Abstract
Introduction and hypothesis
The objective was to investigate the expression of endothelial nitric oxide synthase (eNOS) and phosphodiesterase (PDE) 5 in vaginal tissue of premenopausal women experiencing stress urinary incontinence (SUI) with and without sexual dysfunction.
Methods
Women presenting for treatment of SUI were screened using the Female Sexual Function Index (FSFI) and 10 were selected who met the criteria for female sexual dysfunction (FSD) and 10 asymptomatic controls. Vaginal tissue specimens were obtained from those premenopausal women aged ≥40 years who had had sexual activity ≥2 times every month for the last 6 months and who were scheduled to undergo surgery for SUI. FSD criteria was FSFI scores <18 and arousal domain scores <3. The control group had FSFI scores ≥26 and individual domain scores ≥4. The expressions of eNOS and PDE 5 were compared in the two groups using immunofluorescence staining and western blotting.
Results
The mean total FSFI scores were 30.4 ± 2.6 and 15.3 ± 2.3 in the control and FSD groups respectively. In immunofluorescence staining, eNOS and PDE5 were localized in the vaginal epithelium. In western blotting, the expressions of eNOS and PDE5 were significantly lower in the FSD group than in the control group (p = 0.003 and p = 0.038 respectively).
Conclusions
eNOS and PDE5 in the vagina may play important roles in the pathophysiology of FSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relatively less attention has been paid to female sexual problems than male sexual problems; however, the former are highly prevalent. The prevalence of female sexual problems has been reported to be 32–43 %, even in young women [1, 2]. In women, physiological mechanisms of sexual desire, arousal, and orgasm are known to be associated with vasculogenic, neurogenic, and endocrinological aspects. However, the pathophysiology of female sexual dysfunction (FSD) remains as yet unclear.
Hemodynamic changes and modulation of smooth muscle tone in the female genital organs, such as the clitoris and vagina, are important in female sexual function. Various neurotransmitters, vasoactive agents, and endocrine hormones, which are related to physiological mechanisms of female sexual function, have been investigated in experimental models [3]. Nitric oxide (NO) and phosphodiesterase (PDE) have attracted great attention to the pathophysiology of FSD. NO is a potent vasodilator in female genital organs, and the NO/cyclic guanosine monophosphate (cGMP) pathway mediates clitoral and vaginal blood flow during sexual arousal [4]. NO is generated by endothelial nitric oxide synthase (eNOS) in blood vessels; thus, eNOS could play a key role in vascular function and vasodilation, and it is known to be localized to the vagina [5]. The NO/cGMP pathway has been reported to be involved in the regulation of smooth muscle function in the vagina [6]. cGMP, which mediates smooth muscle relaxation, is degraded by PDE5. PDE5 inhibitors, which increase cGMP levels and enhance vascular and nonvascular smooth muscle relaxation, have been widely used to treat sexual dysfunction. The localization of PDE5 in the anterior wall of the human vagina has been reported histologically [7].
The pathophysiology of FSD has been evaluated in many animal models; however, few human studies have been performed owing to ethical constraints and practical limitations. Additionally, most human studies that evaluated the pathophysiology of FSD were performed in menopausal women [8–10]. It was the aim of the present study to investigate the changes in expression of eNOS and PDE5 in the vaginal tissues of premenopausal women with sexual dysfunction to identify the pathophysiology of FSD.
Materials and methods
Study population
We screened women aged ≥40 years who had had sexual activity ≥2 times every month for the last 6 months and who were scheduled to undergo transvaginal midurethral sling surgery to treat stress urinary incontinence (SUI) at seven centers in South Korea. Menopausal women and women who had a history of vaginal surgery, total hysterectomy, oophorectomy, stage 2 or greater pelvic organ prolapse, diabetes mellitus, depression, or any medications that could affect sexual function were excluded. We enrolled premenopausal women to minimize the chance of obtaining vaginal samples from women with vaginal atrophy or estrogen deficiency. The women completed the Female Sexual Function Index (FSFI) questionnaire. The FSFI questionnaire consists of six domains (desire, arousal, lubrication, orgasm, satisfaction, and pain) with 19 items, and it has been translated into Korean and validated [11, 12]. Women with FSFI scores <18 and arousal domain scores <3 or FSFI scores ≥26 and individual domain scores ≥4 were enrolled in this study. The women were assigned to the one of the following two groups according to the FSFI scores: the FSD group (FSFI scores <18 and arousal domain scores <3) and the control group (FSFI scores ≥26 and individual domain scores ≥4). Although an FSFI total score of 26.55 has been recognized as the cutoff for FSD [13], the classification of female sexual function using a cutoff score of 26 can induce significant errors in the basic research of sexual function. Thus, we excluded women who were considered to have mild FSD (FSFI total score of 18–23) [14] in this study.
All women provided written informed consent before enrollment. This study was performed according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee and Institutional Review Board of each center.
Study design
Vaginal tissue specimens were obtained during midurethral sling surgery from the anterior vaginal wall 3 cm inside the introitus. The specimen measured approximately 0.5 × 1 cm and included smooth muscle. To minimize possible interference from estrogen, the surgery was performed during the follicular phase. We cut the specimen in two including epithelium and smooth muscle with a scalpel. Specimens were frozen in liquid nitrogen for further analysis. The expressions of eNOS and PDE5 in the vaginal tissues were evaluated using immunofluorescence staining and western blotting, and the results were compared in the FSD and control groups.
Immunofluorescence staining
Tissue was embedded in an optimal cutting temperature compound, frozen, and cut into 4-μm sections. The sections were warmed at room temperature and were then fixed in cold acetone. The preparations were dehydrated, washed with phosphate-buffered saline (PBS), and placed in normal horse serum to block nonspecific binding sites on the cells. The primary antibodies (anti-eNOS antibody and anti-PDE5 antibody), were prepared, and eNOS and PDE5 were immunolabeled at 4 °C after draining the blocking solution. Slides were washed three times with PBS. The secondary antibody (goat anti-rabbit IgG-FITC antibody, 1:200) was then applied, and the slides were incubated in a dark chamber. After the slides had been washed three times with PBS, antifade reagent and a cover slip were applied. Staining was visualized using a fluorescence microscope (BX50; Olympus, Tokyo, Japan), and digital images were captured. As the cells expressing eNOS and PDE5 appeared green, the immunoreactivities of eNOS and PDE5 were evaluated as the percentage of green color portion in three random fields per image, using an imaging analysis program (IMT i-solution version 10.1; IMT i-solution. Vancouver, BC, Canada).
Western blotting
Frozen tissues were pulverized with use of a mortar and were then lysed at 4 °C with RIPA buffer. The lysate was centrifuged and protein levels were measured using the Bio-Rad protein assay. Laemmli sample buffer was combined with 50 μg of total protein, and the mixture was incubated and kept on ice before application on 10 % SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes using a semi-dry transblotting technique. Nonspecific binding sites were blocked by incubation in 1× Tris-Tween buffered saline containing 5 % skim milk and 0.1 % Tween 20. The membranes were incubated overnight with eNOS and PDE5 antibodies. Western blotting was performed using rabbit polyclonal antibodies against eNOS and PDE5. After washing, the membranes were incubated with a rabbit IgG secondary antibody. The membranes were then washed, and protein signals were detected using an enhanced chemiluminescence solution. The membranes were also probed with a beta actin monoclonal antibody, followed by a mouse IgG secondary antibody, which was used as an internal loading control. Reactions were noted at 133 kDa (eNOS), 89 kDa (PDE5) and 42 kDa (beta actin). The immunoreactive proteins were detected, and the scanned film was quantified using a gel documentation system (Bio-Rad Laboratories, Hemel Hempstead, UK).
Statistical analysis
All data are reported as means with standard deviations. The means were statically compared using the Mann–Whitney U test for all continuous variables. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 20.0 (IBM, Armonk, NY, USA). A p value <0.05 was considered to indicate a significant difference.
Results
In total, 31 patients were screened. Of these, 20 women were included in the present study. All subjects were Korean and married. The FSD and control groups included 10 women each. The mean total FSFI scores were 15.3 ± 2.3 in the FSD group (n = 10) and 30.4 ± 2.6 in the control group (n = 10). The clinical characteristics, except for the FSFI score, were not different in the FSD and control groups (Table 1).
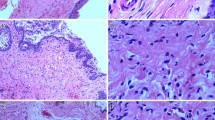
Immunofluorescence staining showed that eNOS and PDE5 were localized in the vaginal epithelium (Fig. 1). The immunofluorescence signals of eNOS (50.5 ± 11.9 % vs 58.4 ± 10.4 %, p = 0.068) and PDE5 (37.5 ± 12.2 % vs 42.9 ± 12.3 %, p = 0.168) assessed using image analysis were not different in the FSD and control groups.
Immunofluorescence staining of eNOS and PDE5. A Endothelial nitric oxide synthase (eNOS) expressions in vagina, B phosphodiesterase 5 (PDE5) expressions in vagina. a epithelium in the control group, b epithelium in the female sexual dysfunction (FSD) group, c smooth muscle in the control group, d smooth muscle in the FSD group. Scale bars: 200 μm
In western blot analysis, the mean relative density of eNOS against beta actin was significantly lower in the FSD group than in the control group (6.04 ± 3.05 vs 18.05 ± 7.82, p = 0.003). Additionally, the mean relative density of PDE5 against beta actin was significantly lower in the FSD group than in the control group (1.93 ± 1.36 vs 3.77 ± 1.68, p = 0.038; Fig. 2).
Discussion
We noted changes in the expressions of eNOS and PDE5 in the vaginal tissue of premenopausal women according to sexual function. The expressions of eNOS and PDE5 in the vagina were significantly lower in women with FSD than in those without FSD on western blot analysis.
It is not easy to identify FSD because the diagnostic criteria and pathophysiology of FSD are still controversial. Although laboratory evaluations of hormones and some methods of physiological assessment of sexual function are used to diagnose FSD, they are rarely accurate, as the methods are not standardized and relationships between the results and sexual function are lacking. Validated sexual function instruments are useful in the diagnosis of FSD. The FSFI is one of the most useful diagnostic and research tools for FSD. Thus, we used FSFI to classify the existence of FSD in this study.
An increase in genital blood flow is an important physiological process in the female sexual response. After sexual stimulation, vaginal blood flow was higher in healthy women than in women with FSD [15]. The NO signaling system plays an important role in regulating vaginal hemodynamics. Kim et al. [16] demonstrated the effects of the NO/cGMP pathway on vaginal blood flow in rabbits. Intravenous administration of an NOS inhibitor resulted in the reduction of vaginal blood flow. A previous study reported that the eNOS levels in the vagina decreased in diabetic rats after a reduction of vaginal blood flow triggered by pelvic nerve stimulation [17]. The present study found that eNOS levels in the vagina were lower in the FSD group than in the control group. Although we did not assess vaginal blood flow in this study, the FSD group had a mean arousal domain score of 1.83 ± 0.49, indicating a definite arousal problem. Thus, eNOS levels in the vagina could reflect the status of female sexual function, including sexual arousal.
The reduction of the NO level in the vagina influences the stimulation of guanylyl cyclase, which is a link between NO and cGMP. Therefore, the cGMP level decreases, suppressing endothelial and smooth muscle relaxation. PDE5, which catalyzes the hydrolysis of cGMP to GMP, should then decrease to maintain the cGMP level in sexual dysfunction, as a negative feedback. We found that the PDE5 levels in the vagina were lower in the FSD group than in the control group. This is supported by the finding that the low cGMP levels, which were caused by low NO levels, led to deactivation of PDE5 and high cGMP levels led to activation of PDE5 in a previous in vivo experiment [18]. A previous report demonstrated that PDE5 expression in the cavernous smooth muscle cells of rats was significantly reduced under anoxia and hypoxia, which represents the situation of sexual dysfunction [19]. Previous histological analyses of human cadaveric vaginal tissue showed that PDE5 was distributed in vascular and nonvascular vaginal smooth muscles, Skene periurethral glands and the vaginal epithelium [7, 20]. Thus, PDE5 in the vagina may play an important physiological role in female sexual function.
Although we found differences in the eNOS and PDE5 levels in the vagina between the FSD and control groups on western blot analysis, there were no differences in the immunofluorescence signals of eNOS and PDE5 between the two groups on immunofluorescence staining. Additionally, the immunoreactivities of eNOS and PDE5 were localized in the vaginal epithelium rather than in the lamina propria or muscle layer. Thus, the relationship between the vaginal epithelium and eNOS or PDE5 could be considered in female sexual function. Stimulating vaginal epithelium in adenylyl cyclase pathway promotes vaginal lubrication during sexual arousal [21]. Sexual arousal in women is characterized by an increase in blood flow to the genital organs, in addition to an increase in vaginal lubrication. An increase in vaginal lubrication results from the passive transfer of proteins and fluids across the capillary network, and NOS has been shown to play an important role in macromolecular permeability [22]. A previous animal study reported that the inhibition of PDE5 increased vaginal lubrication irrespective of the hormonal status [23]. In the present study, the lubrication subscore in the FSFI was significantly lower in the FSD group than in the control group, and this could be explained by the differences in the eNOS and PED5 levels between the FSD and control groups.
The present study had several limitations. We designed the inclusion and exclusion criteria to minimize bias in this study; however, we could not evaluate various socioeconomic and psychological statuses in the women and the characteristics of their partners. Additionally, we only included women with SUI in this study. Urinary incontinence has been reported to affect sexual function owing to emotional and organic factors in premenopausal sexually active women [24]. However, a recent study showed that SUI was not related to any sexual difficulty in specific domains of the FSFI [25]. We used strict FSFI scores for the enrollment of women and assessed the existence of sexual dysfunction to reduce the effect of SUI on sexual function. Estrogen is important for maintaining vaginal blood flow and lubrication, and estrogen could influence the regulation of eNOS expression in the vagina [26]. Although we obtained all vaginal tissue samples during the follicular phase in premenopausal women to minimize the effects of estrogen on the eNOS or PDE5 level, we did not measure the estrogen levels after the vaginal tissue samples had been obtained, and this could be considered a limitation. Furthermore, our study comprised a relatively small sample that would not have enough statistical power to detect differences in eNOS and PDE5 levels in the vagina.
The vagina in premenopausal women expresses eNOS and PDE5, and these levels are low in women with sexual dysfunction and sexual arousal disorder, suggesting that those in the vagina might play important roles in the pathophysiology of FSD. eNOS and PDE5 could be novel biomarkers in the diagnosis of FSD and may aid its pharmacological treatment.
Abbreviations
- cGMP:
-
Cyclic guanosine monophosphate
- eNOS:
-
Endothelial nitric oxide synthase
- FSD:
-
Female sexual dysfunction
- FSFI:
-
Female Sexual Function Index
- NO:
-
Nitric oxide
- PBS:
-
Phosphate-buffered saline
- PDE:
-
Phosphodiesterase
- SUI:
-
Stress urinary incontinence
References
Witting K, Santtila P, Jern P, Varjonen M, Wager I, Hoglund M, et al. Evaluation of the female sexual function index in a population based sample from Finland. Arch Sex Behav. 2008;37:912–24. doi:10.1007/s10508-007-9287-8.
Song SH, Jeon H, Kim SW, Paick JS, Son H. The prevalence and risk factors of female sexual dysfunction in young Korean women: an internet-based survey. J Sex Med. 2008;5:1694–701. doi:10.1111/j.1743-6109.2008.00840.x.
Min K, O’Connell L, Munarriz R, Huang YH, Choi S, Kim N, et al. Experimental models for the investigation of female sexual function and dysfunction. Int J Impot Res. 2001;13:151–6.
Giuliano F, Rampin O, Allard J. Neurophysiology and pharmacology of female genital sexual response. J Sex Marital Ther. 2002;28 Suppl 1:101–21. doi:10.1080/00926230252851230.
Batra S, Al-Hijji J. Characterization of nitric oxide synthase activity in rabbit uterus and vagina: downregulation by estrogen. Life Sci. 1998;62:2093–100.
Giraldi A, Alm P, Werkstrom V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14:271–82. doi:10.1038/sj.ijir.3900886.
D’Amati G, di Gioia CR, Bologna M, Giordano D, Giorgi M, Dolci S, et al. Type 5 phosphodiesterase expression in the human vagina. Urology. 2002;60:191–5.
Pace G, Palumbo P, Miconi G, Silvestri V, Cifone MG, Vicentini C. PDE-5 and NOS II mRNA expression in menopausal women: a molecular biology study. World J Urol. 2011;29:243–8. doi:10.1007/s00345-010-0517-7.
Bertin J, Dury AY, Ouellet J, Pelletier G, Labrie F. Localization of the androgen-synthesizing enzymes, androgen receptor, and sex steroids in the vagina: possible implications for the treatment of postmenopausal sexual dysfunction. J Sex Med. 2014;11:1949–61. doi:10.1111/jsm.12589.
Baldassarre M, Alvisi S, Berra M, Martelli V, Farina A, Righi A, et al. Changes in vaginal physiology of menopausal women with type 2 diabetes. J Sex Med. 2015;12:1346–55. doi:10.1111/jsm.12906.
Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi:10.1080/009262300278597.
Kim HYSH, Park KS, Jeong SJ, Lee JY, Ryu SB. Development of the Korean-version of Female Sexual Function Index (FSFI). Korean J Androl. 2002;20:50–6.
Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi:10.1080/00926230590475206.
Safarinejad MR. Female sexual dysfunction in a population-based study in Iran: prevalence and associated risk factors. Int J Impot Res. 2006;18:382–95. doi:10.1038/sj.ijir.3901440.
Allers KA, Richards N, Sultana S, Sudworth M, Dawkins T, Hawcock AB, et al. I. Slow oscillations in vaginal blood flow: alterations during sexual arousal in rodents and humans. J Sex Med. 2010;7:1074–87. doi:10.1111/j.1743-6109.2009.01465.x.
Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. Role of the nitric oxide-cyclic GMP pathway in regulation of vaginal blood flow. Int J Impot Res. 2003;15:355–61. doi:10.1038/sj.ijir.3901038.
Kim NN, Stankovic M, Cushman TT, Goldstein I, Munarriz R, Traish AM. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006;6:4. doi:10.1186/1472-6793-6-4.
Mullershausen F, Russwurm M, Koesling D, Friebe A. In vivo reconstitution of the negative feedback in nitric oxide/cGMP signaling: role of phosphodiesterase type 5 phosphorylation. Mol Biol Cell. 2004;15:4023–30. doi:10.1091/mbc.E03-12-0890.
Lin G, Xin ZC, Lue TF, Lin CS. Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J Urol. 2003;170:S15–18; discussion S19. doi:10.1097/01.ju.0000075500.11519.e8
Uckert S, Oelke M, Waldkirch E, Stief CG, Albrecht K, Troger HD, et al. Cyclic adenosine monophosphate and cyclic guanosine monophosphate-phosphodiesterase isoenzymes in human vagina: relation to nitric oxide synthase isoforms and vasoactive intestinal polypeptide-containing nerves. Urology. 2005;65:604–10. doi:10.1016/j.urology.2004.10.028.
Sun Q, Huang J, Yang DL, Cao XN, Zhou WL. Activation of beta-adrenergic receptors during sexual arousal facilitates vaginal lubrication by regulating vaginal epithelial Cl(−) secretion. J Sex Med. 2014;11:1936–48. doi:10.1111/jsm.12583.
Mayhan WG. Nitric oxide accounts for histamine-induced increases in macromolecular extravasation. Am J Physiol. 1994;266:H2369–73.
Game X, Roumiguie M, Bouali O, Allard J, Gourdy P, Mazerolles C, et al. Vaginal lubrication after cervicovaginal stimulation is facilitated by phosphodiesterase type 5 inhibition in ovariectomized mice. J Sex Med. 2013;10:1452–60. doi:10.1111/jsm.12127.
Aslan G, Koseoglu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17:248–51. doi:10.1038/sj.ijir.3901296.
Su CC, Sun BY, Jiann BP. Association of urinary incontinence and sexual function in women. Int J Urol. 2015;22:109–13. doi:10.1111/iju.12610.
Musicki B, Liu T, Strong TD, Lagoda GA, Bivalacqua TJ, Burnett AL. Post-translational regulation of endothelial nitric oxide synthase (eNOS) by estrogens in the rat vagina. J Sex Med. 2010;7:1768–77. doi:10.1111/j.1743-6109.2010.01750.x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Cho, K.J., Lee, KS., Choo, MS. et al. Expressions of vaginal endothelial nitric oxide synthase and phosphodiesterase 5 in female sexual dysfunction: a pilot study. Int Urogynecol J 28, 431–436 (2017). https://doi.org/10.1007/s00192-016-3159-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-016-3159-5