5.5 μg/day) [14], which was therefore assumed to be sufficient VitD intake.
Higher VitD intakes may be required, however, to ensure sufficient serum 25(OH)D concentrations, given that serum 25(OH)D concentrations remained insufficient in 64.2% of those whose VitD intake was consistent with the AI in the DRIs (5.5 μg/day or higher). In the present study, an attempt was made to determine the VitD intake required to maintain serum 25(OH)D concentrations at 20 ng/mL or higher as a measure of 25(OH)D sufficiency, which led to the VitD intake for 25(OH)D sufficiency being found to be 11.6 μg/day. Tsugawa reported that VitD intakes of ≥ 12 and ≥ 14 μg/day would be required to reach 25(OH)D concentrations of 50 nmol/L (20 ng/mL) in boys and girls, respectively [8]. This result is similar to the present results in 19 to 29-year-old women. Of note, the RDA for VitD intake in the AI, 5.5 μg/day, is consistent with the median VitD intake among healthy individuals and is not intended as the VitD intake threshold for maintaining bone health, suggesting that a new, separate criterion needs to be established for this VitD intake threshold.
To date, professional societies have similarly defined the RDA for VitD intake as 15–20 μg/day in the 2015 Guidelines for Prevention and Treatment of Osteoporosis [23], 15 μg/day for individuals less than 75 years old, and 20 μg/day for those 75 years old or older (the US and Canadian guidelines for treatment and prevention of VitD deficiency) [3], or 20 μg/day for older adults (IOF position statement) [5]. It is to be noted, however, that these RDAs are each intended as the VitD intake threshold assumed less likely to be associated with an increased risk of bone fracture.
The present study findings suggest that the ideal VitD intake threshold for acquisition of PBM is 11.6 μg/day. Of note, given that the study was conducted in winter, it is less likely that the study results were significantly influenced by the status of cutaneous VitD synthesis among the study subjects. However, it is clear in Fig. 2 that around 40% of subjects who took VitD from foods over 11.6 μg/day had a sufficient serum level of 25(OH)D, suggesting that around 60% of subjects still had an insufficient VitD level. Therefore, the skin production of VitD has significant importance in maintaining proper serum VitD levels, even if VitD intake is sufficient. A negative correlation was observed between serum 25(OH)D and intact PTH in this analysis. Lower intake of VitD is related to an insufficient 25(OH)D level and causes secondary hyperparathyroidism. Previously, it was reported that 25(OH)D and serum PTH independently affected areal BMD [24]. Sufficient intake of VitD may help prevent secondary hyperparathyroidism in the younger population.
The present study had limitations. The first one was its relatively small sample size. The second one was the limited lifestyle of the participants, who were all nursing students. The third is that the seasonal effect, since 25(OH)D is also produced by UV radiation, was not evaluated. The latter limitation together with the limited season during which the serum samples were obtained, however, may contribute to the small variations in the chemical parameters.
In conclusion, the present study findings suggest that the VitD intake recommended in the DRIs in Japan, 5.5 μg/day, may not be sufficient to maintain serum 25(OH)D concentrations for bone health. Again, the present study suggests that a VitD intake of 11.6 μg/day is required to maintain 25(OH)D concentrations at 20 ng/mL or higher. Furthermore, VitD supplementation is considered mandatory in those with VitD deficiency despite cutaneous synthesis and nutritional intake of VitD.
References
Ohta H, Uenishi K, Shiraki M (2016) Recent nutritional trends of calcium and vitamin D in East Asia. Osteoporos Sarcopenia 4:208–213
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011). In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds) Dietary reference intakes for calcium and vitamin D. National Academies Press (US), Washington DC
Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N (2010) IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 21:1151–1154
Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD, Cole DE, Atkinson SA, Josse RG, Feldman S, Kline GA, Rosen C, Guidelines Committee of the Scientific Advisory Council of Osteoporosis Canada (2010) Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. CMA 182:E610–E618
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, Foundation National Osteoporosis (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
Tsugawa N, Uenishi K, Ishida H, Ozaki R, Takase T, Minekami T, Uchino Y, Kamao M, Okano T (2016) Association between vitamin D status and serum parathyroid hormone concentration and calcaneal stiffness in Japanese adolescents: sex differences in susceptibility to vitamin D deficiency. J Bone Miner Metab 34:464–474
Ohta H, Uemura Y, Nakamura T, Fukunaga M, Ohashi Y, Hosoi T, Mori S, Sugimoto T, Itoi E, Orimo H, Shiraki M, Adequate Treatment of Osteoporosis (A-TOP) Research Group (2014) Serum 25-hydroxyvitamin D level as an independent determinant of quality of life in osteoporosis with a high risk for fracture. Clin Ther 36:225–235
Kumamoto K, Nakamura T, Suzuki T, Gorai I, Fujinawa O, Ohta H, Shiraki M, Yoh K, Fujiwara S, Endo N, Matsumoto T (2010) Validation of the Japanese Osteoporosis Quality of Life Questionnaire. J Bone Miner Metab 28:1–7
Kuroda T, Shiraki M, Tanaka S, Ohta H (2009) Contributions of 25-hydroxyvitamin D, co-morbidities and bone mass to mortality in Japanese postmenopausal women. Bone 44:168–172
Roth HJ, Zahn I, Alkier R, Schmidt H (2001) Validation of the first automated chemiluminescence protein-binding assay for the detection of 25-hydroxycalciferol. Clin Lab 47:365–367
Murakami K, Sasaki S, Takahashi Y, Okubo H, Hirota N, Notsu A, Fukui M, Date C (2008) Reproducibility and relative validity of dietary glycaemic index and load assessed with a self-administered diet-history questionnaire in Japanese adults. Br J Nutr 99:639–648
Dietary Reference Intakes (2015). http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf. Accessed 5 Oct 2017 (in English)
Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, Michigami T, Takeuchi Y, Matsumoto T, Sugimoto T (2017) Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society (Opinion). J Bone Miner Metab 35:1–5
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J, IOF Committee of Scientific Advisors (CSA) Nutrition Working Group (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20:1807–1820
Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K (2014) A systematic review of vitamin D status in populations worldwide. Br J Nutr 111:23–45
Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips PJ (2009) Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab 94:1244–1250
Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, Dietrich T, Willett WC (2009) Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 24:935–942
Yoshimura N, Muraki S, Oka H, Morita M, Yamada H, Tanaka S, Kawaguchi H, Nakamura K, Akune T (2013) Profiles of vitamin D insufficiency and deficiency in Japanese men and women: association with biological, environmental, and nutritional factors and coexisting disorders: the ROAD study. Osteoporos Int 24:2775–2787
Tamaki J, Iki M, Sato Y, Kajita E, Nishino H, Akiba T, Matsumoto T, Kagamimori S, For the JPOS Study Group (2017) Total 25-hydroxyvitamin D levels predict fracture risk: results from the 15-year follow-up of the Japanese Population-Based Osteoporosis (JPOS) Cohort Study. Osteoporos Int 28:1903–1913
Nakamura K, Nashimoto M, Tsuchiya Y, Obata A, Miyanishi K, Yamamoto M (2001) Vitamin D insufficiency in Japanese female college students: a preliminary report. Int J Vitam Nutr Res 71:302–305
Guidelines for Prevention and Treatment of Osteoporosis (2015). http://jsbmr.umin.jp/pdf/GL2015.pdf. Accessed 5 Oct 2017 (in Japanese)
Choi SW, Kweon SS, Choi JS, Rhee JA, Lee YH, Nam HS, Jeong SK, Park KS, Ryu SY, Song HR, Shin MH (2016) The association between vitamin D and parathyroid hormone and bone mineral density: the Dong-gu Study. J Bone Miner Metab 34:555–563
Acknowledgements
The authors would like to express their sincere acknowledgement to the people who voluntarily participated in the present study. This study was financially supported by a grant from the Japan Osteoporosis Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.O. received lecture fees from Pfizer. M.S. received consulting fees from Asahi Kasei Pharma and Teijin Pharma. T.K. is an employee of Asahi Kasei Corporation. N.T., Y.O., and T.O. have no conflicts of interest.
About this article
Cite this article
Ohta, H., Kuroda, T., Tsugawa, N. et al. Optimal vitamin D intake for preventing serum 25-hydroxyvitamin D insufficiency in young Japanese women. J Bone Miner Metab 36, 620–625 (2018). https://doi.org/10.1007/s00774-017-0879-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0879-7