Abstract
Our objective was to study the expression of estrogen receptor (ER) isoforms, ER-α and ER-β, in the anterior vaginal wall of menopausal and fertile women with genuine stress incontinence (SI) by immunohistochemistry and Western blot analysis. Eighteen menopausal women with SI who either were or were not taking estrogen/progestin replacement therapy and 14 fertile women with SI who either were or were not taking contraceptives were enrolled in the study. Biopsies from the suburethral anterior vaginal wall were obtained at tension-free vaginal tape (TVT) operation. Monoclonal antibody to ER-α and polyclonal antibody to ER-β were used to stain frozen sections of vaginal tissue. The receptor expressions were scored based on percentage of positive cells. ER-α was detected in vaginal epithelial, stromal and smooth muscle cells. In menopausal SI women ER-α was detected significantly more frequently in the vaginal walls of estrogen/progestin-treated patients than in those who were untreated. Fertile SI women had significantly higher expression of ER-α than menopausal SI women. ER-α was not observed in vaginal blood vessels. ER-β was detected in epithelial and vascular smooth muscle cells of the vagina. No significant difference in ER-β expression was observed between different groups of patients. The expression of ER-α was not correlated with that of ER-β. Both ER-α and -β were detected, indicating a potential role for both types of estrogen receptor in the human vaginal wall. The expression of ER-α, but not of ER-β, in menopausal SI women was regulated by estrogen/progestin replacement therapy. The presence of ER-β in vaginal vascular smooth muscle cells raises the possibility of vascular effects of estrogen on the human vaginal wall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence is a disease with urinary leakage. As the number of elderly in the population is increasing, the prevalence of urinary incontinence is expected to increase in proportion. The vagina is the lowermost segment of the female reproductive tract. One important function of the anterior vaginal wall is to support the urethra and bladder base [1]. Excessive relaxation of the vaginal wall, often caused by pregnancy and delivery, may result in urinary incontinence and uterovaginal prolapse [2, 3]. Lack of estrogen has been suggested to relate to these changes [4, 5]. It is then important to investigate the estrogen receptors in the vagina.
Steroid hormones regulate cell function via specific receptors, members of a superfamily of ligand-activated transcription factors expressed in the target tissues. The classic actions of estrogen are manifested through its initial interaction with specific high-affinity intracellular receptors [6]. The classic estrogen receptor (ER-α) has been cloned [7, 8] and identified in estrogen-sensitive organs [9, 10]. In the late 1990s a new ER complementary DNA, designated and now known as ER-β subtype, was also cloned from many different tissues [11, 12]. Both isoforms of ER have been found in steroid-sensitive tissues [13], indicating the complexity of the tissue response to estrogen. The specific effect of estrogen therapy might be due to the differential expression of these two ER genes, depending on the physiologic and/or physiopathologic state of the target tissues [14].
The aim of the present study was to investigate the expression of ER-α and ER-β in the human vaginal wall of menopausal women with SI who were or were not taking hormone replacement therapy (HRT) and of fertile women with SI who were or were not taking contraceptives.
Materials and methods
The study was approved by the Ethics Committee of Uppsala University. Informed consent was obtained from all participating women. Thirty-two women with genuine SI were enrolled in the study. No patient had previously been exposed to vaginal surgery. Patients with clear clinical evidence of pelvic floor prolapse grade 2 or greater were excluded. Eighteen patients were menopausal and 14 were still in the fertile ages with regular menstruation. The menopausal SI women on HRT had received 2 mg of estradiol plus 5 mg of medroxyprogesterone acetate orally per day and the duration of HRT use was 24±6 months. The fertile SI women on contraceptives had 30 μg ethinylestradiol plus 75 μg of hevonorgestrel orally per day. The fertile women who did not receive medication were operated on at the middle of their menstrual cycle. There was a significant difference in the mean ages of the two groups (Table 1). All women underwent a tension-free vaginal tape operation for urinary SI [15] and the biopsies were taken from the anterior vaginal wall covering the middle part of the urethra. All samples were snap-frozen in liquid nitrogen and stored at −70°C.
Immunohistochemistry
Immunostaining for ER-α and -β proteins was performed on frozen sections, applying the avidin–biotin–peroxidase complex (ABC) method. Serial 5–6 μm cryosections were placed on poly-l-lysine-coated glass microscope slides and fixed in Zamboni´s fixative (pH=7.3) for 3 min. The endogenous peroxidase activity was quenched with 0.3% H2O2 in PBS (vol/vol) for 15 min. The endogenous avidin and biotin were blocked by antiavidin and antibiotin, respectively. For ER-α the slides were then incubated with 2% blocking horse serum and for ER-β with 2% blocking goat serum (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Excess serum was drained and primary antibodies (ER-α, ID5, 200 μg/ml, Dako, Corp. Denmark, 1:200) and ER-β (PA1–313, 1 mg/ml, Affinity Bioreagents Inc., Golden, CO, USA, 1:500) were added to the sections. The polyclonal rabbit antihuman ER-β antibody (PA1–313)corresponds to the C-terminal amino acid residues 467–485 of human ER-β. Sections were incubated overnight at 4°C in a humidified chamber. For the negative control, non-specific mouse and rabbit IgG (Vector Laboratories) were used at the same protein concentrations. Sections were rinsed with PBS and then biotinylated antimouse (1.5 mg/ml, Vector Laboratories) and antirabbit (1.5 mg/ml, Vector Laboratories) antibodies were added at dilutions of 1:1000 and 1:1500 for 45 min at room temperature. The antigen–antibody complex was detected by using an avidin–biotin–peroxidase kit (ABC, Vector Laboratories). Diaminobenzidine (DAB) hydrogen peroxide (Vector Laboratories) was used as the chromogen, and the sections were counterstained with hematoxylin and then mounted with Eukitt (Kebo Lab).
Quantification
A Nikon microscope connected to a computer using Colorvision software (Leica Imaging System Ltd.) was used to quantitatively assess immunostaining by a computer imaging analysis system. Quantification was performed on the digitized images of eight different fields systematically and randomly selected from each section. Cells in the field were assigned a score of 0–3 based on the number of cells specifically stained as follows: 0 = no positive cells; 1 = fewer than 25% positive cells; 2 = 25%–75% positive cells; and 3 = more than 75% positive cells.
Western blot
Frozen tissues were homogenized in five volumes of ice-cold 0.05 M Tris buffer (pH 7.40) containing 0.5 mM phenylmethyl sulfonylfluoride to inhibit proteolysis. The protein concentration was determined by the method of Lowry. SDS-PAGE and Coomassie blue staining were performed for all samples as a control for degradation prior to Western blotting. Each of 30 μg aliquots of proteins extracted from vaginal tissues was separated by 7.5% SDS-polyacrylamide gel and transferred on to a nitrocellulose membrane using an electrophoretic blotter. The membranes were incubated with 5% BSA in PBS for non-specific blocking and then exposed to ER-α (1:100) or ER-β (1:1000) antibody at 4°C overnight. The antigen–antibody complexes were detected with the secondary antibody using the ECL chemiluminescence detection system (Amersham, Arlington Heights, IL, USA). The molecular size of proteins was determined by running marker proteins in an adjacent lane. For controls, ER-α and ER-β antibodies were replaced with non-specific IgG.
Student's t-test or two-way ANOVA followed by the Tukey test for unequal samples was used to determine statistical significance.
Results
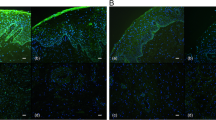
ER-α was indicated by uniform yellow to brown nuclear staining exhibited in epithelial (Fig. 1A), stromal fibroblasts (Fig. 1B and C), and smooth muscle cells (Fig. 1D) of the vagina. ER-α was not seen in vaginal blood vessels (Fig. 1B). In menopausal SI women who were not on HRT the ER-α was limited to the basal cells of the vaginal epithelium (Fig. 1E). When patients had received HRT, the ER-α was detected throughout the epithelium (Fig. 1A). Menopausal SI women on HRT had a significantly higher expression of ER-α than untreated women (Fig. 2A). Contraceptives had no significant effects on vaginal ER-α expression in fertile SI women (Fig. 2B). A significantly higher expression of ER-α was observed in vaginal walls of fertile SI women who were not using contraceptives than in menopausal SI women not on HRT (Fig. 2C). Moreover, there was no significant difference in the expression of ER-α between menopausal SI women on HRT and fertile SI women not taking contraceptives (Fig. 2D). Western blotting for ER-α demonstrated a single band at ~67 kDa which was present in all vaginal samples and to a lesser extent in HRT-untreated samples from menopausal SI women (Fig. 3A). Omission of primary antibody eliminates the signal (data not shown).
Immunohistochemical staining for estrogen receptors (ER)-α and -β in human vaginal wall. ER-α immunoreactivity was localized in the nuclei of vaginal epithelium (Epi) (A a representative sample from postmenopausal SI women with HRT); stromal cells (Str) (B, C a representative sample from fertile SI women without contraceptive); and smooth muscle cells (SM) (D a representative sample from fertile SI women without contraceptive). In postmenopausal women who did not receive HRT, ER-α was restricted to the basal cells of vaginal epithelium (E). Positive staining for ER-β is seen in the vaginal epithelium (F) and smooth muscle cells of blood vessels (BV) (G). Arrowheads indicate positive cells
Immunohistochemical staining score of estrogen receptor (ER)-α in vaginal walls of menopausal (n=18) and fertile (n=14) SI women. ER-α was detected in epithelial (Epi), stromal (Str) and smooth muscle (SM) cells of the vagina. Results were scored in a total of eight non-overlapping areas and expressed as 0: no positive cells, 1: <25% positive cells, 2: 25%–75% positive cells, 3: >75% positive cells. The cells were counted by using an image analysis system.
Western blotting of ER-α and ER-β proteins on human vaginal wall. ER-α and ER-β were identified as a band at 67 kDa and 55 kb, respectively, when 30 μg protein was loaded. Lanes 1–2 samples from fertile SI women with contraceptive; lanes 3–4 samples from postmenopausal SI women without HRT; lanes 5–6 samples from fertile SI women without contraceptive; lanes 7–8 samples from postmenopausal SI women with HRT; lane 9, molecular weight markers; Arrow indicates the dominant protein recognized by the antibodies
ER-β was immunolocalized in vaginal epithelial cells (Fig. 1F) and vascular smooth muscle cells of the vaginal blood vessels (Fig. 1G). There were no significant differences in vaginal ER-β expression between HRT-treated and untreated menopausal SI women (Fig. 4A) nor between fertile SI women with or without contraceptives (Fig. 4B). Statistical significance was not observed in the expression of vaginal ER-β between menopausal and fertile SI women (Fig. 4C). Western blotting demonstrated a protein band with predicted size for ER-β at ~55 kDa (Fig. 2B). Omission of primary antibody eliminates the signal (data not shown). The expression of ER-β was not correlated with that of ER-α.
Immunohistochemical staining score of estrogen receptor (ER)-β in vaginal walls of menopausal and fertile SI women. ER-β was detected in epithelial (Epi), smooth muscle (SM) and vascular smooth muscle cells (VSM) of vagina. Results were scored in a total of eight non-overlapping areas and expressed as 0: no positive cells, 1: <25% positive cells, 2: 25%–75% positive cells, 3: >75% positive cells. The cells were counted by using an image analysis system
Discussion
In this study both ER-α and ER-β were expressed immunohistochemically in the human anterior vaginal wall of fertile and menopausal women with genuine SI. The Western blot analysis using ER-α- and ER-β-specific antisera demonstrated single bands for ER-α and ER-β at ~67 and ~55 kDa, respectively. ER-α was localized in vaginal epithelial, stromal and smooth muscle cells, whereas ER-β was expressed in vaginal epithelial and vascular smooth muscle cells. The presence of mRNA for ER-α and ER-β in the vaginal walls of premenopausal women and the absence of ER-β in postmenopausal women has recently been reported [16]. In rats, mRNA for both ER-α and ER-β in the vagina has also been detected [17, 18].
Estrogen induces epithelial proliferation, stratification and cornification in vaginal epithelium and these processes are mediated by stromal ER-α in the vagina [19]. The decline and eventual cessation of estrogen production by the ovary at menopause is reflected physiologically in tissues with estrogen receptors. The receptor number is an important determinant of the cell's ability to respond to a steroid hormone [20, 21]. We observed that ER-α expression increased significantly in the vaginal walls of estrogen-/progestin-treated menopausal SI women compared with untreated women, suggesting that ER-α levels can be affected by estrogen/progestin therapy.
Both ER-α and ER-β bind 17β-estradiol with high affinity and they also bind to classic estrogen response elements in a similar fashion. However, there are also major differences between ER-α and ER-β with respect to their tissue distribution [22, 23, 24], the phenotype of the corresponding knockout mice [25] and their transcriptional activities [26]. The presence of cell-specific trans-acting factors that mediate tissue/cell-specific ER-β expression has been suggested [27]. From the present study, we could not detect the regulation and variation of ER-β in the human vaginal wall in SI women who were estrogen/progestin treated and untreated, nor with/without contraceptives. Moreover, it seems that ER-β expression does not appear to be dependent on ER-α. At present it is unclear whether and how ER-β is regulated by estrogen.
Estrogens appear to protect women from cardiovascular disease through their effects on lipid metabolism as well as through more direct effects on arterial walls that appear to inhibit atherosclerotic plaque formation. The presence of ER-α in the vascular endothelium of some organs supports the hypothesis that ER-α has direct vascular effects [10, 28, 29, 30, 31, 32]. Studies of vaginal function in postmenopausal women have demonstrated that vaginal blood flow is significantly increased after estrogen replacement therapy [33, 34]. However, in the present study, ER-α was undetectable in both endothelial and smooth muscle cells of vaginal blood vessels. In contrast, ER-β was detected in vaginal vascular smooth muscle cells, suggesting that ER-β may mediate some direct vascular effects of estrogen on the human vaginal wall. Both genomic- and non-genomic-mediated mechanisms of vascular effects of estrogen have been reported. It is possible that the genomic effects of estrogen on human vaginal blood vessels are modulated by ER-β in the vascular tissue. An expression of ER-β in blood vessels of human endometrium [35], pregnant uterus of rhesus monkey [36], rat carotid artery [37] and the male rat aorta [38] has also been demonstrated. It seems that both ER-α and ER-β play a role in the direct vascular effects of estrogen. Probably there is a tissue- or species-dependent difference regarding the expression, the relation and the biological actions of these two receptors.
In conclusion, we have identified both ER-α and ER-β in human vaginal walls. ER-α, but not ER-β, was regulated by HRT in menopausal SI women. In fertile SI women contraceptives had no significant effect on the expression of ER.
Abbreviations
- ER:
-
Estrogen receptor
- SI:
-
Stress incontinence
- TVT:
-
Tension-free vaginal tape
- HRT:
-
Hormone replacement therapy
- ABC:
-
Avidin–biotin–peroxidase complex
- PBS:
-
Phosphate buffered saline
- DAB:
-
Diaminobenzidine
References
Petros PE, Ulmsten U (1995) Urethral pressure increase on effort originates from within the urethra, and continence from musculovaginal closure. Neurourol Urodyn 14:337–346
Winters JC, Cespedes RD, Vanlangendonck R (2000) Abdominal sacral colpopexy and abdominal enterocele repair in the management of vaginal vault prolapse. Urology 56:55–63
Fatton B, Jacquetin B (1999) Pelvic and perineal sequelae of delivery. Rev Prat 49:160–166
Bernier F, Jenkins P (1997) The role of vaginal estrogen in the treatment of urogenital dysfunction in postmenopausal women. Urol Nurs 17:92–95
de Aloysio D, Altieri P, Penacchioni P, Mauloni M, Bottiglioni F (1996) Premenopause-dependent changes. Gynecol Obstet Invest 42:120–127
Sarff M, Gorski J (1971) Control of estrogen binding protein concentration under basal conditions and after estrogen administration. Biochemistry 10:2557–2563
Green S, Walter P, Kumar V et al. (1986) Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139
Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J (1986) Sequence, and expression of human estrogen receptor complementary DNA. Science 231:1150–1154
Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. (1988) Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 67:334–340
Gehrig PA, Van Le L, Olatidoye B, Geradts J (1999) Estrogen receptor status, determined by immunohistochemistry, as a predictor of the recurrence of stage I endometrial carcinoma. Cancer 86:2083–2089
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930
Mosselman S, Polman J, Kijkema R (1996) ER-β: Identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49–53
Katzenellenbogen BS, Korach KS (1997) A new actor in the estrogen receptor drama-enter ER-β. Endocrinology 138:861–862
Benassayag C, Leroy MJ, Rigourd V et al. (1999) Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: pregnancy and leiomyoma. Am J Physiol 276:E1112–1118
Ulmsten U (1997) For female urinary stress incontinence. Women's Health Digest 3:259–262
Ghen GD, Oliver RH, Leung BS, Lin LY, Yeh J (1999) Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil Steril 71:1099–1102
Wang H, Eriksson H, Sahlin L (2000) Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol Reprod 63:1331–1340
Mowa CN, Iwanaga T (2000) Differential distribution of oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of rats as revealed by in situ hybridization. J Endocrinol 165:59–66
Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS (1998) Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification and cornification. Endocrinology 139:4345–4352
Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ (1992) The limits of the cellular capacity to mediate an estrogen response. Mol Endocrinol 6:157–167
Vanderbilt JN, Miesteld R. Maler BA, Yamamoto KR (1987). Intracellular receptor concentration limits glucocorticoid dependent enhance activity. Mol Endocrinol 1:68–74
Saj S, Omoto Y, Shimizu C, Horiguchi S, Watanabe T, Funata N, Hayash S, Custafsson JA, Toi M (2002) Clinical impact of assay of estrogen receptor beta cx in breast cancer. Breast Cancer 9:303–307
Speirs V, Skliris GP, Burdall SE, Carder PJ (2002) Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol 55:371–374
Iro T, Tachibana M, Yamamoto S, Nakashima J, Murai M (2001) Expression of estrogen receptor (ER-alpha and ER-beta) mRNA in human prostate cancer. Eur Urol 40:557–563
Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I (1997) The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology 138:5649–5652
McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS (1998) Transcription activation by the human estrogen receptor subtype β (ERβ) studied with ERβ and ERα receptor Chimeras. Endocrinology 139:4513–4522
Li LC, Yeh CC, Nojima D, Dahiya R (2000) Cloning and characterization of human estrogen receptor beta promoter. Biochem Biophys Res Commun 275:682–689
Suzuma I, Mandai M, Takagi H et al. (1999) 17 Beta-estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci 40:2122–2129
Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME (1998) Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res 83:224–229
Kim-Schulze S, McGowan KA, Hubchak SC et al. (1996) Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation 94:1402–1407
Venkov CD, Rankin AB, Vaughan DE (1996) Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation 94:727–733
Leiberman JR, van Vroonhoven CC, Beckmann I, van der Kwast TH, Wallenburg HC (1990) Uterine artery estrogen receptors in the non-pregnant and pregnant guinea pig. Am J Obstet Gynecol 163:1685–1688
Semmens JP, Tsai CC, Semmens EC, Loadholt CB (1985) Effects of estrogen therapy on vaginal physiology during menopause. Obstet Gynecol 66:15–18
Semmens JP, Wagner G (1982) Estrogen deprivation and vaginal function in postmenopausal women. JAMA 248:445–448
Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M (2001) Presence of estrogen receptor beta in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J Clin Endocrinol Metab 86:1379–1386
Wu WX, Ma XH, Smith GC, Nathanielsz PW (2000) Differential distribution of ERalpha and ERbeta mRNA in intrauterine tissues of the pregnant rhesus monkey. Am J Physiol Cell Physiol 278:C190–198
Makela S, Savolainen H, Aavik E et al. (1999) Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proc Natl Acad Sci USA 96:7077–7082
Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME (1998) Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res 83:224–229
Acknowledgment
The study was supported financially by the Swedish Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Comment: This was an interesting study in that it addressed the expression of estrogen receptors in incontinent women. There is not a control group of incontinent women. Conclusions regarding mechanisms of action or treatment with estrogen for incontinence cannot be made on the basis of the results. At best this study proves there is expression of estrogen receptors in vaginal tissues, which has been done by prior investigators.
Rights and permissions
About this article
Cite this article
Fu, X., Rezapour, M., Wu, X. et al. Expression of estrogen receptor-α and -β in anterior vaginal walls of genuine stress incontinent women. Int Urogynecol J 14, 276–281 (2003). https://doi.org/10.1007/s00192-003-1042-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-003-1042-7