Abstract
Introduction and hypothesis
We verified the presence of single nucleotide polymorphisms (SNP) rs2236479 of the collagen 18 (COL18A1) and rs2862296 of the lysyl oxidase-like 4 (LOXL-4) genes and the association with pelvic organ prolapse (POP) in Brazilian women and determined risk factors for POP development.
Methods
We assessed 532 postmenopausal women divided into POP (stages III and IV) and control (stages 0 and I) groups by examination and peripheral blood sample collection. DNA sequences of interest were analyzed by real-time reverse-transcriptase polymerase chain reaction (RT-PCR). We used logistic regression models for the analyses, with p < 0.005 for significance.
Results
The frequency of homozygous polymorphic alleles (AA) in COL18A1 and (GG) in LOXL-4 were similar in both groups (17.5% and 15.4% for COL18A1 and 18.9% and 20.6% for LOXL-4, respectively). There were no associations between those polymorphisms or other genotypes and POP. Multiple logistic regression analysis identified age [odds ratio (OR) = 1.10, confidence interval (CI) 95% = 1.07; 1.14), number of vaginal births (OR = 1.66, CI 95% = 1.36; 2.03), and family history (OR = 2.55 CI 95% = 1.43; 4.55) as independent risk factors for POP.
Conclusion
Our study suggests lack of association between DNA polymorphisms rs2236479 of COL18A1 and rs2862296 of LOXL-4 with advanced POP in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is defined as the descent of one or more vaginal walls toward or through the vaginal introitus [1]. It is estimated that 11% of women will require surgery to repair prolapse and/or urinary incontinence (UI) by the age of 79 years, with a 29% reoperation rate [2]. Epidemiological studies have described numerous risk factors for POP, such as aging, estrogen deficiency, parity, vaginal delivery, pelvic surgery, bowel dysfunction, connective tissue diseases, and lifestyle (obesity, smoking/chronic obstructive pulmonary disease, heavy lifting). Among them, vaginal delivery is the main risk factor for POP [3]. However, not all women undergoing vaginal delivery develop severe POP, and it is equally intriguing that POP is seen in nulliparous women [4]. It has been reported that sisters of women <55 years with advanced POP have a five times increased risk for POP development [5]. With that, the interest in investigating the potential genetic predisposition to POP has increased [6, 7].
Genetic studies have mainly focused on extracellular matrix (ECM) of the connective tissues that compose pelvic fascia and ligaments [7]. It is believed that disorders of ECM play a role in POP development [8], and its components may be potential markers for POP. Collagen and elastin are among the main proteins in the human body, and the mechanical and physiological properties of both fibers are highly dependent on the lysyl oxidases (LOX), enzymes that act in collagen and elastin fiber maturation and are essential for ECM remodeling [8, 9].
Allen–Brady et al. reported the results of a genome-wide association study (GWAS) that assessed Caucasian women with POP and a strong family history of POP (n = 115) and matched controls (n = 2976) that identified six DNA single nucleotide polymorphisms (SNPs) significantly associated with POP [10]. One was rs2236479, an intronic A/G variant of the collagen XVIII gene (COL18A1), in which the allele G is the ancestral (guanine nucleotide) mapped to the chromosomal region 21q22.3 [10, 11]. Endostatin is a protein derived from carboxy-terminal proteolytic fragment of collagen XVIII. It is a broad-spectrum angiogenesis and neovascularization inhibitor, and together with collagen XVIII precursor plays a role in the structural organization of basement membranes [12]. Another genomic-linkage study by Allen–Brady et al. identified chromosome 10q24-26 as possibly related to POP in women from Utah with high-risk POP pedigrees [13]. Lysyl oxidase like-4 (LOXL-4), an isoenzyme of the LOX family, was one of the candidate genes in this region [9, 13]. The SNP rs2862296 is located in the promoter region of LOXL-4, of which wild-type allele is A (adenine nucleotide) [14].
It is believed that common diseases such as POP may be due to genetic variants (SNPs) that frequently occur in multiple genes. Due the importance of the collagen and LOX enzymes in pelvic floor support [8] and findings of previous genetic investigations in Caucasian women [10, 11, 13], we developed this study to test the hypothesis that rs2236479 (COL18A1) and rs2862296 (LOXL-4) polymorphisms may be associated with POP in the Brazilian population, which is influenced by various genetic backgrounds.
Methods
The local Research and Ethics Board of the Federal University of São Paulo approved this case–control study (CEP: 47208/12).
Study population and assessment
Women who attended the General Gynecology Clinic and the Urogynecologic Clinic at Federal University of São Paulo, Brazil, were consecutively assessed for eligibility between July 2012 and July 2016. The patients underwent an interview, clinical examination, gynecological evaluation, and Pelvic Organ Prolapse Quantification (POP-Q) system assessment [1]. Candidate women were invited to contribute to the study, and an informed consent form was subsequently signed by participants. Two groups were formed: the case group consisted of postmenopausal women with stage III or IV POP, while the control group consisted of postmenopausal women with stage 0 or I who had never undergone prior pelvic surgery. Demographic and clinical data were then obtained: age, body mass index (BMI), obstetric history, age at menopause, family history of POP, history of respiratory diseases with chronic cough, and connective tissue or collagen diseases. The Brazilian population is composed of various ethnic backgrounds. Participants were classified as white, black, Asian, or mixed. Mixed includes those with physical features and background that do not fit in any other category, which therefore prevents a precise differentiation among ethnic groups. A substantial part of the Brazilian population resulted from racial miscegenation.
Sampling and genotyping
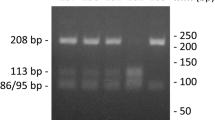
We collected 10 ml of peripheral blood from each participant for genetic evaluation, which was then sent to the laboratory for analysis. Genomic DNA was extracted from peripheral leukocytes and purified using a commercially available kit (IllustraTM blood genomic Prep Mini Spin Kit, GE Healthcare, Buckinghamshire, UK). Identification of rs2236479 (COL18A1) and rs2862296 (LOXL-4) polymorphisms was done using real-time reverse transcriptase polymerase chain reaction (RT-PCR) (Step One Plus Real Time PCR System, Applied Biosystems, Foster City, CA, USA) using 400 ng DNA for each reaction. For amplification, Taqman® Universal PCR Master Mix II (Applied Biosystems) and commercially obtained test solution containing the polymorphism were used. Fifty cycles of fluorescent amplification were performed in the reactions. At the conclusion of the RT-PCR reaction, genotyping results were plotted in a graph of the Step One Plus Real Time PCR System software and exported to an Excel file.
Statistical analysis
Sample and effect sizes were not calculated due to common issues on studies of genetic population: allele frequencies are largely unknown, as were SNPs in our study; common diseases such as POP may be due to genetic variants (SNPs) that frequently occur in multiple genetic factors, each contributing only small or moderate effects to disease manifestation. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 24.0. Categorical demographic variables were described in proportions and compared using the χ2 test. Variables with normal distribution were described in mean and standard deviation (SD), and the variables with nonnormal distribution were described in median and 25th and 75th percentiles (P25 and P75). Comparisons between groups were made using Student’s t test for two independent samples and the Mann–Whitney test, respectively. To verify that no evolutive factor affected the study population and that allele frequencies did not alter along the generations, Hardy–Weinberg equilibrium was calculated using the χ2 test, with a significance level of 5%. Association between polymorphism genotype and POP was estimated as odds ratio (OR) and respective 95% confidence intervals (CI 95%) using binary logistic regression models. The nonadjusted model was without adjustment for possible confounding variables; the adjusted model was adjusted for age, parity, number of normal births, and weight of the largest newborn. Women with undetermined genotype were excluded from the analysis. Since this proportion was relatively low (< 3%), it is expected that these exclusions would not have a relevant impact on results. To verify whether variables that were different between groups at baseline could be considered independent risk factors for the development of the disease, OR and respective 95% CI were calculated using binary logistic regression models with adjustment for variables of interest. The level of significance was 5% (p ≤0.05).
Results
A total of 532 women were assessed: 285 (53.6%) cases and 247 (46.4%) controls. Eight patients (2 cases and 6 controls) refused to participate. The clinical and demographic characteristics are described in Table 1. Univariate analyses have shown that mean age, parity, number of vaginal deliveries, weight of the largest newborn, prevalence of varicose veins and UI, and family history of POP were higher in the POP group. There were no statistical differences between groups with regard to ethnicity, age at menopause, BMI, alterations related to collagen (such as hernias and collagenosis), and chronic cough.
According to logistic regression analysis that included all variables not homogeneous between groups at baseline, age, parity, number of vaginal deliveries, and family history of POP were positively and significantly associated with POP in the unadjusted model. After adjustment for all variables, significant associations were only found for age, number of vaginal births, and family history of POP. The highest association was related to POP family history [OR = 2.55; CI 95% (1.43–4.55)], followed by vaginal delivery [OR = 1.66; CI 95% (1.36–2.03)], and age [OR = 1.10; CI 95% (1.07–1.14)] (Table 2).
The study population was stable (not in evolution) according to allelic frequencies. Hardy–Weinberg equilibrium was reached by calculating the χ2, with p value referring to 1 degree of freedom. The frequency of homozygous (wild-type or polymorphic variant) genotypes was similar in both POP and control groups [17.5% and 15.4% for (AA) COL18A1, and 18.9% and 20.6% for (GG) LOXL-4, respectively]. Heterozygous (GA) COL18A1 and (AG) LOXL-4 genotypes were predominant (Table 1).
Results of the multivariate logistic regression analysis concerning the association between COL18A1 and LOXL-4 genotypes and POP are described in Table 3. Two regression models were calculated: the first with no adjustment for possible confounding factors; the second with adjustment for age, parity, number of normal deliveries, and weight of the largest newborn. In both analyses, there were no significant associations between possible genotypes and POP phenotype. The adjusted model showed OR = 0.76 (95% CI 0.43–1.35) in women presenting homozygous polymorphic variant for LOXL-4 (GG) and OR = 0.69 (95% CI 0.41–1.17) in women presenting homozygous polymorphic variant COL18A1 (AA). Those results suggest lack of association between rs2236479 (COL18A1) and rs2862296 (LOXL-4) polymorphisms and POP stages III or IV.
Discussion
POP constitutes a “hidden epidemic,” with profound functional consequences for affected women. It is believed that POP development is a result of multiple risk factors acting on the pelvic floor [15]. In our study, we identified aging as an independent risk factor for POP: a 1-year increase in age corresponded to a 10% increase in the risk of POP development. Corroborating our finding, an Italian study found OR for POP of 1.3 (95% CI 1.1–1.5) and 1.7 (95% CI 1.5–2.0), respectively, for women aged 52–55 and ≥56 years compared with women aged ≤51 years [16].
Vaginal delivery is associated with POP and may confer a 4- to 11-fold increase in POP risk [4, 17]. In our study, we verified that the increase of one vaginal delivery can lead to a 66% increase in the risk of POP development. A systematic review showed that women with POP are substantially more likely to have family members with the same condition compared with women without POP, demonstrating that a positive family history is an important risk factor [18]. Our study showed that women with a positive family history had a 2.55-fold higher risk of developing the dysfunction compared with patients without a family history.
Predisposition to POP may potentially occur at the genetic level as a result of the millions of alleles that provide each person with their phenotypic individuality [5]. In recent years, different groups have searched for genetic markers of POP using GWAS, linkage, and SNP studies [6, 10, 11, 13]. Potential candidates are genes related to ECM metabolism, which have previously been identified in pelvic floor tissues of women with POP [6]. Both endostatin and collagen XVIII appear to interfere with growth factors, participate in structural organization of basement membranes, and act in collagen remodeling during a wound-healing process [12]. In the pelvic floor, endostatin and collagen XVIII may play a role in tissue organization and response to both minor and major insults, such as vaginal delivery. We chose to study the rs2236479 polymorphism of the COL18A1 gene based on findings from the GWAS by Allen–Brady et al. [9] that investigated African American and Hispanic women from the Women’s Health Initiative Hormone Therapy Study, which described positive association between SNP and POP in a Caucasian population of women with a high risk for POP. However, Giri et al. [19] could not reproduce these findings, and found OR close to the null for both groups of women with moderate POP (n = 1274; stage II and III) and controls with no POP (n = 317). Similar to our candidate-gene study, Khadzhieva et al. did not observe significant association between rs2236479 of COL18A1 and POP development by investigating Russian women with advanced (n = 210) and with no (n = 292) POP [11]. Potential reasons for the different results among those studies are that genetic variants for POP may differ across racial/ethnic populations or power, as the minor allele frequency variants may vary across geographic populations. In addition, the studies are of different sample size, inclusion criteria, and methodologies. Even though we found no association of the COL18A1 rs2236479 with POP in Brazilian women, attention should be given to the potential protective factor of the polymorphic allele (AA) for POP phenotype, which is a subject for further investigations.
LOX-family genes are plausible functional candidates since they are essential for the mechanical stability and maintenance of the vascular structure without which weakening of the connective tissues occurs [14, 20, 21]. Differently from other members of LOX [20,21,22], LOXL-4 has not been well explored in POP investigation. The unique study by Shynlova et al. failed to detect change in LOXL-4 gene expression in vaginal tissues of postmenopausal patients with severe POP [23]. We investigated LOXL-4 gene based on the relevant findings of Allen–Brady et al. by studying families of Caucasian women with members affected by POP [13]. Those authors reported that genes related to POP may be on or near chromosome 10q24-26 region, where LOXL-4 is located. In our study, we found no association between the SNP rs2862296 and POP in Brazilian women. However, our study suggests that the homozygous genotype for the polymorphic allele (GG) may attribute a protective factor for POP occurrence, even though results did not reach statistical significance. This subject requires additional investigations.
Sample size is an important aspect in genetic studies and a large sample is ideal and required [24]. However, most studies included limited participants. By definition, SNPs occur in at least 1% of the population [25], and small samples tend to conclude that values are not significant. We acknowledge that our results may be biased by the small sample size and that our findings should be interpreted with caution.
The Brazilian population is influenced by a great ethnic miscegenation, which may have impacted on our results. As genetic changes are population-dependent, it becomes an interesting challenge for future investigations to determine whether ethnic-specific genotype frequencies of SNPs contribute to a higher prevalence of POP. We consider this is an important study, since these polymorphisms of collagen XVIII and LOXL-4 genes have still been poorly explored in POP. We believe the study can contribute to enhance literature in this field, especially when grouped with other investigations in meta-analyses. However, further studies are needed relating COL18A1 and LOXL-4 genes to pelvic floor dysfunctions and to address the functionality of those SNPs.
Other SNPs located at 4q21 (rs1455311), 8q24 (rs1036819), 9q22 (rs430794), 15q11 (rs8027714), 20p13 (rs1810636), and 21q22 (rs2236479) have been previously described to be associated with POP in Caucasian high-risk familial cases [10]. Those still require additional replication in Brazilian and other populations. Multicentric collaboration is encouraged to overcome issues of access to a large number of participants and economic burden to encourage genetic screening tests for POP.
Conclusion
Age, number of vaginal deliveries, and family history are associated with POP. Our study suggests a lack of association between polymorphisms rs2236479 of the COL18A1 and rs2862296 of the LOXL-4 genes and advanced POP in the Brazilian mixed population.
References
Haylen BT, Maher CF, Barber MD, et al. An international Urogynecological association (IUGA) / international continence society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J. 2016;27:165–94.
Olsen A, Smith V, Bergstrom J, Colling J, Clark A. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6.
Fornell EU, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2002;83:383–9.
Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford family planning association study. Br J Obstet Gynecol. 1997;104(5):579–85.
Jack GS, Nikolova G, Vilain E, Raz S, Rodriguez LV. Familial transmission of genitovaginal prolapse. Int Urogynecol J. 2006;17(5):498–501.
Bortolini MAT, Rizk DEE. Genetics of pelvic organ prolapse: crossing the bridge between bench and bedside in Urogynecologic research. Int Urogynecol J. 2011;22(10):1211–9.
Cartwright R, Kirby AC, Tikkinen KAO, et al. Systematic review and metaanalysis of genetic association studies of urinary symptoms and prolapse in women. Am J Obstet Gynecol. 2015;212(2):199.e1–199.e24.
Kerkhof MH, Hendriks L, Brölmann HA. Changes in connective tissue in patients with pelvic organ prolapse--a review of the current literature. Int Urogynecol J. 2009;20(4):461–74.
Mäki J.: Lysyl Oxidases: Cloning and Characterization of the Fourth and the Fifth Human Lysyl Oxidase Isoenzymes, and the Consequences of a Targeted Inactivation of the First Described Lysyl Oxidase Isoenzyme in Mice. 2002. http://jultika.oulu.fi/files/isbn9514267397.pdf. Accessed 02 Sept 2017.
Allen-Brady K, Cannon-Albright L, Farnham JM, et al. Identification of six loci associated with pelvic organ prolapse using genome-wide association analysis. Obstet Gynecol. 2011;118(6):1345–53.
Khadzhieva MB, Kolobkov DS, Kamoeva SV, Ivanova AV, Abilev SK, Salnikova LE. Verification of the chromosome region 9q21 association with pelvic organ prolapse using RegulomeDB annotations. Biomed Res Int. 2015;2015:1–9.
Seppinen L, Sormunen R, Soini Y, Elamaa H, Heljasvaara R, Pihlajaniemi T. Lack of collagen XVIII accelerates cutaneous wound healing, while overexpression of its endostatin domain leads to delayed healing. Matrix Biol. 2008;27(6):535–46.
Allen-Brady K, Cannon-Albright LA, Farnham JM, Norton PA. Evidence for pelvic organ prolapse predisposition genes on chromosomes 10 and 17. Am J Obstet Gynecol. 2015;212(6):771.e1–7.
Akagawa H, Narita A, Yamada H, et al. Systematic screening of lysyl oxidase-like (LOXL) family genes demonstrates that LOXL2 is a susceptibility gene to intracranial aneurysms. Hum Genet. 2007;121(3-4):377–87.
Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin N Am. 1998;25:723–46.
Group PMIS. Risk factors for genital prolapse in non-hysterectomized women around menopause. Eur J Obstet Gynecol Reprod Biol. 2000;93(2):135–40.
Patel DA, Xu X, Thomason AD, Ransom SB, Ivy JS, DeLancey JO. Childbirth and pelvic floor dysfunction: an epidemiologic approach to the assessment of prevention opportunities at delivery. Am J Obstet Gynecol. 2006;195(1):23–8.
Lince SL, van Kempen LC, Vierhout ME, Kluivers KB. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int Urogynecol J. 2012;23(10):1327–36.
Giri A, Wu JM, Ward RM, et al. Genetic determinants of pelvic organ prolapse among African American and Hispanic women in the Women’s health initiative. PLoS One. 2015;10(11):e0141647.
Liu G, Daneshgari F, Li M, Lin D, Lee U, Li T, et al. Bladder and urethral function in pelvic organ prolapsed lysyl oxidase like-1 knockout mice. BJU Int. 2007;100(2):414–8.
Alperin M, Debes K, Abramowitch S, Meyn L, Moalli PA. LOXL1 deficiency negatively impacts the biomechanical properties of the mouse vagina and supportive tissues. Int Urogynecol J. 2008;19(7):977–86.
Alarab M, Bortolini MA, Drutz H, Lye S, Shynlova O. LOX family enzymes expression in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Int Urogynecol J. 2010;21(11):1397–404.
Shynlova O, Bortolini MAT, Alarab M. Genes responsible for vaginal extracellular matrix metabolism are modulated by women’s reproductive cycle and menopause. Int Braz J Urol. 2013;39(2):257–67.
Roberts R, Wells GA, Stewart AFR, Dandona S, Chen L. The genome-wide association study—a new era for common polygenic disorders. J Cardiovasc Transl Res. 2010;3(3):173–82.
Wang DG, Fan JB, Siao CJ, et al. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280(5366):1077–82.
Author information
Authors and Affiliations
Contributions
Conception and design: Castro, Bortolini.
Data acquisition: Santos, Pepicelli, Batista, Carvalho.
Data analysis and interpretation: Santos, Pepicelli, Batista, Carvalho, Bortolini.
Manuscript drafting: Santos, Bortolini.
Manuscript revision: Bortolini, Castro.
Supervision: Carvalho, Bortolini, Castro.
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
dos Santos, R.G.M., Pepicelli, F.C.A., Batista, N.C. et al. Collagen XVIII and LOXL-4 polymorphisms in women with and without advanced pelvic organ prolapse. Int Urogynecol J 29, 893–898 (2018). https://doi.org/10.1007/s00192-018-3597-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3597-3