Abstract
Purpose
This study was designed to explore whether zoledronic acid could prevent expected loss of bone mineral density (BMD) in postmenopausal women with pre-existing osteopenia or osteoporosis who were initiating adjuvant letrozole therapy for primary breast cancer.
Methods
Between June 2006 and July 2007, 60 postmenopausal women with estrogen and/or progesterone receptor-positive breast cancer and a BMD T-score ≤−2.0 were enrolled. Participants received letrozole 2.5 mg and vitamin D 400 IU daily, calcium 500 mg twice daily, and zoledronic acid 4 mg every 6 months for a maximum of 5 years or until disease progression. BMD at the lumbar spine and femoral neck was recorded at the start of the study and annually for 5 years. Patients were evaluated for fractures every 6 months for the duration of the trial.
Results
After 5 years, mean BMD increased significantly by 11.6 % (p = 0.01) at the lumbar spine and by 8.8 % (p = 0.01) at combined sites. Femoral neck BMD increased by 4.2 %, although this was not significant (p = 0.23). At the end of the trial, BMDs were consistent with osteoporosis in 7 % and osteopenia in 36 % of the patients. A total of six fractures were reported after 417 individual assessments.
Conclusions
Zoledronic acid appears to prevent further bone loss in postmenopausal breast cancer patients with osteopenia and osteoporosis starting treatment with letrozole. These findings were maintained at 5 years and support concurrent initiation of bisphosphonate and aromatase inhibitor therapy in this high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatase inhibitors (AIs) have been consistently shown to provide benefit for patients with hormone-positive breast cancer and are frequently incorporated into treatment both in adjuvant and metastatic settings. By inactivating the enzyme responsible for production of estrogens from androgens, AIs reduce plasma estrogen levels [1]. While helpful in the treatment of breast cancer, this suppression of estrogen has consequences for bone mineral density (BMD). Estrogen promotes osteoclast inactivation and apoptosis, which minimizes bone mineral resorption [2, 3]. When plasma levels of estrogen are suppressed, women are susceptible to loss of BMD and development of osteoporosis, which is an adverse effect associated with AI use that has been observed in several clinical trials [4–10].
Treatment for osteoporosis often includes bisphosphonates, which are indicated for postmenopausal women with BMD T-score of ≤−2.5 or T-score of ≤−2.0 if there is presence of risk factors for ongoing bone loss. Zoledronic acid (ZA) is a potent intravenously administered bisphosphonate that has been used to promote skeletal stability in patients at particularly high risk for fracture, including those with cancer metastatic to the bone and multiple myeloma. ZA is also known to preserve BMD in postmenopausal women with T-scores better than −2.0 who are undergoing adjuvant AI therapy [11–14].
In contrast to prior studies, which have assessed the effect of ZA on prevention of osteopenia and osteoporosis for women with BMD T-scores better than −2.0 who were being treated with AIs, this study was designed to evaluate the impact of ZA for women who possessed osteopenia or osteoporosis at the start of AI therapy. An initial report from this work illustrated that administration of ZA was associated with a statistically significant 2.66 % increase in BMD after 1 year of treatment [15]. However, longer-term follow-up was needed to confirm the durability of these results. The current report provides 5-year follow-up data from this trial.
Patients and methods
This study was conducted by the Mayo Clinic Cancer Research Consortium (MCCRC), which was recently renamed as Academic and Community Cancer Research United (ACCRU). The study was approved by the institutional review boards at all participating sites. All participants provided written consent prior to enrollment. Funding was provided by Novartis, whose involvement was limited to review of the original study protocol.
Study population and design
The design of this study has previously been described [15]. Briefly, the study population consisted of postmenopausal women with newly diagnosed stage I–IIIa estrogen receptor (ER) and/or progesterone receptor (PR)-positive, localized breast cancer who had an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–2 and a life expectancy of at least 5 years. Upon study entry, patients were required to have a baseline total lumbar spine (LS) or femoral neck (FN) BMD T-score ≤−2.0, and any oral bisphosphonate use must have been discontinued at least 3 weeks prior to registration. Important exclusion criteria included treatment with drugs known to affect skeletal stability within the past 2 weeks; prior treatment with intravenous bisphosphonates or AIs; exposure to anabolic steroids or growth hormone within the past 6 months; or additional malignancies within the past 5 years, with the exception of adequately treated non-melanoma skin cancer and/or cervical carcinoma in situ.
In this open-label, single-arm, observational trial, participants were prescribed letrozole 2.5 mg daily, calcium 500 mg twice daily, and vitamin D 400 IU daily. ZA 4 mg was given intravenously over 15 min, every 6 months (with dose adjustments for creatinine clearance, if necessary) until disease progression or a maximum of 5 years. BMD was measured by dual-energy X-ray absorptiometry (DXA) once at the start of the trial and then annually for 5 years. Patients who discontinued letrozole or ZA for any reason were taken off study, and further evaluation was not performed. Patients were evaluated for disease progression at each visit, and if disease progression was found, they were similarly excluded. Patients who discontinued calcium or vitamin D for any reason were allowed to continue with the study.
The primary endpoint for the original analysis was prospectively defined as the mean intra-patient change in BMD measured in the LS from baseline to 12 months (1 year). The endpoints for this follow-up analysis were mean intra-patient changes in BMD measured in the LS from baseline to 24, 36, 48, and 60 months after study commencement (2–5 years). Secondary endpoints included the mean intra-patient change in FN BMD or either LS or FN at these same time points. Annual incidence rates for osteopenia, osteoporosis, and bone fractures were also assessed, and the NCI’s Common Terminology Criteria for Adverse Events (version 3.0) was used to calculate the severity of medication-related toxicity.
Statistical analysis
The primary and secondary endpoints were calculated by subtracting the baseline LS or FN BMD values from values at years 1 through 5 and converting the differences to a percent of baseline. Data were tested for normality, and if normal, a single sample t test was utilized to determine whether the percent change was statistically significant at each time point. If non-normal, the Wilcoxon sign rank test was utilized. Osteopenia (LS T-score between −2.0 and −2.5) and osteoporosis (LS T-score less than −2.5) statuses were assigned to each patient. Summary statistics (frequency, percentages) were calculated for these two endpoints and number of bone fractures. Toxicity grade was summarized similarly. This study did not follow a formal study design as it was primarily observational, but given a sample size of 60, it was estimated to provide statistical accuracy within 13 %, with 95 % confidence.
Results
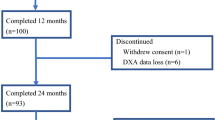
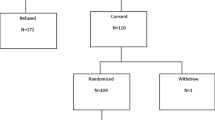
Between June 2006 and July 2007, 60 patients were enrolled. Of these, one patient cancelled, one patient had a major violation in treatment, and five patients were ineligible; thus, 53 patients were potentially evaluable for the endpoints. The baseline characteristics of the patients who initiated treatment are shown in Table 1. There were 30 patients evaluable for the primary BMD endpoint in the original manuscript [15], and there were 11 patients evaluable for the LS measured BMD at 5 years. Figure 1 illustrates the dropout of patients over time. The most common reason for dropout was refusal of further treatment (12 patients) due to personal preference. Two patients were taken off study due to treatment-related toxicity (one patient with musculoskeletal pain and one patient with elevated creatinine), and one patient died during the course of the trial. The remaining patients were removed for a variety of reasons, such as conflicting treatment of other medical conditions and relocation away from study centers.
BMD changes over time
Changes in BMD over time are shown in Table 2 and Fig. 2, indicating that there were increases in BMD over 5 years. The percent change in BMD from baseline increased consistently over time as measured at the LS, and at each year, the change was statistically significant. At 5 years, an 11.6 % increase from baseline (p = 0.01) was observed. The percent change using the FN measurements peaked at year 2 and then decreased. The 5-year measurement for FN BMD was not statistically significant. Assessment of change in either LS or FN BMD (whichever were available) yielded statistically significant results at all time points.
Osteopenia, osteoporosis, and bone fractures
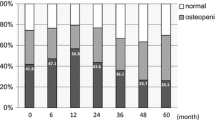
Patients were evaluated for fracture at 6-month intervals during the course of the study. Over 5 years, a total of six fractures were reported after 417 individual assessments. Participants were also monitored annually for osteopenia and osteoporosis (Table 3). After 1 year, 60 % were observed to have osteopenia and 10 % osteoporosis. Among the 14 patients evaluable for these conditions after 5 years, 36 % had osteopenia and 7 % had osteoporosis.
Toxicity profile
Toxicities thought to be at least possibly related to therapy are shown in Table 4, understanding that, without a placebo arm, nocebo effects could not be determined. Consistent with data reported after 1 year [15], the most common toxicity was arthralgia, a well-established adverse effect associated with AIs. The total number of patients reporting this toxicity was unchanged from the 1-year data. Hot flashes, another frequent AI-related side effect, were noted by three patients. Mild-to-moderate creatinine elevation was observed in a total of nine patients, an effect which could possibly be related to ZA administration. No patients experienced osteonecrosis of the jaw during this study.
Discussion
The 5-year follow-up of this single-arm study supports the notion that loss of BMD in women with osteopenia or osteoporosis is stabilized with concurrent initiation of an AI and ZA. These findings build on the data previously reported from year 1 of the trial and strengthen the argument for use of ZA in a high-risk population starting hormone therapy known to cause bone loss and increase fracture risk. Although a control arm was not available to compare the magnitude of BMD improvement, 5 years of AI therapy has previously been associated with a median decrease in BMD of 6 % at the LS and 7 % at the hip [16], which provides context for the benefit observed with ZA administration in this trial.
The prevalence of osteoporosis among women over age 50 in the USA is estimated to be about 10 %, with an additional 50 % possessing low BMD, defined as a T-score less than or equal to −1.0 [17]. When patients in this population develop hormone-positive breast cancer, initiation of an AI may be met with some hesitation, given the risk for added bone loss and fractures. Breast cancer itself may increase skeletal fragility and fracture risk, even in the absence of bone metastases, further complicating this decision [18]. In the first year after initiation of AI therapy, BMD has been shown to fall by as much as 3 %, with further decreases observed in subsequent years [8, 19]. In the ATAC trial, this decrement in BMD translated into a fracture incidence of 11.0 % versus 7.7 % in the tamoxifen arm over a median follow-up of 68 months [6], with similarly elevated fracture rates observed in the other trials of AIs with the longest follow-up [5, 7, 19]. In the MA.17 study exploring the efficacy of 5 years of letrozole therapy in postmenopausal women following 5 years of tamoxifen, there was a significant increase in new rates of osteoporosis (8 versus 6 % for placebo) at a median follow-up of 30 months [10]. In light of these data, tamoxifen might be considered a favorable alternative for a sub-population at high risk for skeletal instability, given its partial estrogenic activity, which has been shown to prevent bone loss in postmenopausal women [20]. However, AIs are commonly used as first-line hormonal therapy for this population based on substantial clinical trial data demonstrating its superiority over tamoxifen in terms of disease-free survival [21]. Therefore, prevention of ongoing bone loss and protection against fractures in postmenopausal women receiving AIs has become a subject of important investigation.
Among the variety of agents available for treatment of osteoporosis, bisphosphonates are considered a good choice for patients with breast cancer. ZA belongs to a subset of bisphosphonates possessing a nitrogen-containing substituent that is thought to confer superior anti-resorptive activity and has proven effective in reducing fractures in postmenopausal women with osteoporosis [22]. In preclinical trials, ZA was consistently shown to provide superior inhibition of osteoclast activity, with the largest therapeutic ratio of desired inhibition of calcium resorption and unwanted inhibition of mineralization. Further, ZA has been shown to directly inhibit breast cancer cell invasion in a dose-dependent manner, making it a natural choice in postmenopausal women with a history of breast cancer who are at risk for ongoing bone loss. A North Central Cancer Treatment Group (N03CC) trial and the Zometa-Femara Adjuvant Synergy sister trials (Z-FAST, ZO-FAST, and E-ZO-FAST) were designed to investigate whether ZA would be effective for prevention of bone loss and fractures in postmenopausal women without osteopenia or osteoporosis (i.e., T-score ≥−2.0) [11–14]. These trials evaluated whether upfront ZA administration for patients starting AI therapy would lead to BMD preservation and reduced fracture incidence, compared with delayed ZA administration (i.e., after T-score fell below −2.0 or fracture was sustained). An improvement in BMD was observed in patients receiving early ZA, though reduction in fracture risk was not seen in the studies assessing this endpoint [11–13]. The absence of fracture risk reduction has made routine early administration of ZA a controversial practice. When making this decision, the potential benefit must be weighed against cost and toxicities, which in the present study were reasonably benign, though not non-existent.
In contrast to prior trials, which excluded women with known osteopenia or osteoporosis, this is the first study to provide extended follow-up of the effect of concurrent AI and ZA administration on BMD in postmenopausal women with pre-existing low BMDs. These data support the concept that treatment with ZA decreases the risk of further bone loss classically associated with AI use. Whether the increase in BMD correlates with reduced fracture risk for this population remains unclear. Six participants reported fractures during the course of this 5 year study, after a total of 417 individual assessments. This corresponds to a fracture rate of 11 % of the original study population; however, by the end of 5 years, only 28 patients were evaluated for this finding. A true fracture rate is, thus, difficult to calculate. Additionally, without a comparison arm, this trial was not specifically designed to identify reduction in fracture risk by ZA treatment.
The improvement in BMD observed in this trial is consistent with data from prior studies evaluating use of oral bisphosphonates in a similar population [23, 24]. In the SABRE trial, women with T-score <−2.0 who were treated with anastrozole and risedronate experienced a significant 3.0 % increase in LS and total hip BMD at 24-month follow-up [23]. In the 5-year follow-up of the ARIBON trial that treated osteoporotic postmenopausal women with early breast cancer with anastrozole and ibandronate, a 9.65 % increase in LS BMD was observed, along with a 2.72 % increase in total hip BMD [24]. This trial also randomized osteopenic patients to 2 years of anastrozole and ibandronate, or anastrozole and placebo. At the 2-year assessment, the mean LS BMD had increased 3.19 % in the 20 patients evaluable. For the remaining 3 years of the study, ibandronate was discontinued in 17 of these 20 patients as BMD T-scores had risen above −2.0. The cessation of bisphosphonate therapy resulted in loss of the accrued benefit by the end of the trial, with 15 patients ending with T-scores at least in the osteopenic range. It seems increasingly clear that addition of a bisphosphonate offers a bone stabilizing effect for postmenopausal women with established bone loss initiating AI therapy. However, intravenous bisphosphonates have not been compared with oral bisphosphonates in a head-to-head manner for this purpose. Additionally, substantive data on fracture outcomes is lacking, making it difficult to select a preferred route of administration. While ZA is generally considered a potent bisphosphonate, a recent meta-analysis has found a mortality benefit in breast cancer patients using bisphosphonates, regardless of sub-type, elevating the importance of convenience and toxicity profile when deciding on a particular agent [25].
It should be noted that patients in this study received, in addition to ZA, calcium and vitamin D supplementation, which has been shown to decrease bone loss in healthy postmenopausal women with osteoporosis [26–30]. To our knowledge, no study has been specifically designed to evaluate the efficacy of calcium and vitamin D in preserving BMD in postmenopausal women with osteoporosis who are initiating AI therapy. Importantly, however, in the companion study to the NCIC CTG MA.17 trial [8], which was designed to evaluate bone loss in women receiving letrozole versus placebo, all patients were provided with calcium and vitamin D supplementation. In spite of this intervention, women in the letrozole arm experienced a significant decrease in BMD. Similarly, in the N03CC, E-ZO-FAST, and ZO-FAST trials, calcium and vitamin D supplementation was provided to all patients, with BMD results favoring those groups additionally receiving ZA [11, 12, 14]. Based on these prior data, it is unlikely that the improvement in BMD observed in the present study is attributable to calcium and vitamin D administration alone; though, without a comparator arm, the influence of calcium and vitamin D on the results cannot be fully evaluated.
The findings of this study are limited by the small sample size and the high dropout rate. Although the reason for dropout appeared to be largely due to personal preference rather than toxicity or disease progression, attrition at the rate observed in this trial introduces the possibility of bias. The statistically significant improvement in BMD observed in the remaining evaluable patients must therefore be interpreted cautiously. Additionally, the absence of a control arm prevents discussion of compared benefit of ZA for this population, with historical controls providing only an imperfect means of comparison. Such a study could not ethically have been performed in a randomized, controlled fashion, given the standard use of bisphosphonate therapy in high-risk osteopenic patients.
The impact of osteoporosis on the healthcare system is already considerable, with incidence of hip fracture in the USA projected to increase from 1.66 million in 1990 to 6.26 million in 2050 [31]. This is expected to contribute US$131.5 billion in healthcare expenditure to the global economy [32]. For the patient, hip and vertebral fractures translate into 5-year survival rates 80 % of age-matched controls [33]. The increased use of hormone therapy known to decrease BMD and increase fracture risk in a high-risk sub-population can be expected to compound this problem. This heightens the necessity of discovering an optimal strategy to combat ongoing bone loss and reduce fracture rates. This trial supports the argument for continued use of AI therapy in combination with ZA in postmenopausal women with established bone loss. Further work should be designed to determine fracture risk and to compare efficacy of intravenous versus oral bisphosphonate in this population.
References
Smith IE, Dowsett M (2003) Aromatase inhibitors in breast cancer. N Engl J Med 348(24):2431–2442. doi:10.1056/NEJMra023246
Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF (1996) Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med 2(10):1132–1136
Chen FP, Wang KC, Huang JD (2009) Effect of estrogen on the activity and growth of human osteoclasts in vitro. Taiwan J Obstet Gynecol 48(4):350–355. doi:10.1016/S1028-4559(09)60323-5
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349(19):1793–1802. doi:10.1056/NEJMoa032312
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350(11):1081–1092. doi:10.1056/NEJMoa040331
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62. doi:10.1016/S0140-6736(04)17666-6
Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26):2747–2757. doi:10.1056/NEJMoa052258
Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, Shenkier TN, Tozer RG, Palmer MJ, Shepherd LE, Liu S, Tu D, Goss PE (2006) Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol 24(22):3629–3635. doi:10.1200/JCO.2005.05.4882
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25(5):486–492. doi:10.1200/JCO.2006.08.8617
Goss PE (2007) Letrozole in the extended adjuvant setting: MA.17. Breast Cancer Res Treat 105(suppl 1):45–53. doi:10.1007/s10549-007-9698-1
Hines SL, Mincey B, Dentchev T, Sloan JA, Perez EA, Johnson DB, Schaefer PL, Alberts S, Liu H, Kahanic S, Mazurczak MA, Nikcevich DA, Loprinzi CL (2009) Immediate versus delayed zoledronic acid for prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen-N03CC. Breast Cancer Res Treat 117(3):603–609. doi:10.1007/s10549-009-0332-2
Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, Deleu I, Miller J, Schenk N, Neven P (2012) Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer 12(1):40–48. doi:10.1016/j.clbc.2011.08.002
Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, Jin L, Warsi G, Argonza-Aviles E, Hohneker J, Ericson SG, Perez EA (2012) Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 118(5):1192–1201. doi:10.1002/cncr.26313
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24(2):398–405. doi:10.1093/annonc/mds277
Hines SL, Sloan JA, Atherton PJ, Perez EA, Dakhil SR, Johnson DB, Reddy PS, Dalton RJ, Mattar BI, Loprinzi CL (2010) Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy. Breast 19(2):92–96. doi:10.1016/j.breast.2009.12.001
Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G (2008) Effect of anastrozole on bone mineral density: 5-year results from the Anastrozole, Tamoxifen, Alone or in Combination Trial 18233230. J Clin Oncol 26(7):1051–1057. doi:10.1200/JCO.2007.11.0726
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526. doi:10.1002/jbmr.2269
Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T (1999) A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 79(7–8):1179–1181. doi:10.1038/sj.bjc.6690188
Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 8(2):119–127. doi:10.1016/S1470-2045(07)70003-7
Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S (1996) Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 14(1):78–84
Forbes J, Dowsett M, Bradley R, Ingle JN, Aihara T, Bliss JM, Boccardo F, Coates AS, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thurlimann B, van de Velde C, Davies C, Gray R (2014) Patient-level meta-analysis of randomized trials of aromatase inhibitors (AI) versus tamoxifen (Tam). J Clin Oncol 32(5s)
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822. doi:10.1056/NEJMoa067312
Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, Barlow D, Makris A, Eastell R (2010) Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol 28(6):967–975. doi:10.1200/jco.2009.24.5902
Lester JE, Dodwell D, Brown JE, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, Horsman JM, Coleman RE (2012) Prevention of anastrozole induced bone loss with monthly oral ibandronate: final 5 year results from the ARIBON trial. J Bone Oncol 1(2):57–62. doi:10.1016/j.jbo.2012.06.002
Coleman R, Gnant M, Paterson A, Powles T, von Minckwitz G, Pritchard K, Bergh J, Bliss J, Gralow J, Anderson S, Evans V, Pan H, Bradley R, Davies C, Gray R (2013) Abstract S4-07: effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: A meta-analysis of individual patient data from randomised trials. Cancer Res 73(24s). doi:10.1158/0008-5472.sabcs13-s4-07
Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S (1990) A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 323(13):878–883. doi:10.1056/NEJM199009273231305
Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ (1993) Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med 328(7):460–464. doi:10.1056/NEJM199302183280702
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE (1997) Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337(10):670–676. doi:10.1056/NEJM199709043371003
Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354(7):669–683. doi:10.1056/NEJMoa055218
Reid IR, Mason B, Horne A, Ames R, Reid HE, Bava U, Bolland MJ, Gamble GD (2006) Randomized controlled trial of calcium in healthy older women. Am J Med 119(9):777–785. doi:10.1016/j.amjmed.2006.02.038
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2(6):285–289
Johnell O (1997) The socioeconomic burden of fractures: today and in the 21st century. Am J Med 103(2As):20s–25s
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137(9):1001–1005
Conflict of interest
The authors declare that they have no competing interests. All primary data is under the control of the investigators and is available for review by the journal if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majithia, N., Atherton, P.J., Lafky, J.M. et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: a 5-year follow-up. Support Care Cancer 24, 1219–1226 (2016). https://doi.org/10.1007/s00520-015-2915-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2915-2