Abstract
Summary
Daily dosing with vitamin D often fails to achieve optimal outcomes, and it is uncertain what the target level of 25-hydroxyvitamin D should be. This study found that large loading doses of vitamin D3 rapidly and safely normalize 25OHD levels, and that monthly dosing is similarly effective after 3–5 months. With baseline 25OHD > 50 nmol/L, vitamin D supplementation does not reduce PTH levels.
Introduction
There is concern that vitamin D supplementation doses are frequently inadequate, and that compliance with daily medication is likely to be suboptimal.
Methods
This randomized double-blind trial compares responses to three high-dose vitamin D3 regimens and estimates optimal 25-hydroxyvitamin D (25OHD) levels, from changes in parathyroid hormone (PTH), and procollagen type I amino-terminal propeptide (P1NP) in relation to baseline 25OHD. Sixty-three elderly participants were randomized to three regimens of vitamin D supplementation: a 500,000-IU loading dose; the loading dose plus 50,000 IU/month; or 50,000 IU/month.
Results
The Loading and Loading + Monthly groups showed increases in 25OHD of 58 ± 28 nmol/L from baseline to 1 month. Thereafter, levels gradually declined to plateaus of 69 ± 5 nmol/L and 91 ± 4 nmol/l, respectively. In the Monthly group, 25OHD reached a plateau of ~80 ± 20 nmol/L at 3–5 months. There were no changes in serum calcium concentrations. PTH and P1NP were only suppressed by vitamin D treatment in those with baseline 25OHD levels <50 and <30 nmol/L, respectively.
Conclusions
Large loading doses of vitamin D3 rapidly and safely normalize 25OHD levels in the frail elderly. Monthly dosing is similarly effective and safe, but takes 3–5 months for plateau 25OHD levels to be reached.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adequate circulating concentrations of 25OHD are important for optimal bone health. Vitamin D status is related to bone density [1–5], bone turnover markers [6, 7], falls risk functional stability and strength [4, 8], and to the occurrence of hip fractures [9–11]. Intervention studies with vitamin D suggest beneficial effects on fractures, particularly in institutionalized individuals and when calcium is co-administered [12–14]. The elderly are particularly susceptible to vitamin D insufficiency [3, 15, 16] as a result of reductions in mobility, time spent outdoors, sun exposure (contributed to by increased skin coverage), intrinsic skin response to UV radiation, and dietary vitamin D intake [17, 18]. The consequences of vitamin D deficiency are potentially severe in the elderly since it is likely to contribute to their high fracture risk and to the mortality that is associated with fractures in this age group [19, 20].
Vitamin D deficiency is thought to contribute to bone loss by stimulating PTH secretion, resulting in increased bone resorption [21, 22]. Consequently, determinations of the optimal level of 25OHD have been based on estimates of the level of 25OHD required to minimize PTH concentrations, most frequently from cross-sectional studies. These analyses suggest that the optimal 25OHD concentration lies between 25 and 122 nmol/L [23]. This variability may result from other factors that impact on PTH levels (such as dietary calcium intake, renal function) and from assumptions made in fitting curves through widely scattered data points. An approach that circumvents some of these problems is to study the impact of vitamin D supplementation on PTH concentrations longitudinally, in order to identify a level of 25OHD above which supplementation has no effect on PTH. There are only three such longitudinal investigations, and these have produced more consistent estimates of optimal 25OHD, ranging from 40 to 50 nmol/L [23–25]. Further corroboration of these results is needed.
Most people requiring vitamin D supplementation take this in the form of a daily tablet, but there is concern that the doses used are frequently inadequate, and that compliance with daily medication is likely to be suboptimal. The long half-life of 25OHD following oral calciferol supplementation [26] means that larger, less frequent doses are a practical alternative to daily supplementation. Despite this, systematic evaluation of regimens using high doses of vitamin D is lacking. The present study seeks to address this need by comparing 25OHD responses to three different high-dose cholecalciferol regimens. In addition, we have also used this dataset to determine the optimal level of 25OHD in these subjects, by assessing the relationships between baseline 25OHD and the responses of PTH and the bone formation marker P1NP (procollagen type I amino-terminal propeptide) to vitamin D supplementation.
Materials and methods
Participants
Patients aged ≥65 years in the general medical wards of a metropolitan hospital were recruited between October 2005 and May 2006. Exclusion criteria were a creatinine clearance <20 mL/min, current glucocorticoids use for >6 months, recent calciferol treatment at doses >600 IU/day, disorders or drugs that might influence vitamin D or PTH metabolism, and a life expectancy of <6 months. Sixty-three of 183 eligible participants agreed to participate. The main reason given for potential subjects declining was their and their spouses’ deteriorating health. All participants provided written informed consent and the study procedures were approved by the local ethics committee.
Treatment
Participants were randomized into one of three treatment groups using a minimization algorithm to ensure balanced numbers of men and women between groups. One group (Loading) took 10 × 50,000 IU vitamin D3 tablets (12.5 mg) at the beginning of the study followed by a monthly placebo tablet for 8 months. A second group (Loading + Monthly) took 10 × 50,000 IU vitamin D3 tablets at study entry, followed by a 50,000 IU (1.25 mg) vitamin D3 tablet monthly. The third group (Monthly) took one vitamin D plus 9 placebo tablets at baseline followed by a vitamin D3 tablet monthly. The baseline doses were administered by study personnel, resulting in 100% compliance. Compliance with subsequent doses was assessed by tablet counts. Participants and investigators were blinded to the treatment assignments throughout. Trial medication was supplied by API Consumer Brands, Auckland
Assays
Plasma and serum were stored at −80°C until the completion of the study, when assays for the measurement of plasma intact PTH, serum 25OHD and serum P1NP were undertaken. PTH and P1NP were measured using automated Roche electrochemiluminescence immunoassays, and 25OHD was measured using the DiaSorin radioimmunoassay. Each participant’s samples were measured in the same batch. Inter-assay coefficients of variation were 6.1% for PTH, 7.1% for P1NP and 5.7% for 25OHD. Creatinine clearance was estimated using the Cockroft–Gault formula.
Clinical assessment
Details of medical and pharmacological history were taken from hospital medical records and during an interview conducted at baseline. Time spent outdoors, from an average of the times in mid-summer and mid-winter, was recorded. Calcium and vitamin D intakes were assessed using a food frequency questionnaire [27]. Additional vitamin-D-containing foods were added to this questionnaire, and intake was quantified using food composition data from the Foodfiles 2004© (Revision 18) database (New Zealand Institute for Crop and Food Research Ltd., Christchurch, New Zealand) accessed through FoodWorks Professional Edition 4 (Xyris Software (Australia) Pty. Ltd., Brisbane, Australia). Calcium and vitamin D intakes include that derived from supplements. Grip strength was measured in triplicate in the dominant hand at baseline and at 9 months.
Statistical analysis
Data were analyzed on an intention to treat basis using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Two-way (month × dose) mixed model analysis of variance (ANOVA) was applied to examine changes in the dependent variables 25OHD, PTH, and P1NP. Differences in the areas under the curves for these variables were analyzed using one-way ANOVA. Post-hoc multiple comparisons are Bonferroni-adjusted. Linear relationships between 25OHD, PTH, P1NP and their changes are reported as Pearson’s correlation coefficients. Data are reported as mean ± SEM unless otherwise stated. All tests were two-tailed and P < 0.05 was considered significant.
Results
Participants
The study subjects were in hospital at the time of recruitment for the following reasons: vascular disease [26], collapse or falls [15], rehabilitation following orthopedic surgery [10], infections [6], respiratory disease [3], and other problems [3]. Prior to hospital admission, 53 lived at home, seven in a retirement village, and three in residential care. At the time of the 1-month assessment, 81% were independent with respect to mobility and self-cares, and at 9 months this was the case for 89%. Treatment groups were comparable in terms of baseline characteristics (Table 1). Despite their frailty, age, and reported minimal use of vitamin D supplementation, mean baseline levels of 25OHD were 58 nmol/L, though the range was wide and some subjects were markedly deficient.
Six participants withdrew within the first month because of ill health. Four withdrew subsequently for health reasons. One subject moved away from the area, and five died during the study. Of the 47 participants who completed the study, one completed a 4-week course of oral steroids for a bronchial infection and another was started on an oral bisphosphonate during the study. Their data are included in the analyses, except where noted otherwise. Compliance, defined as the number of monthly tablets taken as a percentage of the number of monthly tablets that should have been taken, was <80% in only two subjects, and the median was 100% in all groups.
25OHD, PTH and P1NP relationships at baseline
Baseline 25OHD showed a similar variation with month as we have previously described in a much larger cohort [28], though it was not significant in this smaller number of subjects (data not shown). There was an inverse correlation between 25OHD and PTH for the individual data (r = −0.38, P = 0.003), but inspection of the data grouped in 10 nmol/L intervals of 25OHD indicates that there was little decline in PTH above 25OHD levels of 50 nmol/L. Baseline 25OHD was also negatively correlated with age (r = −0.28, P = 0.03) and positively with total vitamin D intake (r = 0.45, P = 0.001). Baseline P1NP and 25OHD were not related (r = −0.15; Figure 1).
25OHD responses to supplementation
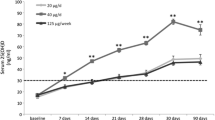
The Loading and Loading + Monthly groups showed similar patterns of change, with rapid increases in 25OHD to 1 month (mean increase 58 nmol/L) followed by gradual declines to respective plateaus of 69 ± 5 nmol/L and 91 ± 4 nmol/l. The decay half-time from peak to plateau for the Loading group was 1.3 months (95% CI 1.7, 5.3). In the Monthly group, 25OHD rose gradually to a plateau of about 80 nmol/L at 5 months. The ANOVA model confirmed that the group (F (2,57) = 7, P = 0.002) and month effects (F (5,241) = 58, P < 0.001), and their interaction (F (10,241) = 13, P < 0.001) were all significant. Areas under these curves calculated for those who completed the study were different between groups (F (2,38) = 5, P = 0.02), with post-hoc tests showing a difference between the Loading + Monthly and Monthly groups (26,000 ± 1000 and 20,000 ± 1,000 nmol days/L, respectively). Response to vitamin D supplementation might be affected by BMI and season, but the groups were comparable with respect to these variables at baseline, and there was no significant interaction between them and study group (Figure 2).
Because these high-dose replacement regimens would usually be used in patients with low vitamin D levels, we re-assessed these data in subjects with baseline 25OHD levels below 50 nmol/L (Fig. 3). The upper panel of this figure demonstrates the efficacy of the loading dose in rapidly correcting low levels of 25OHD, mean values increasing from 31 nmol/L (range 12–48 nmol/L) at baseline, to 101 nmol/L (range 46–141 nmol/L) 1 month later. The Loading + Monthly group then declined to a plateau of 86 nmol/L at 5 months, whereas the Loading group progressively decreased, reaching 58 nmol/L at 9 months. The Monthly regimen was also effective, but took 3 months to reach the plateau of ~70 nmol/L.
Effects of three regimens of supplementation with vitamin D3 on serum levels of 25-hydroxyvitamin D (25OHD), according to whether baseline concentrations of 25OHD were (a) below, or (b) above 50 nmol/L. In both cohorts, there were significant time-treatment interactions in the 25OHD response (P < 0.001; a F(10,99) = 11, b F(105,130) = 63). Data are mean ± SEM
With the more widespread use of vitamin D supplementation, assessment of the response in non-deficient subjects is also of interest to determine the likelihood of hypercalcemia. Following the loading dose, the increase to 1 month was 50 (95% CI, 38–63) nmol/L, somewhat less than the increase of 71 (95% CI 58–84) nmol/L seen in the deficient group (P = 0.026). Mean 25OHD peaked at ~130 nmol/L, and the highest individual 25OHD level at 1 month was 220 nmol/L, in a subject with a baseline value of 136 nmol/L. At 3 months, the maximum level was 160 nmol/L. Serum calcium (corrected for albumin) was measured at baseline, 1, 5, and 9 months. There was no significant increase in serum calcium concentrations from baseline during the study, and no individual values were above the upper end of the reference range. The mean (±SD) values at these time-points were: 2.32 ± 0.13, 2.35 ± 0.12, 2.28 ± 0.11 and 2.24 ± 0.08 mmol/L in the Loading group; 2.36 ± 0.09, 2.31 ± 0.08, 2.31 ± 0.08, and 2.28 ± 0.09 in the Loading + Monthly group; 2.39 ± 0.09, 2.37 ± 0.09, 2.31 ± 0.10, and 2.28 ± 0.08 mmol/L in the Monthly group.
PTH and P1NP responses to supplementation as indicators of optimal 25OHD
Serum PTH concentrations decreased by almost 1 pmol/L at 1 month in the two groups receiving a loading dose of vitamin D (P = 0.0075 for change from baseline in the pooled groups). At 3 months, there was a similar decrease (−1.2 ± 0.6 pmol/L) in the group receiving monthly supplementation. These changes were maintained in the groups receiving monthly supplementation over the remainder of the study. We have utilized these data to estimate the optimal serum 25OHD concentration, using the method of Malabanan [24]. This involves identifying a level of 25OHD above which supplementation with vitamin D causes no further suppression of circulating PTH concentrations. To this end, we have pooled PTH data from all study subjects and identified the visits at which 25OHD is ≥20 nmol/L above baseline for that subject. The changes in PTH at these visits are shown in Fig. 4, in relation to baseline 25OHD levels. When baseline 25OHD was <20 nmol/L, there were substantial reductions in PTH as a result of vitamin D supplementation. For baseline 25OHD levels between 20 and 50 nmol/L, PTH decreased by about 1 pmol/L. However, at higher 25OHD levels, supplementation with vitamin D did not affect PTH. This suggests that there is no advantage, in terms of minimizing PTH, in achieving circulating 25OHD concentrations >50 nmol/L.
These decreases in PTH would be expected to be accompanied by more gradual reductions in bone turnover, assessed here by P1NP. These data from the subjects given the 500,000 IU loading dose were plotted over time, dividing the cohort at cut-points of baseline 25OHD of 20, 30, 40, 50, and 60 nmol/L (data not shown). With the 30 nmol/L cut-point (Fig. 5), there was no mean change in P1NP in subjects whose baseline 25OHD was ≥30 nmol/L, whereas P1NP declined in those with baseline 25OHD < 30 (P = 0.05). The fall in P1NP was greater in those with baseline 25OHD below the cut-point (P = 0.01). With higher cut-points, there were no differences in change in P1NP between those above or below the cut-points. P1NP data from ten subjects were excluded from this analysis: seven with recent orthopedic procedures, one who was immobilized following a stroke, and in two who had markedly elevated baseline values without apparent bone pathology.
Change from baseline in procollagen type I amino-terminal propeptide (ΔP1NP) in subjects who received a loading dose of calciferol at baseline (i.e. the Loading and Loading + Monthly groups), divided according to whether the baseline 25OHD was above or below 30 nmol/L. There was a significant interaction between baseline 25OHD and time, with respect to the changes in P1NP (P = 0.01; F (4,94) = 3). Data are mean ± SEM
Grip strength
Grip strength in the entire cohort increased 2.6 ± 0.6 kg from baseline to the end of the study (P < 0.01), but there were no between-groups differences. Increases in individual subjects were not related to the change in 25OHD (r = −0.1). In subjects in whom 25OHD increased by ≥20 nmol/L, there was no effect of baseline 25OHD levels (grouped as above or below 50 nmol/L) on the change in grip strength.
Discussion
The present study demonstrates the efficacy and safety of three regimens utilizing a 50,000-IU tablet of vitamin D3. Individuals with initial 25OHD levels < 50 nmol/L who received a 500,000 IU loading dose of vitamin D3 showed increases in levels of 25OHD at 1 month to around 100 nmol/L. In contrast, with monthly 50,000 IU dosing, it took ~3 months for those with low initial vitamin D status to attain satisfactory levels. Both regimens are potentially useful. A loading dose is likely to be desirable for individuals with a high fracture risk and probability of poor vitamin D status, because it rectifies these low levels promptly, though there is still a 3–5 month delay in the bone marker response to the increased 25OHD levels. Rapid repletion of vitamin D is mandatory in deficient patients wishing to receive intravenous bisphosphonates post-fracture [29], since these drugs can cause sustained hypocalcemia in such individuals [30]. Individuals with osteomalacia or myopathy secondary to vitamin D deficiency will also require rapid repletion. In contrast, many individuals whose fracture risk is lower or in whom vitamin D status is uncertain can be managed satisfactorily with 50,000 IU monthly. If compliance with a monthly regimen is in question, the present results suggest that single doses of 500,000 IU every 6–12 months might provide a similar outcome.
Several other studies have assessed the effects of large intermittent doses of vitamin D. We have previously shown that a single dose of 500,000 IU vitamin D3 is safe and effective in older women with serum 25OHD levels <25 nmol/L, and that it produces a 15% reduction in PTH concentrations [26]. Dosing with 300,000 IU vitamin D3 leads to a rapid rise in 25OHD, peaking at 17 days and declining with a half-life of 90 days [26]. Adams [31] administered vitamin D2 500,000 IU over 5 weeks to deficient patients resulting in a mean increase in 25OHD of 60 nmol/L, and Przybelski [32] gave 600,000 IU of D2 over 4 weeks with an increase of 47 nmol/L, both results similar to that found in the present study. There were no safety issues in either study. Diamond administered 600,000 IU of D3 as a single intramuscular injection to 50 subjects. Primary hyperparathyroidism was subsequently diagnosed in one person and mild hypercalcemia was found in two others at 12 months, raising uncertainty regarding its etiology [33]. Others have shown that vitamin D2 7,000–10,000 IU/week will prevent falls [34] and 4–12-monthly doses of vitamin D may prevent fractures [35, 36].
Heaney’s group has investigated the time-course and dose–response to daily oral vitamin D3 in healthy young men with initial 25OHD levels of ~70 nmol/L [37, 38]. After 8–20 weeks dosing, 25OHD increased by 0.015–0.03 nmol/L for each IU/day of vitamin D3, similar to the increases seen with monthly dosing in the present study. Comparing these increments with those after the loading dose used here, suggests that this loading dose produces an increment at 1 month equivalent to a long-term daily dose of about 4,000 IU [38].
The number of subjects in the present study with normal 25OHD levels was unexpected, and is likely to indicate that they had been taking vitamin D supplements which they did not report, prior to their hospital admission. This has provided the opportunity to assess the effect of high-dose supplementation in individuals whose levels are ≥50 nmol/L. In this group, treatment with 50,000 IU monthly had a minimal effect, in contrast to those with baseline 25OHD < 50 nmol/L, in whom 25OHD increased by around 40 nmol/L. Providing replete individuals with a single 500,000-IU dose resulted in increases in 25OHD of around 50 nmol/L. Though substantial, these increases were again not as great as when baseline 25OHD was <50 nmol/L. A similar observation was made by Viljakainen et al. who provided vitamin D3 (200–800 IU/day) to elderly women and noted that the response was almost doubled when initial 25OHD levels were below the median level of 47 nmol/L [39]. Vieth has suggested that homeostatic control mechanisms, possibly hepatic, prevent large increases in 25OHD [40]. Our observations support this possibility, though uptake into adipose tissue could also contribute to this effect.
Levels of 25OHD which constitute a risk of hypercalcemia are not clear, and safety issues associated with high levels cannot be ignored. Vieth has pointed out that studies which document both vitamin D dose and 25OHD levels have demonstrated hypercalcemia at intakes >10,000–40,000 IU/day, and these intakes are usually associated with 25OHD levels >500 nmol/L [40–42]. Despite this, upper reference limits for 25OHD are often set at much lower levels of 150–250 nmol/L. The highest level observed in any individual in the present study was 220 nmol/L at 1 month, which appears to be well within the safe range, particularly since this peak was transient. This contention is supported by the normal serum calcium levels throughout this period. However, we would not recommend loading doses in subjects who are vitamin D sufficient. In contrast, in vitamin-D-deficient subjects, the loading dose was efficient in normalizing 25OHD and again did not result in values above the reference range.
The present data provide the opportunity to assess prospectively the optimal concentration of 25OHD, by identifying levels above which supplementation does not further suppress PTH and indices of bone turnover. The PTH data from the present study suggest that optimal levels of 25OHD are around 50 nmol/L, and the P1NP analyses suggest the optimal value might be even lower. Both these parameters might be affected by the subjects’ mobility, though only 11–19% had significant mobility impairment during the study period and their exclusion did not affect this analysis. This matter is subject to considerable controversy [43–45] and much of the evidence supporting higher levels is from cross-sectional studies. Such estimates are problematic because of the considerable variability in PTH levels within each study, meaning that the levels of 25OHD associated with a PTH nadir are not evident from simple inspection of scatter-plots of PTH versus 25OHD. As a result, statistical assumptions are necessary to develop models which define the relationship between PTH and 25OHD. The nature of these assumptions is often arbitrary, and will impact substantially on the results found.
Only three studies have used a longitudinal design to assess optimal 25OHD, as in the present study. All have arrived at optimal levels for 25OHD of around 50 nmol/L, even though they have varied in age group, race, country and duration [23–25]. Malabanan [24] assessed responses to 50,000 IU/week vitamin D2 over 8 weeks in 35 subjects with a mean age of 76 years. They found that PTH was not further suppressed in those whose baseline 25OHD concentrations were >50 nmol/L. Lips utilized data from the placebo group of a multi-national clinical trial in osteoporotic women, and found that in response to calcium (500 mg/day) and vitamin D3 (400–600 IU/day) over 6 months, subjects only showed a significant decrease in PTH if their baseline 25OHD was <50 nmol/L [25]. Aloia studied healthy, postmenopausal African–American women given vitamin D3 800–2,000 IU/day over 3 years, and found that the threshold for PTH suppression was a 25OHD level between 40 and 50 nmol/L [23]. Thus, there is substantial consistency between the present study and the others which have used a similar robust design in identifying a threshold of 25OHD for bone health. This does not mean that this is the optimal 25OHD level for the other tissues on which vitamin D is active [46], nor does it take into account the seasonal variation in 25OHD levels. We have recently shown that to ensure a nadir level of 25OHD in winter >50 nmol/L, the summer peak must be 60–70 nmol/L in women, and 70–90 nmol/L in men [47].
A limitation of this study is that there is no placebo-only group. When planning this study, we felt that it would be unethical to fail to provide any vitamin D treatment to frail elderly people who were deficient. It is therefore possible that some of the changes in 25OHD were due to factors other than the interventions provided, such as altered behavior relating to sun exposure or dietary modification. If this were the case, it is likely that the effect would be consistent across all treatment groups and small, given the clear differences in between-group responses. Furthermore, it is unlikely that increased ultraviolet exposure would have affected our results relating to changes in PTH and P1NP since these changes would have occurred irrespective of the cause of the 25OHD increases. In addition, we were not able to control calcium intake nor fluctuations in renal function, but these are likely to be lesser confounders in a longitudinal study than they would be using a cross-sectional design. The generalizability of these findings to less frail or younger individuals remains to be determined, though the studies of Lips and Aloia were in much younger individuals and yet found similar relationships.
In conclusion, our results show that a large single dose of vitamin D3 rapidly and safely normalizes 25OHD levels in the frail elderly. Monthly 50,000 IU dosing is similarly effective and safe, but takes 3–5 months for plateau 25OHD levels to be reached. The single high-dose regimen provides a convenient option for the rapid correction of vitamin D deficiency, and monthly dosing is likely to increase compliance in this population group.
References
von Muhlen DG, Greendale GA, Garland CF, Wan L, Barrett-Connor E (2005) Vitamin D, parathyroid hormone levels and bone mineral density in community-dwelling older women: The Rancho Bernardo Study. Osteoporos Int 16:1721–1726
Khaw KT, Sneyd MJ, Compston J (1992) Bone density parathyroid hormone and 25-hydroxyvitamin D concentrations in middle aged women. BMJ 305:273–277
Melin AL, Wilske J, Ringertz H, Saaf M (1999) Vitamin D status, parathyroid function and femoral bone density in an elderly Swedish population living at home. Aging Clin Exp Res 11:200–207
Pfeifer M, Begerow B, Minne HW et al (2001) Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes 109:87–92
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B (2004) Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639
Scharla SH, Scheidt-Nave C, Leidig G et al (1996) Lower serum 25-hydroxyvitamin D is associated with increased bone resorption markers and lower bone density at the proximal femur in normal females: a population-based study. Exp Clin Endocrinol Diabetes 104:289–292
Jesudason D, Need AG, Horowitz M, O'Loughlin PD, Morris HA, Nordin BEC (2002) Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 31:626–630
Bischoff-Ferrari HA, Dietrich T, Orav EJ et al (2004) Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr 80:752–758
Lips P, Netelenbos JC, Jongen MJ et al (1982) Histomorphometric profile and vitamin D status in patients with femoral neck fracture. Metab Bone Dis Relat Res 4:85–93
Dixon T, Mitchell P, Beringer T et al (2006) An overview of the prevalence of 25-hydroxy-vitamin D inadequacy amongst elderly patients with or without fragility fracture in the United Kingdom. Curr Med Res Opin 22:405–415
Garnero P, Munoz F, Sornay-Rendu E, Delmas PD (2007) Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone 40:716–722
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264
Avenell A, Gillespie WJ, Gillespie LD, O'Connell DL (2005) Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev CD000227
Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P (2007) Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab 92:1415–1423
Webb AR, Pilbeam C, Hanafin N, Holick MF (1990) An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr 51:1075–1081
Dawson-Hughes B, Harris SS, Dallal GE (1997) Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr 65:67–71
Holick MF (1995) Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr 61:638S–645S
Heaney RP (2006) Barriers to optimizing vitamin D3 intake for the elderly. J Nutr 136:1123–1125
Roy DK, O'Neill TW, Finn JD et al (2003) Determinants of incident vertebral fracture in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 14:19–26
Schroder HM, Erlandsen M (1993) Age and sex as determinants of mortality after hip fracture: 3,895 patients followed for 2.5–18.5 years. J Orthop Trauma 7:525–531
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Holick MF (2002) Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 9:87–98
Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK (2006) Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 84:602–609
Malabanan A, Veronikis IE, Holick MF (1998) Redefining vitamin D insufficiency. Lancet 351:805–806
Lips P, Duong T, Oleksik A et al (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221
Wu F, Staykova T, Horne A et al (2003) Efficacy of an oral, 10-day course of high-dose calciferol in correcting vitamin D deficiency. N Z Med J 116:U536
Angus RM, Sambrook PN, Pocock NA, Eisman JA (1989) A simple method for assessing calcium intake in Caucasian women. J Am Diet Assoc 89:209–214
Lucas JA, Bolland MJ, Grey AB et al (2005) Determinants of vitamin D status in older women living in a subtropical climate. Osteoporos Int 16:1641–1648
Lyles KW, Colon-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Rosen CJ, Brown S (2003) Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N Engl J Med 348:1503–1504
Adams JS, Kantorovich V, Wu C, Javanbakht M, Hollis BW (1999) Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab 84:2729–2730
Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N (2008) Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int 19:1621–1628
Diamond TH, Ho KW, Rohl PG, Meerkin M (2005) Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Med J Aust 183:10–12
Flicker L, MacInnis RJ, Stein MS et al (2005) Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc 53:1881–1888
Trivedi DP, Doll R, Khaw KT (2003) Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326:469
Heikinheimo RJ, Inkovaara JA, Harju EJ et al (1992) Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int 51:105–110
Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF (1998) Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int 8:222–230
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204–210
Viljakainen HT, Palssa A, Kärkkäinen M, Jakobsen J, Lamberg-Allardt C (2006) How much vitamin D3 do the elderly need? J Am Coll Nutr 25:429–435
Vieth R (1999) Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 69:842–856
Vieth R, Chan PC, MacFarlane GD (2001) Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 73:288–294
Vieth R (2006) Critique of the considerations for establishing the tolerable upper intake level for vitamin D: critical need for revision upwards. J Nutr 136:1117–1122
Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R (2005) Estimates of optimal vitamin D status. Osteoporos Int 16:713–716
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Bouillon R, Norman AW, Lips P (2007) Vitamin D deficiency. N Engl J Med 357:1980–1981
Holick MF (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S
Bolland MJ, Grey AB, Ames RW et al (2007) The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on diagnosis of vitamin D sufficiency. Am J Clin Nutr 86:959–964
Acknowledgments
The authors are grateful to API Consumer Brands for providing the study medication.
Funding
This study was supported by the Health Research Council of New Zealand
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bacon, C.J., Gamble, G.D., Horne, A.M. et al. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 20, 1407–1415 (2009). https://doi.org/10.1007/s00198-008-0814-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0814-9