Abstract
Introduction and hypothesis
We aimed to systematically review the literature on pelvic organ prolapse (POP) surgery with uterine preservation (hysteropexy). We hypothesized that different hysteropexy surgeries would have similar POP outcomes but varying adverse event (AE) rates.
Methods
MEDLINE, Cochrane, and clinicaltrials.gov databases were reviewed from inception to January 2018 for comparative (any size) and single-arm studies (n ≥ 50) involving hysteropexy. Studies were extracted for participant characteristics, interventions, comparators, outcomes, and AEs and assessed for methodological quality.
Results
We identified 99 eligible studies: 53 comparing hysteropexy to POP surgery with hysterectomy, 42 single-arm studies on hysteropexy, and four studies comparing stage ≥2 hysteropexy types. Data on POP outcomes were heterogeneous and usually from <3 years of follow-up. Repeat surgery prevalence for POP after hysteropexy varied widely (0–29%) but was similar among hysteropexy types. When comparing sacrohysteropexy routes, the laparoscopic approach had lower recurrent prolapse symptoms [odds ratio (OR) 0.18, 95% confidence interval (CI) 0.07–0.46), urinary retention (OR 0.05, 95% CI 0.003–0.83), and blood loss (difference −104 ml, 95% CI −145 to −63 ml) than open sacrohysteropexy. Laparoscopic sacrohysteropexy had longer operative times than vaginal mesh hysteropexy (difference 119 min, 95% CI 102–136 min). Most commonly reported AEs included mesh exposure (0–39%), urinary retention (0–80%), and sexual dysfunction (0–48%).
Conclusions
Hysteropexies have a wide range of POP recurrence and AEs; little data exist directly comparing different hysteropexy types. Therefore, for women choosing uterine preservation, surgeons should counsel them on outcomes and risks particular to the specific hysteropexy type planned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is a common disorder, with an 11% lifetime risk of requiring surgery [1]. Given the large aging population, the number of surgeries for POP is expected to grow 43% in the coming three decades, increasing the surgical demand to >245,000 surgeries per year by 2050 [2], more than double the present annual rate of reconstructive mastectomy for breast cancer [3]. Women are increasingly choosing apical POP surgeries that preserve the uterus, a set of procedures also known as hysteropexies [4]. Past survey data indicate that more than a third of women will choose uterine-preserving POP surgery provided outcomes are similar [5], and systematic reviews demonstrate that patients may enjoy some safety benefits with hysteropexy as opposed to POP surgery with hysterectomy [6,7,8].
To meet this rising demand for uterine-preserving POP surgery, surgeons must improve their knowledge of hysteropexy in order to provide adequate surgical counseling for interested patients. Given the wide variety of hysteropexy approaches described in the literature and the heterogeneity of research methods used to investigate these surgeries, synthesis of this knowledge is challenging. No systematic reviews or guidelines exist to guide choice of hysteropexy type. Clinicians need coherent, evidence-based counseling points for patients regarding the risks and advantages of various hysteropexy approaches.
The Systematic Review Group of the Society for Gynecologic Surgeons (SGS) recently conducted a systematic review of randomized and nonrandomized studies comparing uterine-preserving surgeries for apical repair of POP with POP surgeries involving hysterectomy, with clinical practice guidelines (CPGs) on the choice between these procedures [8]. Here, we describe in greater detail data from comparative and large single-arm studies investigating hysteropexy, including studies comparing different types of hysteropexy.
Methods
We searched MEDLINE, clinicaltrials.gov, and the Cochrane database from inception to January 2018. The searches included terms for various relevant procedures (Appendix 1). These are further analyses of our previously published systematic review utilizing the same search strategy to compare uterine preservation with hysterectomy in POP surgery [8]. Eligible studies had to include at least one group of adult women with a uterus in place and POP as their primary pathology who underwent uterine-preserving POP surgery with an apical support procedure. Studies had to report on one or more relevant outcomes in four categories: prolapse outcomes, other pelvic floor outcomes, perioperative outcomes, and adverse event (AEs). We included randomized controlled trials (RCTs) or nonrandomized comparative studies (nRCSs) of any size that compared one type of uterine-preserving apical POP surgery to another, as well as prospective or retrospective single-group studies (in which all women had uterine-preserving surgery) with at least 50 participants. RCTs and nRCSs that had a single arm of women receiving uterine-preserving surgery (e.g., compared with hysterectomy) were treated as single-group studies for the purposes of this manuscript. Publications could be in any language or any format (e.g., poster, abstract) that allowed for eligibility determination and outcome extraction.
Study selection and data extraction
Abstracts and full texts were independently screened for eligibility in duplicate by 12 reviewers using the online software Abstrackr (http://abstrack.cebm.brown.edu/) [9]. Discrepancies were resolved by a third reviewer. Data extraction was completed by the same 12 independent reviewers, with each study extracted by two reviewers, at least one of whom had prior systematic review experience [10, 11]. We extracted data on study design, surgical interventions, population characteristics, and rates of outcomes of interest.
Assessment of risk of bias
We assessed the methodologic quality of each study using predefined criteria from a three-tier system in which studies were graded as good (A), fair (B), or poor (C) based on scientific merit, the likelihood of biases, and the completeness of reporting. This grading was founded on the evaluators’ impression of the study’s risk of bias according to the Cochrane Risk of Bias tool and relevant questions from the Newcastle-Ottawa Scale [12, 13]. Qualities of individual outcomes were separately graded within each study based on adequate outcome description, reproducibility and reliability, and outcome importance from the patient perspective.
Data synthesis
We categorized surgeries as:
-
(1)
open mesh sacrohysteropexy (SHP);
-
(2)
laparoscopic or robotic-assisted mesh SHP;
-
(3)
other open abdominal hysteropexy procedures (non-SHP);
-
(4)
other laparoscopic or robotic-assisted hysteropexy procedures (non-SHP);
-
(5)
transvaginal mesh hysteropexy (VMHP);
-
(6)
transvaginal native-tissue hysteropexy (VNTHP) such as sacrospinous hysteropexy (SSHP) or uterosacral hysteropexy (USHP);
-
(7)
the Manchester procedure;
-
(8)
LeFort colpocleisis. Based on reporting by eligible studies and prioritization by the review group, we evaluated the following outcomes:
-
(1)
repeat surgery for prolapse;
-
(2)
prolapse recurrence, allowing any stated definition;
-
(3)
prolapse recurrence symptoms;
-
(4)
any postoperative urinary incontinence (UI), when necessary prioritizing stress UI (SUI), if reported;
-
(5)
de novo postoperative UI, again prioritizing SUI, if reported;
-
(6)
postoperative urinary retention or voiding dysfunction, allowing any definition;
-
(7)
mesh exposure;
-
(8)
sexual dysfunction, including dyspareunia;
-
(9)
estimated blood loss (EBL);
-
(10)
operating time (OT); and
-
(10)
hospital stay duration.
-
(1)
We analyzed the percentages of women following each surgery type who experienced each categorical outcome and average values of continuous outcomes. When at least two studies reported the same outcome associated with the same type of surgery, we meta-analyzed results regardless of the degree of heterogeneity. Meta-analyses were conducted in OpenMeta (www.cebm.brown.edu/openmeta) [14] for either the arcsine transformed proportion [15] or mean value (for single surgery type analyses), or the odds ratio (OR) or difference in mean values (for comparative studies). We meta-analyzed with random-effects-model-restricted maximum likelihoods. The I2 statistic was calculated for all meta-analyses to evaluate statistical heterogeneity.
In data synthesis and reporting, we considered methodologic quality, consistency of results across studies, directness of evidence, and other factors, such as imprecision or sparseness of evidence, to determine an overall quality of evidence in accordance with the Grades for Recommendation, Assessment, Development and Evaluation system, which uses four potential quality ratings: high, moderate, low, and very low [16]. If adequate data existed to make guideline statements or recommendations regarding the evidence for one type of hysteropexy over another, we were prepared to develop guideline statements incorporating the balance between benefits and harms of surgeries on which comparative data were available. All guideline statements would include a level of strength (strong or weak) based on the quality of relevant evidence and significance of medical benefit. Strong recommendations are worded as “we recommend” and indicate what most practitioners would do in a given clinical scenario. Weak recommendations are worded as “we suggest” and imply that the magnitude of the benefits are less certain. Support for recommendations could come from high-, moderate-, or low-quality studies (A, B, and C) independent of recommendation strength.
Results
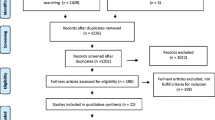
In the original search in 2016, we found 7324 citations, of which 337 abstracts were eligible and screened by full text. Of these screened full texts, 103 underwent data extraction, with 94 studies included in the final review. Five additional eligible studies were found in the updated search January 2018, resulting in 99 studies included in this publication: 53 studies comparing uterine-preserving POP surgery to a POP surgery involving hysterectomy (previously reported in prior review) [8], and 46 studies that included only arms describing a uterine-preserving POP surgery (Fig. 1). Within these 46 studies, there were 42 case series of ≥50 patients regarding a uterine-preserving POP surgery (35 reconstructive hysteropexy and 7 LeFort colpocleisis) and four nRCSs that compared one type of uterine-preserving surgery with another [17,18,19,20]. The 46 trials that included only uterine-preserving surgery arms are described in Tables 1, 2, 3, and 4; the nature and quality of the 53 trials comparing uterine-preserving POP surgery to POP surgery with hysterectomy have been previously described in or did not meet criteria for inclusion in the prior review [8]. The overall quality of evidence was moderate for all 99 trials which included at least one group of women having a uterine-preserving POP surgery. However, the overall quality of evidence from the 46 trials that only included hysteropexy arms was low, as the majority were single-group studies of low quality.
Abdominal or laparoscopic/robotic sacrohysteropexy or other abdominal hysteropexies
Two retrospective cohort studies, one of high [18] and one of moderate [17] quality, compared some approach of mesh SHP to vaginal hysteropexy. Gutman et al. compared laparoscopic SHP to VMHP [18], and Kow et al. compared multiple different arms including open- abdominal SHP, robotic SHP, laparoscopic SHP, robotic or laparoscopic uterosacral suspension, VMHP, and various types of VNTHP (Table 1) [17]. Two retrospective nRCSs [17, 19], both of moderate quality, compared the open approach to SHP with a laparoscopic/robotic approach. Paek et al. compared laparoscopic or robotic SHP to open abdominal SHP [19], and Kow et al. compared multiple approaches to abdominal hysteropexy (native tissue suspension or mesh SHP), as mentioned earlier [17].
Eight single-arm studies, all of low quality, described some approach to SHP [21,22,23,24,25,26,27,28]. Five of these investigated a laparoscopic approach [22,23,24, 26, 28], two a robotic approach [21, 25], and one an open approach [27]. We also utilized the SHP arms from 12 publications representing 11 studies, described in our prior publication, which compared SHP to POP surgeries with hysterectomy [8].
One study of low quality compared open and laparoscopic approaches to an abdominal native-tissue hysteropexy (pectineal ligament suspension) [20]. Four case series, all of low quality, investigated an abdominal approach to hysteropexy that was not SHP (Table 1) [29,30,31,32]. Four additional studies detailed in our prior review compared some type of non-SHP abdominal hysteropexy to POP surgery with hysterectomy, and the hysteropexy arms from these studies were utilized in these analyses [8].
Prolapse recurrence outcomes following SHP and other abdominal hysteropexies, either with open or laparoscopic approaches, were quite heterogeneous (I2 ranges from 31 to 86%, Table 5). Prolapse outcomes were mostly investigated in the short- to medium-term (6–64 months, Table 1), with a total range of follow-up from 6 months [33] to 12 years [31]. The prevalence of recurrent POP in these time frames varied from 0 to 28% for repeat surgery for prolapse, 0–32% for prolapse recurrence by set definitions, and 5–30% for subjective return of prolapse or recurrent prolapse symptoms (Table 5). Prolapse recurrence by objective definitions was common following laparoscopic/robotic SHP (2 studies, moderate quality, mean 20.9%, 95% CI 4.4–45.4%), but recurrent prolapse symptoms (5 studies, low quality, mean 10.1%, 95% CI 6.6–14.2%) or repeat prolapse surgery (10 studies, moderate quality, mean 3.9%, 95% CI 1.9–6.6%) were less common following this procedure.
UI outcomes, whether any postoperative or de novo UI, were also highly heterogeneous regarding abdominal hysteropexy by laparoscopic or open approaches (I2 0–93%), although many procedures and trials lacked data on this outcome (Table 5). Most data on UI was regarding SHP (laparoscopic or open), where the prevalence of postoperative UI ranged from 0 to 42% overall (6 studies, low quality, mean 16.9%, 95% CI 5.8–27.9%), and de novo UI ranged from 1 to 10% (4 studies, low quality, mean 4.4%, 95% CI 0.2–8.5%).
AE rates for abdominal hysteropexies varied widely and were scantily reported, but the most commonly reported AEs other than UI included urinary retention (described in some trials as objective or subjective voiding dysfunction), mesh exposure, and sexual dysfunction (usually reported in the form of dyspareunia). Urinary retention had a wide variety of definitions and time frames, as indicated by a prevalence range of 0–79%, but the mean rate was lower for laparoscopic (3 studies, moderate quality, mean 2.9%, 95% CI 1.0–5.6%) than for open (4 studies, moderate quality, mean 25.5%, 95% CI 2.4–61.6) approaches to SHP, with direct comparison in only one trial (moderate quality, OR 0.05, 95% CI 0.003–0.83) [19]. Mesh exposure in relevant surgeries ranged from 0 to 6.8%, with similar rates described with open (6 studies, moderate quality, mean 3.8%, 95% CI 1.5–7.0%) and laparoscopic (9 studies, moderate quality, mean 1.8%, 95% CI 0.7–3.4%) approaches, with the direct comparison made in three trials (moderate quality, OR 0.33, 95% CI 0.05–2.41) [17, 19, 20]. Sexual dysfunction was fairly common following these procedures, ranging from 0 to 16%, and relatively high for SHP at a mean prevalence of 8.5% (5 studies, low quality, 95% CI 3.2–13.9%) for open and laparoscopic approaches combined.
Comparison of different sacrohysteropexy approaches
In comparison with laparoscopic versus open abdominal SHP, similar outcomes were seen in repeat surgery for POP (3 studies, moderate quality, OR 0.97, 95% CI 0.22–4.23) [17, 19, 20] and recurrence of prolapse by objective criteria (1 trial, moderate quality, OR 0.97, 95% CI 0.41–4.71) [17]. However, for symptoms of recurrent POP, a lower chance of prolapse recurrence with the laparoscopic SHP was demonstrated in one trial (moderate quality, OR 0.18, 95% CI 0.07–0.46) [19]. The laparoscopic approach had lower odds of urinary retention (1 trial, moderate quality, OR 0.05, 95% CI 0.003–0.83) [19] and lower EBL (2 trials with 3 comparisons, moderate quality, mean difference −104 ml, 95% CI −145 to −63 ml) [17, 19] compared with the open approach. Data comparing laparoscopic and open SHP regarding mesh exposure had low heterogeneity and narrow CIs, and no significant difference was seen between approaches. Also, no significant differences were seen in sexual dysfunction, EBL, or hospital stay between approaches, although CIs and heterogeneity measures around these data were large. There was sufficient evidence from these three comparative trials to generate a guideline about the approach to SHP (Table 7).
Vaginal mesh hysteropexy
As mentioned above, two studies of moderate quality compared VMHP with an abdominal SHP [17, 18], with the Kow et al. study also including data allowing for comparison of VMHP with other vaginal surgery. Eleven case series (Table 2), all of low quality, studied some type of VMHP [34,35,36,37]. Two studies compared VMHP with some other type of POP surgery (vaginal hysterectomy or sacrocolpopexy of the vaginal vault), and the VMHP arms of these were utilized in these analyses [38, 39]. An additional 15 publications on 14 studies described in our prior review compared VMHP with POP surgery plus hysterectomy; VMHP arms were integrated in these analyses as well [8].
Data regarding prolapse outcomes for VMHP were also heterogeneous (I2, 77–87%) around prolapse recurrence, with a range of recurrent POP by set definitions from 2 to 33% (13 studies, moderate quality, mean 9.5%, 95% CI 5.3–14.7%) and a range of recurrent prolapse symptoms from 3 to 16% (3 trials, low quality, mean 8.3%, 95% CI 2.4–17.2%). However, for repeat POP surgery following VMHP, data was less heterogeneous (I2 5%) for the 12 trials reporting this outcome, with a range of repeat POP surgery from 3 to 29% (12 studies, moderate quality, mean 4.5%, 95% CI 3.1–6.1%). Most of these trials studied POP outcomes in the short to medium term (12–24 months, Table 2), with a wide total range of follow-up from 2 months [40] to 5 years [41].
AEs most commonly reported for VMHP were also UI, mesh exposure, sexual dysfunction, and urinary retention (Table 6). Postoperative UI after VMHP ranged from 3 to 12% (2 studies, low quality, mean 7.5%, 95% CI 1.4–17.8%) and de novo UI from 1.5 to 29% (6 studies, low quality, mean 5.9%, 95% CI 1.9–11.7%), but heterogeneity around these data was high (I2 68–81%). The prevalence of urinary retention after VMHP also ranged widely, from 0 to 40% (6 studies, moderate quality, mean 15.9%, 95% CI 7.9–26%), as did the prevalence of sexual dysfunction, from 0 to 48% (5 studies, low quality, mean 8.7%, 95% CI 0.5–25.7%), with high data heterogeneity for these outcomes as well (I2 82 and 92%, respectively). The rate of mesh exposure was more consistent (I2 31%), with 17 trials (moderate quality) reporting a range of 0–19% (mean 5.4%, 95% CI 4–7%). Operating time (OT), hospital stay, and EBL were all heterogeneous (I2 92–100%) for VMHP, with wide ranges from 58 to 171 min for OT (14 trials, moderate quality, mean 112 min, 95% CI 91–133 min), 1–6 days hospital stay (10 trials, moderate quality, mean 3.4 days, 95% CI 2.1–4.6 days), and 49–161 ml of EBL (14 trials, moderate quality, mean 117 ml, 95% CI 98–135 ml).
Vaginal mesh hysteropexy compared with sacrohysteropexy
The two studies that compared VMHP with laparoscopic/robotic SHP [17, 18] demonstrated that VMHP decreased OT compared with laparoscopic SHP (2 studies, moderate quality, mean difference 119 min, 95% CI 102–136 min). However, other outcomes investigated, including repeat POP surgery, mesh exposure, sexual dysfunction, EBL, and hospital stay, were similar (Table 6). There was sufficient evidence to make a clinical guideline regarding VMHP versus laparoscopic/robotic SHP hysteropexy (Table 7).
Vaginal native-tissue hysteropexy, including Manchester procedure
Six case series of low quality described outcomes with VNTHP (Table 3) [42,43,44,45,46,47]. Of the case series, four investigated SSHP [42,43,44, 47]; one USHP [45], and one mixed uterine-preserving POP surgeries of a transvaginal approach without further specification of surgical methods [46]. Thirteen studies described in our prior review, four RCTs and nine nRCSs, compared transvaginal native-tissue hysteropexy with POP surgeries plus hysterectomy, and the hysteropexy arms of these studies were utilized in this analysis of VNTHP [8]. Of these 13 comparative studies, all except two (one describing USHP [48] and one describing various transvaginal methods [49]), had SSHP as the hysteropexy arm.
Five single-arm studies of low quality described outcomes after the Manchester procedure (Table 3), in which the cervix is removed and the uterosacral ligaments plicated across the midline to suspend the new vaginal apex [50,51,52,53,54]. Six nRCSs reviewed in our prior publication compared the Manchester procedure with POP surgery plus hysterectomy, and the Manchester arms of these trials were utilized in these analyses [8].
Heterogeneous data (I2, 49–87%) were seen regarding prolapse recurrence after VNTHP, such as SSHP and USHP (Table 6). The range of repeat surgery for prolapse after VNTHP was 0–12% (6 studies, moderate quality, mean 4.1%; 95% CI 1.7–7.5%), and recurrent prolapse by set definitions ranged from 0 to 50% (11 studies, low quality, mean 19%, 95% CI 10.2–29.8%). Only one trial (low quality) investigated recurrent prolapse symptoms (3.3%) after VNTHP specifically [44]. Trials that investigated prolapse outcomes after VNTHP had follow-up times from 4 [55] to 86 months after VNTHP [56], although two trials had unclear data on the timing of follow-up [46, 47] and five [46, 49, 57,58,59] did not measure prolapse outcomes following surgery.
For the Manchester procedure, POP outcomes were just as heterogeneous (I2, 79–98%, Table 6) despite the small number of trials investigating this procedure. Repeat surgery for POP after Manchester ranged from 1.1 to 5.4% (5 studies, low quality, mean 2.8%; 95% CI 1.3–4.8%) and recurrent POP by set definitions from 5.4 to 19.4% (4 studies, low quality, mean 12.7%, 95% CI 6.7–20.3%). Three trials with a large number of patients investigated the prevalence of repeat POP symptoms after Manchester, which ranged from 2.2 to 21.4% (low quality, mean 7.6%, 95% CI 0.7–20.6%). These studies followed up patients from 45 days to 9 years postoperatively [50, 54], with two trials having no prolapse outcomes [50, 60] and one lacking data on follow-up duration [53].
AEs after VNTHPs were poorly investigated in the literature, with only five trials reporting heterogeneous data (I2 85%) on postoperative UI (4 on SSHP or USHP; 1 on Manchester), with a wide range of 0–48%. De novo UI was only reported in two studies on the Manchester procedure (low quality, mean 8.5%, 95% CI 6–11%), and urinary retention reported on seven (low quality, mean 17.8%, 95% CI 12.4–24%). Sexual dysfunction ranged from 4.1 to 16.3%, with data from two studies on SSHP or USHP surgery and two on the Manchester (low quality).
OT, hospital stay, and EBL were all heterogeneous (I2 85–100%) for VNTHP, with means ranging from 51 to 160 min for OT time, 3–8 days hospital stay, and 46–350 ml EBL.
LeFort colpocleisis
One retrospective study of moderate quality compared LeFort colpocleisis outcomes with and without a midurethral sling procedure, in which both arms were combined for this analysis [61]. Seven case series of low quality described populations of women following LeFort colpocleisis (Table 4) [62,63,64,65,66,67,68]. One nRCS detailed in our prior review compared LeFort colpocleisis with POP surgery plus hysterectomy, and the LeFort arm from was included in our analyses [8].
Prolapse recurrence, as expected, was very low after LeFort colpocleisis, with less heterogeneity (I2 0–29%) around data than in reconstructive procedures. Repeat POP by set definitions ranged from 4 to 9.3% (3 studies, low quality, mean 6.2%, 95% CI 3.4–9.4%), repeat symptoms of POP from 2.5 to 5.7% (4 studies, low quality, mean 3.3%, 95% CI 1.6–5.5%), and repeat surgery was rare (3 studies, low quality, mean 0.6%, 95% CI 0.1–1.5%). Few studies on LeFort colpocleisis defined prolapse recurrence well (Table 4); one study lacked data on the length of follow-up [64], while two others did not measure prolapse outcomes [61, 63]. Studies measuring prolapse outcomes and had information on follow-up length hd follow-up times rangig from 6 months to 22 years [65, 67].
Urinary symptoms after LeFort colpocleisis were frequently reported but poorly defined. Postoperative UI was common, ranging from 10.2 to 15.6% (4 studies, low quality, mean 9.6%, 95% CI 9.7–16%), and de novo UI was common, at 4–12.8% (2 studies, low quality, mean 7.4%, 95% CI 1.3–17.7%). Urinary retention was prevalent, ranging from 2 to 18% (2 studies, low quality, mean 8.2%, 95% CI 0–32%). LeFort procedures were brief (mean OT 76–90 min), with low EBL (mean 74–180 ml). However, hospital stay was long, ranging from 4 to 12 days in the 3 studies that adequately reported this outcome [62, 64, 69].
Discussion
This planned secondary analysis of a systematic review describes the outcomes and AEs associated with uterine-preserving procedures for POP (hysteropexies) and found very limited comparative data to inform the choice of one type of hysteropexy over another. Furthermore, most data on hysteropexy is of low quality, as many trials are case series or have poorly reported or defined outcomes.
Those few trials that do compare different approaches to hysteropexy emphasize that minimally invasive (laparoscopic or robotic) SHP approaches have advantages over open abdominal approaches but have a longer OT, with a similar AE rates to VMHP. This difference is likely more attributable to the route of surgery than to the specific uterine fixation technique used. Data on prolapse recurrence comparing one type of hysteropexy with another was limited and indicates few differences between approaches. However, some low-quality descriptive data exist on outcomes and AEs of individual uterine-preserving surgeries, which can help surgeons when counseling patients on those surgeries.
This review is aimed at informing the patient who has made the decision to have a uterine-preserving POP surgery and is exploring various surgical options with her physician. Our previous review described that most high- or moderate-quality literature on the topic of hysteropexy explores the risks and benefits of whether or not to preserve the uterus (vs. hysterectomy) in POP surgery [8]. This updated review notes that studies with hysteropexy only as the focus are usually lower quality (case series) and often do not utilize the most modern surgical options. Comparative studies in this analysis were more recent and reported on current options that patients often consider and surgeons often offer, such as laparoscopic/robotic SHP, vaginal mesh hysteropexy, and LeFort colpocleisis [17, 18, 61]. Despite the fact that surgical options for POP are often changing and continue to expand due to overwhelming demand [2, 70], it is encouraging that data collected by this review is relevant to the contemporary surgical climate. Moreover, the choices informed by these data (such as choice between laparoscopic/robotic SHP and VMHP for patients who want a reconstructive procedure), fits with current surgical training for POP surgeons [71, 72].
Differences found between hysteropexy approaches in this trial are consistent with past knowledge about advantages and limitations of minimally invasive procedures in benign gynecological surgery. Past systematic review has demonstrated that benign hysterectomy through a minimally invasive route (laparoscopic) has longer OT than open abdominal or vaginal routes but less morbidity and recovery time than open abdominal approaches [73]. This has prompted the American College of Obstetrics and Gynecology (ACOG) to recommend the vaginal route as the preferred approach to hysterectomy, with laparoscopic routes as the second choice [74]. However, in POP surgery, there are no such recommendations on surgical approach, and routes that avoid morbidity or reduce costs may have different rates of POP recurrence, making consistent decisions difficult [75]. In the context of uterine-preserving POP surgery, where hysterectomy is not part of the procedure but surgical approach choices are similar to hysterectomy options, this review demonstrates that insufficient data exist to consistently choose one approach to hysteropexy or for use by large societies to recommend a preferred approach. Even CPGs used in this report, which suggest the sensible approach of avoiding longer or more invasive surgery, are based on moderate evidence and cannot be applied to all patients.
In most investigations of one hysteropexy approach versus another, mostly comparing laparoscopic/robotic SHP to the open abdominal route or VMHP, prolapse outcomes demonstrate no significant differences between approaches. These results, however, need to be taken in the context of the limited number of studies (2–3 studies making similar comparisons), the moderate quality of these studies (nRCSs), and varying definitions of prolapse recurrence. The prolapse outcome that was most reliable across studies (lowest heterogeneity and most consistently reported and defined) was repeat surgery for recurrent prolapse, and this was similar in all direct hysteropexy comparisons. However, all POP retreatment rates must be interpreted considering follow-up time from the index surgery, which varied in relevant trials. The one disparate prolapse outcome between hysteropexy approaches found in this review was the increased prevalence of recurrent prolapse symptoms in the Paek et al. trial, which used a nonvalidated questionnaire to assess this outcome [19]. Clearly, limitations in these data highlight the fact that insufficient evidence exists to recommend one type of mesh hysteropexy over another to improve POP outcomes, and no comparative data exists on nonmesh hysteropexy procedures. Therefore, clinical suggestions made in this review are based on differences in surgery risk and morbidity with the assumption that differences in POP outcomes after mesh hysteropexy either do not exist or have yet to be demonstrated. Prospective, randomized trials comparing one type of uterine-preserving surgery would be invaluable to the field.
This review incorporated data on both obliterative and reconstructive approaches to uterine-preserving POP repair; this may aid surgeons counseling women who are unsure about future sexual activity. Descriptive data from this review demonstrates that LeFort colpocleisis is a surgery with a short operative time, low EBL, and consistently low rates of POP recurrence. No studies compare LeFort colpocleisis with reconstructive hysteropexy, and such investigations are usually not possible or meaningful, as women who choose LeFort are not typically of a similar population as those who choose reconstructive hysteropexy [68, 76]. Specifically, women who are younger, have more optimal health, and desire future sexual activity often elect for reconstructive procedures, so data on the LeFort often involves women who are older, less healthy, and are not prioritizing sexual function following surgery [77]. This selection bias is best exemplified in the long hospital admission times observed after LeFort surgery despite the low EBL and OT, even in recent publications [63, 64]. The poor health of the population getting this low-morbidity surgery limits ethical feasibility of comparing LeFort procedures to anything other than different obliterative procedures.
Strengths of this study include incorporation of all published data on the topic up to the date of this review, including non-English publications, posters, and abstracts. This review also utilized a large team of experts in pelvic surgery, including experienced systematic review researchers, which allowed for optimal adherence to a rigorous systematic review protocol and enhanced data accuracy. Furthermore, inclusion of studies in all languages allowed a more worldwide perspective on surgical choices; most systematic reviews only consider English studies and may disregard surgeries commonly performed in non-English-speaking locales. We ensured data was collected on AEs that highly affect patient quality of life, including urinary symptoms, mesh erosion, and sexual function. The integration of all comparative and noncomparative studies with uterine-preserving POP arms is particularly important in describing frequencies of AEs, which are vital to informed consent conversations.
Limitations of this review are mostly linked to the paucity of comparative literature on the topic and the poor quality of most studies that describe uterine-preserving POP surgery. As noted above, most comparative literature on uterine-preserving POP surgery aims to compare hysteropexy with POP surgery plus hysterectomy, as opposed to comparing different surgical choices for hysteropexy. Case series inherently contain a large amount of selection, follow-up, and measurement bias, so results from such studies must be interpreted with this in mind. Furthermore, most studies are short-term in their follow-up, making accurate conclusions about long term POP outcomes in these patients difficult. Finally, as noted in our prior review on this topic [8], most studies investigating hysteropexy do not measure outcomes that patients who choose these surgeries may prioritize, such as body image, future fertility, and future uterine/cervical malignancy risk. The two moderate- to high-quality comparative studies in this review [17, 18], one of which provided years of follow-up, report some vital information for future directions of investigation, and it is our hope that future randomized trials will compare one type of uterine-preserving POP surgery with another. For appropriate patients who are considering uterine-preserving POP surgery, such data are vital to inform them of the most safe and effective approach. Furthermore, such data would allow our field to expand or alter surgeon training to include superior uterine-preserving techniques in POP surgery.
In summary, this secondary review discovered that literature focusing on hysteropexy, which assumes a patient has already made the choice to preserve their uterus during POP surgery, is mostly of lower quality, finds few differences in outcomes between hysteropexy approaches, and often does not compare or describe the most modern hysteropexy choices. Therefore, surgeons with a patient who desires uterine preservation during POP surgery should be cautious about offering one type of hysteropexy over another. However, women should be counseled that minimally invasive approaches, such as laparoscopic or vaginal approaches, limit morbidity or, in the case of vaginal surgery, operating time. If surgical choices are restricted due to surgeon training or patient medical conditions, women should be counseled on data particular to the surgery being selected, including the risk of prolapse return, urinary symptoms, and AEs.
References
Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–6. https://doi.org/10.1097/AOG.0000000000000286.
Dieter AA, Wilkins MF, Wu JM. Epidemiological trends and future care needs for pelvic floor disorders. Curr Opin Obstet Gynecol. 2015;27(5):380–4. https://doi.org/10.1097/GCO.0000000000000200.
Sisco M, Kyrillos AM, Lapin BR, Wang CE, Yao KA. Trends and variation in the use of nipple-sparing mastectomy for breast cancer in the United States. Breast Cancer Res Treat. 2016;160(1):111–20. https://doi.org/10.1007/s10549-016-3975-9.
Madsen AM, Raker C, Sung VW. Trends in Hysteropexy and apical support for Uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365–71. https://doi.org/10.1097/SPV.0000000000000426.
Korbly NB, Kassis NC, Good MM, Richardson ML, Book NM, Yip S, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209(5):470.e471–6. https://doi.org/10.1016/j.ajog.2013.08.003.
Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2013;24(11):1803–13. https://doi.org/10.1007/s00192-013-2171-2.
Ridgeway B, Frick AC, Walter MD. Hysteropexy. A review Minerva Ginecol. 2008;60(6):509–28.
Meriwether KV, Antosh DD, Olivera CK, Kim-Fine S, Balk EM, Murphy M, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219(2):129–46 e122. https://doi.org/10.1016/j.ajog.2018.01.018.
Wallace BC, Trikalinos TA, Lau J, Brodley C, Schmid CH. Semi-automated screening of biomedical citations for systematic reviews. BMC Bioinformatics. 2010;11:55. https://doi.org/10.1186/1471-2105-11-55.
Rahn DD, Ward RM, Sanses TV, Carberry C, Mamik MM, Meriwether KV, et al. Vaginal estrogen use in postmenopausal women with pelvic floor disorders: systematic review and practice guidelines. Int Urogynecol J. 2015;26(1):3–13. https://doi.org/10.1007/s00192-014-2554-z.
Olivera CK, Meriwether K, El-Nashar S, Grimes CL, Chen CC, Orejuela F, et al. Nonantimuscarinic treatment for overactive bladder: a systematic review. Am J Obstet Gynecol. 2016;215(1):34–57. https://doi.org/10.1016/j.ajog.2016.01.156.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Wallace BCD, J I, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational Back-end. J Stat Softw. 2012;49(5):5085.
Trikalinos TA, Hoaglin DC, Schmid CH (2013). In: Empirical and Simulation-Based Comparison of Univariate and Multivariate Meta-Analysis for Binary Outcomes. AHRQ Methods for Effective Health Care. Rockville (MD),
Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. 2004;4(1):38. https://doi.org/10.1186/1472-6963-4-38.
Kow N, Goldman HB, Ridgeway B. Uterine conservation during prolapse repair: 9-year experience at a single institution. Female Pelvic Med Reconstr Surg. 2016;22(3):126–31. https://doi.org/10.1097/SPV.0000000000000221.
Gutman RE, Rardin CR, Sokol ER, Matthews C, Park AJ, Iglesia CB, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38 e31–11. https://doi.org/10.1016/j.ajog.2016.08.035.
Paek J, Lee M, Kim BW, Kwon Y. Robotic or laparoscopic sacrohysteropexy versus open sacrohysteropexy for uterus preservation in pelvic organ prolapse. Int Urogynecol J. 2016;27(4):593–9. https://doi.org/10.1007/s00192-015-2869-4.
Joshi VM, Otiv SR, Dagade VB, Borse M, Majumder RN, Shrivastava M, et al. Pectineal ligament Hysteropexy for uterine prolapse in premenopausal women by open and laparoscopic approach in Indian urban and rural centers. Female Pelvic Med Reconstr Surg. 2015;21(4):215–9. https://doi.org/10.1097/SPV.0000000000000179.
Mourik SL, Martens JE, Aktas M. Uterine preservation in pelvic organ prolapse using robot assisted laparoscopic sacrohysteropexy: quality of life and technique. Eur J Obstet Gynecol Reprod Biol. 2012;165(1):122–7. https://doi.org/10.1016/j.ejogrb.2012.07.025.
Costantini E, Lazzeri M, Zucchi A, Bini V, Mearini L, Porena M. Five-year outcome of uterus sparing surgery for pelvic organ prolapse repair: a single-center experience. Int Urogynecol J. 2011;22(3):287–92. https://doi.org/10.1007/s00192-010-1342-7.
Rahmanou P, White B, Price N, Jackson S. Laparoscopic hysteropexy: 1- to 4-year follow-up of women postoperatively. Int Urogynecol J. 2014;25(1):131–8. https://doi.org/10.1007/s00192-013-2209-5.
Fayyad AM, Siozos CS. Safety and one year outcomes following vaginally assisted laparoscopic uterine sacropexy (VALUES) for advanced uterine prolapse. Neurourol Urodyn. 2014;33(3):345–9. https://doi.org/10.1002/nau.22433.
Grimminck K, Mourik SL, Tjin-Asjoe F, Martens J, Aktas M. Long-term follow-up and quality of life after robot assisted sacrohysteropexy. Eur J Obstet Gynecol Reprod Biol. 2016;206:27–31. https://doi.org/10.1016/j.ejogrb.2016.06.027.
Kupelian AS, Vashisht A, Sambandan N, Cutner A. Laparoscopic wrap round mesh sacrohysteropexy for the management of apical prolapse. Int Urogynecol J. 2016;27(12):1889–97. https://doi.org/10.1007/s00192-016-3054-0.
Khan A, Jaleel R, Nasrullah FD. Sacrohysteropexy performed as uterus conserving surgery for pelvic organ prolapse: review of case files. Pak J Med Sci. 2016;32(5):1174–8. https://doi.org/10.12669/pjms.325.10307.
Krause HG, Goh JT, Sloane K, Higgs P, Carey MP. Laparoscopic sacral suture hysteropexy for uterine prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(4):378–81. https://doi.org/10.1007/s00192-005-0019-0.
Hsieh CH. A new laparoscopic technique for uterine prolapse: one-sided uterine fixation through the round ligament. Int Urogynecol J. 2011;22(2):213–9. https://doi.org/10.1007/s00192-010-1269-z.
Rimailho J, Talbot C, Bernard JD, Hoff J, Becue J. Anterolateral hysteropexy via abdominal approach. Results and indications. Apropos of a series of 92 patients. Ann Chir. 1993;47(3):244–9.
Khanam RA, Rubaiyat A, Azam MS. Sling for correcting uterine prolapse: twelve years experience. Mymensingh Med J. 2014;23(1):13–7.
Veit-Rubin N, Dubuisson JB, Lange S, Eperon I, Dubuisson J. Uterus-preserving laparoscopic lateral suspension with mesh for pelvic organ prolapse: a patient-centred outcome report and video of a continuous series of 245 patients. Int Urogynecol J. 2016;27(3):491–3. https://doi.org/10.1007/s00192-015-2859-6.
Diwan A, Rardin CR, Strohsnitter WC, Weld A, Rosenblatt P, Kohli N. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):79–83. https://doi.org/10.1007/s00192-005-1346-x.
Khandwala S, Williams C, Reeves W, Dai J, Jayachandran C. Role of vaginal mesh hysteropexy for the management of advanced uterovaginal prolapse. J Reprod Med. 2014;59(7–8):371–8.
Sheng Q, Ma N, Huang H, Xu B, He C, Song Y. Significance of preoperative calculation of uterine weight as an indicator for preserving the uterus in pelvic reconstructive surgery. Int J Clin Exp Pathol. 2015;8(1):900–5.
Jirschele K, Seitz M, Zhou Y, Rosenblatt P, Culligan P, Sand P. A multicenter, prospective trial to evaluate mesh-augmented sacrospinous hysteropexy for uterovaginal prolapse. Int Urogynecol J. 2015;26(5):743–8. https://doi.org/10.1007/s00192-014-2564-x.
Khandwala S. Transvaginal mesh surgery for pelvic organ prolapse: one-year outcome analysis. Female Pelvic Med Reconstr Surg. 2013;19(2):84–9. https://doi.org/10.1097/SPV.0b013e31827de6de.
Geoffrion R, Hyakutake MT, Koenig NA, Lee T, Cundiff GW. Bilateral sacrospinous vault fixation with tailored synthetic mesh arms: clinical outcomes at one year. J Obstet Gynaecol Can. 2015;37(2):129–37.
Del Amo E, Burcet G, Vellvé K, Hernández J, Carreras R. Quality of life and patients satisfaction after genital prolapse surgery: vaginal hysterectomy versus mesh hysteropexy. Abstracts of the 44th annual meeting of the international continence society (ICS) 20-24 October, 2014, Rio de Janeiro, Brazil. Neurourol Urodyn. 2014;33(6):631–1071. https://doi.org/10.1002/nau.22655.
de Landsheere L, Ismail S, Lucot JP, Deken V, Foidart JM, Cosson M. Surgical intervention after transvaginal Prolift mesh repair: retrospective single-center study including 524 patients with 3 years’ median follow-up. Am J Obstet Gynecol. 2012;206(1):83 e81–7. https://doi.org/10.1016/j.ajog.2011.07.040.
Malandri M, Iordanidou E, Takou M, Moraitis B, Balaxis D. A randomized comparison of two vaginal procedures for the treatment of stage two, or higher uterine prolapse: hysterectomy with mesh versus only mesh implantation. Neurourol Urodyn. 2012;31(6):855.
Lin TY, Su TH, Wang YL, Lee MY, Hsieh CH, Wang KG, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104(4):249–53.
Dietz V, Huisman M, de Jong JM, Heintz PM, van der Vaart CH. Functional outcome after sacrospinous hysteropexy for uterine descensus. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):747–52. https://doi.org/10.1007/s00192-007-0520-8.
Dietz V, de Jong J, Huisman M, Schraffordt Koops S, Heintz P, van der Vaart H. The effectiveness of the sacrospinous hysteropexy for the primary treatment of uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1271–6. https://doi.org/10.1007/s00192-007-0336-6.
Dubernard G, Rouzier R, Haddad B, Dubois P, Paniel BJ. Correction of uterine prolapse by the vaginal route using the uterosacral ligaments: Shirodkar procedure. Eur J Obstet Gynecol Reprod Biol. 2003;109(2):214–8.
Bohoussou E, Adjoussou SA, Letouzey V, Fatton B, de Tayrac R. Should we perform intra-operative endometrial biopsy during pelvic reconstructive surgery with uterine preservation? J Gynecol Obstet Biol Reprod (Paris). 2014;43(1):40–5. https://doi.org/10.1016/j.jgyn.2013.10.011.
Abdulsid ATG, Jani M, Elsapagh K, Allam M. Sacrospinous fixation, keep or remove the uterus? That is the question Gynecol Surg. 2016;13(Suppl 1):S1–S453.
Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23(5):625–31. https://doi.org/10.1007/s00192-011-1635-5.
Farthmann J, Watermann D, Erbes T, Roth K, Nanovska P, Gitsch G, et al. Functional outcome after pelvic floor reconstructive surgery with or without concomitant hysterectomy. Arch Gynecol Obstet. 2015;291(3):573–7. https://doi.org/10.1007/s00404-014-3435-x.
Nava y Sanchez RM, Acosta RU, Ruiz Velasco V, Garcia TL. Manchester’s operation. I. Morbimortality and early complications. Ginecol Obstet Mex. 1973;33(198):347–60.
Oversand SH, Staff AC, Spydslaug AE, Svenningsen R, Borstad E. Long-term follow-up after native tissue repair for pelvic organ prolapse. Int Urogynecol J. 2014;25(1):81–9. https://doi.org/10.1007/s00192-013-2166-z.
Ayhan A, Esin S, Guven S, Salman C, Ozyuncu O. The Manchester operation for uterine prolapse. Int J Gynaecol Obstet. 2006;92(3):228–33. https://doi.org/10.1016/j.ijgo.2005.12.002.
Conger GT, Keettel WC. The Manchester-fothergill operation, its place in gynecology; a review of 960 cases at university hospitals, Iowa City. Iowa Am J Obstet Gynecol. 1958;76(3):634–40.
Tipton RH, Atkin PF. Uterine disease after the Manchester repair operation. J Obstet Gynaecol Br Commonw. 1970;77(9):852–3.
Carey MP, Slack MC. Transvaginal sacrospinous colpopexy for vault and marked uterovaginal prolapse. Br J Obstet Gynaecol. 1994;101(6):536–40.
Lo TS, Pue LB, Hung TH, Wu PY, Tan YL. Long-term outcome of native tissue reconstructive vaginal surgery for advanced pelvic organ prolapse at 86 months: hysterectomy versus hysteropexy. J Obstet Gynaecol Res. 2015;41(7):1099–107. https://doi.org/10.1111/jog.12678.
Dietz V, CH vV, APM H, SE S-K. Vaginal hysterectomy versus sacrospinous hysteropexy as primary treatment of prolapse: a randomized controlled trial (RCT), a preliminary report (abstract number 285). Int Urogynecol J. 2006;17(Suppl. 2):S171–359.
Jeng CJ, Yang YC, Tzeng CR, Shen J, Wang LR. Sexual functioning after vaginal hysterectomy or transvaginal sacrospinous uterine suspension for uterine prolapse: a comparison. J Reprod Med. 2005;50(9):669–74.
van Brummen HJ, van de Pol G, Aalders CI, Heintz AP, van der Vaart CH. Sacrospinous hysteropexy compared to vaginal hysterectomy as primary surgical treatment for a descensus uteri: effects on urinary symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(5):350–355; discussion 355. https://doi.org/10.1007/s00192-003-1084-x.
Kalogirou D, Antoniou G, Karakitsos P, Kalogirou O. Comparison of surgical and postoperative complications of vaginal hysterectomy and Manchester procedure. Eur J Gynaecol Oncol. 1996;17(4):278–80.
Catanzarite T, Rambachan A, Mueller MG, Pilecki MA, Kim JY, Kenton K. Risk factors for 30-day perioperative complications after Le fort colpocleisis. J Urol. 2014;192(3):788–92. https://doi.org/10.1016/j.juro.2014.03.040.
Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of LeFort’s operation. Obstet Gynecol. 1973;42(3):415–20.
Mueller MG, Ellimootil C, Abernethy MG, Mueller ER, Hohmann S, Kenton K. Colpocleisis: a safe, minimally invasive option for pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2015;21(1):30–3. https://doi.org/10.1097/SPV.0000000000000114.
Szczesniewska A, Szpakowski M, Wladzinski J, Wilczynski JR. (un)forgotten Neugebauer-Le fort operation. Paramedian closure of the vagina--safe and effective surgical procedure for treating of pelvic organ prolapse in older women. Ginekol Pol. 2015;86(3):198–202.
Marin Ardila L. Le Fort’s colpocleisis. 10-year study in the gynecology and obstetrics Department of the Hospital san Juan de Dios in Bogota. Rev Colomb Obstet Ginecol. 1966;17(6):415–24.
Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12(1):31–5.
Falk HC, Kaufman SA. Partial colpocleisis: the Le fort procedure; analysis of 100 cases. Obstet Gynecol. 1955;5(5):617–27.
Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort Colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24(3):415–9. https://doi.org/10.1016/j.jmig.2016.12.015.
Denehy TR, Choe JY, Gregori CA, Breen JL. Modified Le fort partial colpocleisis with Kelly urethral plication and posterior colpoperineoplasty in the medically compromised elderly: a comparison with vaginal hysterectomy, anterior colporrhaphy, and posterior colpoperineoplasty. Am J Obstet Gynecol. 1995;173(6):1697–701 discussion 1701-1692.
Geynisman-Tan J, Kenton K. Surgical updates in the treatment of pelvic organ prolapse. Rambam Maimonides Med J. 2017;8(2). https://doi.org/10.5041/RMMJ.10294.
Younger A, Rac G, Clemens JQ, Kobashi K, Khan A, Nitti V, et al. Pelvic organ prolapse surgery in academic female pelvic medicine and reconstructive surgery urology practice in the setting of the Food and Drug Administration public health notifications. Urology. 2016;91:46–51. https://doi.org/10.1016/j.urology.2015.12.057.
Elterman DS, Chughtai BI, Vertosick E, Maschino A, Eastham JA, Sandhu JS. Changes in pelvic organ prolapse surgery in the last decade among United States urologists. J Urol. 2014;191(4):1022–7. https://doi.org/10.1016/j.juro.2013.10.076.
Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;8:CD003677. https://doi.org/10.1002/14651858.CD003677.pub5.
Committee on Gynecologic P. Committee opinion no 701: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129(6):e155–9. https://doi.org/10.1097/AOG.0000000000002112.
Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376. https://doi.org/10.1002/14651858.CD012376.
Practice Bulletin No. 176 Summary: Pelvic Organ Prolapse. Obstet Gynecol. 2017;129(4):763–5. https://doi.org/10.1097/AOG.0000000000002008.
FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Ann Weber for the Pelvic Floor Disorders N. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(3):261–71. https://doi.org/10.1007/s00192-005-1339-9.
De Vita D, Araco F, Gravante G, Sesti F, Piccione E. Vaginal reconstructive surgery for severe pelvic organ prolapses: a ‘uterine-sparing’ technique using polypropylene prostheses. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):245–51. https://doi.org/10.1016/j.ejogrb.2008.01.013.
Huang KH, Chuang FC, Fu HC, Kung FT. Polypropylene mesh as an alternative option for uterine preservation in pelvic reconstruction in patients with uterine prolapse. J Obstet Gynaecol Res. 2012;38(1):97–101. https://doi.org/10.1111/j.1447-0756.2011.01647.x.
Milani AL, Withagen MI, Vierhout ME. Outcomes and predictors of failure of trocar-guided vaginal mesh surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2012;206(5):440 e441–8. https://doi.org/10.1016/j.ajog.2012.01.039.
Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol. 2007;177(1):192–5. https://doi.org/10.1016/j.juro.2006.08.100.
Feiner B, Gietelink L, Maher C. Anterior vaginal mesh sacrospinous hysteropexy and posterior fascial plication for anterior compartment dominated uterovaginal prolapse. Int Urogynecol J. 2010;21(2):203–8. https://doi.org/10.1007/s00192-009-1012-9.
Fink K, Shachar IB, Braun NM. Uterine preservation for advanced pelvic organ prolapse repair: anatomical results and patient satisfaction. Int Braz J Urol. 2016;42(4):773–8. https://doi.org/10.1590/S1677-5538.IBJU.2015.0656.
Li BH, Huang HJ, Song YF. Modified Prolift procedure without trachelectomy or hysterectomy for the treatment of advanced pelvic organ prolapse complicated with cervical elongation. Zhonghua Fu Chan Ke Za Zhi. 2016;51(3):174–9. https://doi.org/10.3760/cma.j.issn.0529-567X.2016.03.003.
Acknowledgments
This work is supported by the Society of Gynecologic Surgeons (SGS), whose members comprise the Systematic Review Group (SRG) performing this review. SGS supports the SRG with provision of meeting space and oversight, and aids in the public dissemination of study findings to its members. SGS funds Dr. Balk as a paid methodological consultant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
KVM is a textbook editor for Elsevier publications and has not yet received any royalties for that publication. The other authors have no conflicts to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Study Registration
Registration with PROSPERO (CRD42017067899) and full protocol can be found at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017067899
Paper Presentation Information
Society of Gynecologic Surgeons Annual Scientific Meeting, San Antonio, TX, USA, 26–29 March 2017
Appendix 1: literature search strategy
Appendix 1: literature search strategy
Among the Medical Subject Headings (MeSH) searched were uterine prolapse, pelvic organ prolapse, prolapse, descensus, vaginal prolapse, pelvic floor, rectocele, cystocele, sacrocolpopexy, sacropexy, colpopexy, hysteropexy, uterine preservation, Manchester, colpocleisis, Fothergill, LeFort, randomized trial, longitudinal studies, clinical trial, controlled clinical trial, comparative study, prospective study, retrospective study, meta-analysis, and systematic review. Included studies could be in any published format (e.g., journal article, abstract, poster) as long as data could be extracted from the form in which it was published. We did not attempt to identify unpublished articles or abstracts, and we did not contact study authors. The search was limited to humans and included any language. Studies in languages that were not fluently read by one of our group members were interpreted with the assistance of a fluent speaker in the medical field or with professional translational software to extract relevant findings. Reference lists of selected articles and review papers were screened for additional eligible references.
Rights and permissions
About this article
Cite this article
Meriwether, K.V., Balk, E.M., Antosh, D.D. et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J 30, 505–522 (2019). https://doi.org/10.1007/s00192-019-03876-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-03876-2