Abstract
The purpose of this study was to compare changes in bone mineral density (BMD) in premenopausal patients with node-positive early breast cancer treated with goserelin (Zoladex) or cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Patients (n=1640) were randomized to goserelin (3.6 mg every 28 days for 2 years) or CMF (six×28-day cycles) treatment. In a protocoled sub-study involving 96 patients from eight centers (goserelin: n=53; CMF: n=43), lumbar spine (L2–L4) and femoral neck BMD were assessed by dual X-ray absorptiometry at baseline and then annually for 3 years. At the end of the 2-year goserelin-treatment period, mean BMD losses for goserelin-treated and CMF-treated patients were −10.5% and −6.5% (P=0.0005) for lumbar spine and −6.4% and −4.5% (P=0.04) for femoral neck, respectively. At 3 years, partial recovery of BMD was observed in goserelin recipients. In contrast, mean BMD losses for the CMF group indicated persistent BMD loss. No significant differences in BMD were observed between groups at the 3-year assessment of the spine or femoral neck. In the CMF group, based on amenorrhea status at 48 weeks, BMD losses at the lumbar spine were greater for amenorrheic than non-amenorrheic patients. Ovarian suppression resulting in amenorrhea was closely related to BMD loss in both treatment groups. Overall, patients who received CMF did not show recovery of BMD throughout follow-up, whereas partial recovery was observed 1 year after cessation of goserelin therapy, associated with the return of ovarian function in the majority of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of amenorrhea in premenopausal patients has been linked to an improved outcome in the treatment of early breast cancer [1,2]. Both chemotherapy with cyclophosphamide, methotrexate and 5-fluorouracil (CMF) and treatment with goserelin (Zoladex) induce amenorrhea. However, amenorrhea induced by goserelin is reversible in the majority of patients following cessation of therapy but permanent in most patients treated with CMF [3]. Thus, goserelin treatment avoids continuation of the menopausal side effects and, potentially, the long-term risks associated with permanent ovarian ablation.
The efficacy and tolerability of goserelin have been established in several major international trials along with chemotherapy or tamoxifen, the current standard treatments in premenopausal patients with early breast cancer. These ongoing trials indicate that the combination of goserelin and tamoxifen has similar [4] or superior efficacy [5] to CMF in premenopausal women with hormone receptor-positive tumors. Additionally, goserelin, with or without tamoxifen, can add benefit in premenopausal patients with hormone receptor-positive tumors following chemotherapy [6], or when given in addition to standard adjuvant therapy [7].
Most recently, the large ZEBRA (Zoladex in Early Breast Cancer Research Association) trial involving 1640 premenopausal patients ≤50 years of age with node-positive early breast cancer was the first study to compare directly the efficacy and tolerability of estrogen suppression with goserelin monotherapy or CMF chemotherapy following initial surgery (with or without radiotherapy). This study has previously reported that goserelin is as effective as CMF in patients with estrogen receptor (ER)-positive tumors and has a favorable side-effect profile [3] with significant improvements in patients’ quality of life [8,9]. Similar results have been obtained in two trials of adjuvant goserelin combined with tamoxifen versus CMF in the treatment of premenopausal, hormone-responsive breast cancer [7,10,11].
In normal healthy women, bone mass begins to decline after the menopause with approximately 20% bone loss occurring during the first 10 years [12]. The risk of hip fracture has been shown to increase 3-fold for each 10-year increase in age [13]. Other factors frequently associated with excessive bone loss include declining physical activity, impaired absorption of calcium, and the adverse effects of other medical conditions or drugs [14].
It is well recognized that an early menopause or prolonged amenorrhea, from whatever cause, are major risk factors for bone loss and osteoporosis [15,16]. Osteoporosis currently affects more than 25 million women world-wide [17], with approximately 1.5 million osteoporotic fractures occurring annually in the United States, including 700,000 vertebral fractures, 300,000 hip fractures, 250,000 wrist fractures, and more than 300,000 fractures at other sites [18].
This paper reports a protocoled sub-study of the ZEBRA trial, which was designed to compare BMD levels during and following adjuvant treatment with goserelin or CMF.
Materials and methods
Study design
The ZEBRA study is an international, multicenter, open, randomized study initiated in 1990 to compare the efficacy and tolerability of goserelin with CMF in pre-/perimenopausal women with histologically proven, node-positive, early breast cancer [3]. Patients were recruited over a 6-year period, between 1 October 1990 and 30 December 1996, from 102 centers in 15 countries. Following local therapy for breast cancer (mastectomy or breast conserving therapy with or without radiotherapy, according to local practice), patients were randomized in a 1:1 ratio to receive goserelin or CMF chemotherapy.
In a protocoled ZEBRA sub-study, BMD was also assessed in a sub-group of patients from eight centers. The objectives were to compare and quantify the loss in BMD between patients receiving goserelin or CMF, and to assess BMD changes and possible recovery of BMD during follow-up. The study was designed to include 196 patients with at least 69 patients evaluable per group.
Patients and methods
Patients were eligible for inclusion in the study if they were pre-/perimenopausal and ≤50 years of age, had undergone surgery (breast conserving or mastectomy), had histologically proven operable invasive breast cancer with no evidence of metastatic disease, and had not received previous systemic therapy. All patients gave their informed consent to participate in the sub-study.
Patients were randomized to receive either goserelin 3.6 mg depot subcutaneously (every 28 days for 2 years, i.e. 26 depots) or a standard regimen of CMF chemotherapy (six cycles, each cycle planned to be 28 days [19]. A cycle of CMF consisted of: cyclophosphamide (500 mg/m2 given intravenously on days 1 and 8, or 100 mg/m2 given orally on days 1–14), methotrexate (40 mg/m2 given intravenously on days 1 and 8, and 5-fluorouracil (600 mg/m2 given intravenously on days 1 and 8).
BMD was assessed from 1 March 1992 in the selected patients consenting to the sub-protocol. Patients with BMD measurements at the lumbar spine or femoral neck or both were included in the BMD population. Patients were excluded from protocoled BMD assessments if they had a history of traumatic fracture or spinal abnormalities, were obese (body mass index >30 kg/m2), had serious metabolic disease, or were receiving cortiocosteroids, vitamin D, calcium supplements, bisphosphonates or other drugs known to affect calcium metabolism. If a patient developed skeletal metastases in the L2–L4 lumbar spine or neck of femur, all assessments after the development of these metastases were excluded.
Patient characteristics
Of the patients recruited in the ZEBRA study, 364 had been randomized from 12 selected centers by the time of the start of the BMD protocol on 1 March 1992. However, of these 12 centers, two did not include any patients in the BMD sub-protocol, two provided BMD measurements that were not of acceptable quality, leaving patients from eight centers of which one entered only patients treated with goserelin in the study. Furthermore, many of the patients did not give consent for BMD measurements or were not willing to accept any additional measurements after the first investigation and were therefore excluded or withdrawn. While this sub-study does not meet the requirements of a randomized trial, the characteristics of the final study population, which comprised two groups, of which 53 patients were treated with goserelin and 43 patients were treated with CMF, were similar in terms of baseline demographics, tumor characteristics and the local therapy they received (Table 1). However, seven patients in the goserelin group had a hysterectomy compared with none in the CMF group. Both groups were similar with respect to baseline BMD data for both lumbar spine and neck of femur sites (Table 2).
Treatment compliance
Of the 53 patients who received goserelin, 45 patients (84.9%) received the full course of 26 depots, four patients (7.5%) received 27 depots, two patients (3.8%) stopped treatment early (≤12 depots) and two (3.8%) received 22–25 depots. Of the 43 patients in the CMF group, 41 (95.3%) completed all six cycles of CMF; the remaining two patients (4.7%) stopped treatment early after three and five cycles.
BMD assessments
BMD of the lumbar spine (L2–L4) and neck of femur were assessed by dual X-ray absorptiometry (DXA) scans using three different types of machine [Lunar (Madison, Wisc., USA), Hologic (Bedford, Mass., USA) and Norland (White Plains, N.Y., USA)] at baseline then annually for up to 3 years post-randomization. All DXA scans were independently reviewed at a central quality control center that was blinded to trial treatment and outcome. Scans were assessed in terms of technical quality, whether the same scanner had been used throughout and whether adequate instrument quality control had been performed.
Units for lumbar spine BMD for different manufacturer’s machines were converted to standardized BMD (units mg/cm2) using the equations published by Steiger [20]. For neck of femur BMD, the equations published by Genant et al. [21] were used to convert Hologic and Norland figures to equivalent figures on the Lunar BMD scale (units g/cm2).
Statistical analyses
Patients were included in the statistical analysis if they had a baseline measurement of BMD of acceptable quality as assessed by the central independent quality control center, and at least one post-baseline measurement at the same site of acceptable technical quality within the protocoled time windows (between 12 weeks before and 4 weeks after the start of trial therapy, 12±2 months, 24±3 months, 36±4 months).
BMD values were log-transformed and analysis of covariance (ANCOVA) was used to compare change from baseline at each assessment time between the two treatment groups. Covariates in the model were treatment, age and baseline BMD (log-transformed).
Results
Percentage change in BMD from baseline
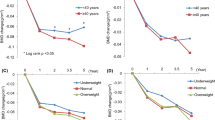
During the study, there was a mean loss of BMD in both treatment groups at the lumbar spine and neck of femur (Fig. 1). At 1 year from the start of adjuvant therapy, mean percentage losses in BMD were significantly greater in the goserelin group than in the CMF group at the lumbar spine (−8.2% versus −4.5%; P=0.00008), but equal at the neck of the femur (−4.5% versus −4.4%; P=0.70). At the end of the 2-year goserelin treatment period, the BMD losses for goserelin-treated patients were significantly greater at both sites compared with CMF-treated patients (−10.5% versus −6.5%; P=0.0005 for lumbar spine and −6.4% versus −4.5%; P=0.04 for neck of femur).
At the 3-year assessment, 1 year after cessation of treatment with goserelin, partial recovery of BMD was observed. In contrast, BMD losses in the CMF group persisted at 3 years at both the lumbar spine and the femoral neck, although some individual patients showed increases in BMD (Fig. 2). As a result, no significant differences in BMD were observed between the two treatment groups at 3 years (−6.2% with goserelin versus −7.2% with CMF; P=0.26) and neck of femur (−3.1% with goserelin versus −4.6% with CMF; P=0.48).
Recovery of BMD loss was also examined in a subset of patients who had completed or nearly completed trial therapy (i.e. patients who had received at least 24 out of 26 depots of goserelin or at least five out of six planned cycles of CMF) and had BMD assessments at baseline, 1, 2 and 3 years at the lumbar spine (goserelin, n=24; CMF, n=16) and/or neck of femur (goserelin, n=25; CMF, n=16). A similar profile of BMD loss was observed in this subset of patients. Once again, partial recovery of BMD was observed in the goserelin group following cessation of therapy, whereas BMD losses persisted in the CMF group.
Induction of amenorrhea
During the study, 100% of goserelin-treated patients became amenorrheic during treatment compared with 69.4% of CMF-treated patients at 2 years (Table 3). Menses returned in almost 73% of goserelin-treated patients on cessation of therapy, whereas amenorrhea was permanent in most CMF patients (76.5% amenorrheic at 3 years).
In the CMF group, a retrospective indirect comparison based on amenorrhea status at 48 weeks, showed mean BMD losses at the lumbar spine were greater among amenorrheic patients than non-amenorrheic patients (−6.4% versus −1.6% at 1 year, −9.7% versus −2.0% at 2 years and −9.1% versus −3.4% at 3 years, respectively) (Fig. 3a). BMD losses at the neck of femur were also greater among amenorrheic patients than non-amenorrheic patients (−5.2% versus −3.1% at 1 year and −5.1% versus −4.0% at 3 years, respectively) (Fig. 3b). During the follow-up, some of the patients in both groups who had recovered their pre-menopausal status became amenorrheic through natural menopause.
Discussion
Previous studies have shown that approximately 70% of premenopausal women receiving adjuvant chemotherapy experience premature menopause and a significant decrease in BMD compared with patients not receiving adjuvant chemotherapy [22] or those maintaining normal menses [23]. As more patients are surviving a relatively long time after diagnosis, osteoporosis as a result of early menopause caused by adjuvant chemotherapy is an increasing problem with considerable morbidity and reduction in quality of life [24].
The results of this study demonstrate that ovarian suppression resulting in amenorrhea was associated with considerable BMD loss in both groups. Two years following the start of trial therapy, there was a significant decrease in BMD in patients who received goserelin that was not at the time observed in those who received CMF. However, the return of menses in the majority of patients was associated with partial recovery of BMD in nearly all patients 1 year after the completion of goserelin treatment. In contrast, patients in the CMF group did not show recovery of BMD at 3 years after the commencement of trial therapy, and no significant difference in BMD loss was observed between the two treatment groups at the 3-year assessment.
Partial recovery of BMD following cessation of treatment has also been observed in patients receiving 6-month courses of goserelin and other luteinizing hormone-releasing hormone (LHRH) analogs for endometriosis [25,26,27]. In a review of several major studies, a 6-month course of a LHRH analog resulted in a small reduction in trabecular bone density that was partially or completely reversible on withdrawal of treatment [25]. Further monitoring will indicate whether transient estrogen deficiency with long-term goserelin treatment will result in clinically relevant bone loss and an elevated risk of osteoporosis.
Previous clinical trials have demonstrated that amenorrhea induced by chemotherapy is accompanied by a loss of BMD of 2–7%, a similar loss to that which occurs during the natural menopause [16,28]. In one study, women who became permanently amenorrheic as a result of chemotherapy had BMD measurements 14% lower than women who maintained menses after chemotherapy [23]. Similarly, in the present study BMD losses in CMF-treated amenorrheic patients were markedly greater than in those who did not become amenorrheic. As with normal menopause, trabecular bone (such as lumbar spine) was affected more than cortical bone (such as femoral neck). These changes in BMD, if maintained in the long-term, would increase the risk of osteoporotic fractures. Adjuvant chemotherapy may precipitate osteoporotic fractures by 10 years in a considerable proportion of women cured of premenopausal breast cancer [22], and it is likely that this premature increase in the risk of osteoporotic fractures is confined to those patients who were rendered amenorrheic during chemotherapy.
Longer-term follow-up is being carried out. Meanwhile the observed recovery of BMD in goserelin-treated patients is an encouraging observation, whereas it seems likely that BMD will further fall in those patients who were amenorrheic after CMF treatment. Therefore it is possible that the risk of osteoporotic fractures previously reported with chemotherapy could be avoided with treatments that permit a return to menses, such as goserelin. The results of the ZEBRA BMD sub-study are therefore encouraging as they could have future implications for quality of life. Coupled with recent follow-up data from the ZEBRA study, which show that adjuvant goserelin has a similar effect on disease-free and overall survival in premenopausal women with ER-positive tumors [29], and initial data showing that goserelin was better tolerated than CMF [3] and improved patients’ quality of life [8,9], data from the BMD sub-study add further support to the use of goserelin as an alternative to chemotherapy in this patient population. However, longer-term follow-up is required to fully document the effects of goserelin treatment on long-term survival and patients’ risk of an osteoporotic fracture.
References
Del Mastro L, Venturini M, Sertoli MR, Rosso R (1997) Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: prognostic role and clinical implications. Breast Cancer Res Treat 43:183–190
Pagani O, O’Neill A, Castiglione M, Gelber RD, Goldhirsh A, Rudenstam CM et al. (1998) Prognostic impact of amenorrhea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer 34:632–640
Jonat W, Kaufmann M, Sauerbrei W, Blamey R, Cuzick J, Namer M et al. (2002) Goserelin versus cyclophosphamide, methotrexate and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex Early Breast Cancer Research Association study. J Clin Oncol 20:4628–4635
Boccardo F, Rubagotti A, Amoroso D, Mesiti M, Romeo D, Sismondi P et al. (2000) Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: results of the Italian Breast Cancer Adjuvant Study Group 02 randomized trial. J Clin Oncol 18:2718–2727
Jakesz R, Hausmaninger H, Samonigg H et al. (2001) Complete endocrine blockade with tamoxifen and goserelin is superior to CMF in the adjuvant treatment of premenopausal, lymph node-positive and -negative patients with hormone-responsive breast cancer. Breast 10:S10 (abstract S26)
Davidson N, O’Neill A, Vukov A, Osborne S, Martino D, White M.D (1999) Effect of chemohormonal therapy in premenopausal, node (+), receptor (+) breast cancer: an Eastern Cooperative Oncology Group Phase III Intergroup trial. Proc ASCO 18:67a (abstract 249)
Baum M, Houghton J, Odling-Smee W et al. (2001) Adjuvant Zoladex in premenopausal patients with early breast cancer: results from the ZIPP trial. Breast 10:S32–S33 (abstract P64)
de Haes H and the ZEBRA Trialists Group (2000) Comparison of quality of life in pre/perimenopausal women treated with Zoladex or CMF as adjuvant therapy for the management of node-positive early breast cancer: results from the ZEBRA study. Breast Pharmacokinet 39:27–48
Olschewski M, de Haes H (2001) The ZEBRA study: early benefits in quality of life in goserelin-treated vs CMF-treated pre/perimenopausal patients with node-positive early breast cancer. Eur J Cancer 37:S40 (abstract Q115)
Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T et al. (2002) Randomized adjuvant trial of tamoxifen plus goserelin versus cyclophosphamide, methotrexate and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer. J Clin Oncol 20:4621–4627
Boccardo F, Rubagotti A, Amoroso D, Mesiti M, Romeo D, Aldrighetti D et al. (2001) CMF vs tamoxifen (TAM) plus ovarian suppression (OS) as adjuvant treatment of ER-positive (ER+) pre/perimenopausal breast cancer (BCA) patients. Breast 10:S32 (abstract P62)
Trevisan C, Ortolani S, Bianchi ML, Caraceni MP, Ulivieri FM, Gandolini G, Polli EE (1991) Age, time since menopause, and body parameters as determinants of female spinal bone mass: a mathematical model. Calcif Tiss Int 49:1–5
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK et al. (1993) Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75
Tannirandorn P, Epstein S (2000) Drug-induced bone loss. Osteoporos Int 11:637–659
Fogelman I (1996) The effects of estrogen deficiency on the skeleton and its prevention. Br J Obstet Gynaecol 103 (suppl 14):5–9
Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I (1997) Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol 15:1341–1347
Melton LJ, Thamer M, Ray NF, Chan JK, Chesnut CH, Einhorn TA et al. (1997) Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res 12:16–23
Peterson JA (2001) Osteoporosis overview. Geriatr Nurs 22:17–21
Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C et al. (1976) Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 294:405–410
Steiger P (1995) Standardization of spine BMD measurements. J Bone Miner Res 10:1602–1603
Genant HK, Grampp S, Gluer CC, Faulkner KG, Jergas M, Engelke K et al. (1994) Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 9:1503–1514
Bruning PF, Pit MJ, de Jong-Bakker M, van den Ende A, Hart A, van Enk A (1990) Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer 61:308–310
Headley JA, Theriault RL, LeBlanc AD, Vassilopoulou-Sellin R, Hortobagyi GN (1998) Pilot study of bone mineral density in breast cancer patients treated with adjuvant chemotherapy. Cancer Invest 16:6–11
Delmas PD, Fontana A (1998) Bone loss induced by cancer treatment and its management. Eur J Cancer 34:260–262
Fogelman I (1992) Gonadotropin-releasing hormone agonists and the skeleton. Fertil Steril 57:715–724
Paoletti AM, Serra GG, Cagnacci A, Vacca AM, Guerriero S, Solla E, Melis GB (1996) Spontaneous reversibility of bone loss induced by gonadotropin-releasing hormone analog treatment. Fertil Steril 65:707–710
Taga M, Minaguchi H (1996) Reduction of bone mineral density by gonadotropin-releasing hormone agonist, nafarelin, is not completely reversible at 6 months after the cessation of administration. Acta Obstet Gynecol Scand 75:162–165
Powles TJ, McCloskey E, Paterson AH, Ashley S, Tidy VA, Nevantaus A et al. (1998) Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst 90:704–708
Namer M, Jonat W, Kaufmann M, Blamey R, Cuzick J, Fogelman I et al. (2002) Survival data from the ZEBRA study. Ann Oncol 13 (suppl 5):38 (abstract 135P)
Acknowledgements
The ZEBRA study is supported by a grant from AstraZeneca, Macclesfield, UK. Participating investigators: M. Geberth, Heidelberg, Germany; B. Lisboa, Hamburg, Germany; A.X. Izquierdo, Gerona, Spain; P. Firat, Ankara, Turkey; S.J. Leinster, Liverpool, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fogelman, I., Blake, G.M., Blamey, R. et al. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos Int 14, 1001–1006 (2003). https://doi.org/10.1007/s00198-003-1508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-003-1508-y