Abstract
Purpose
The roles of estrogen receptor α (ERα) in stress urinary incontinence (SUI) remain elusive. This study was conducted to understand the molecular mechanism of ERα against SUI.
Methods
Wild-type (ERα+/+) and ACTB-cre ERα knockout (ERα−/−) female mice were generated. Urethral function and protein expression were measured. Leak point pressures (LPP) and maximum urethral closure pressure (MUCP) were assessed in mice under urethane anesthesia. After the measurements, the urethras were removed for proteomic analysis using the two-dimensional differential gel electrophoresis and liquid chromatography–mass spectrometry technology. Interaction between these ERα pathway proteins was further analyzed by using MetaCore. Lastly, Western blot and immunochemistry (IHC) were used to confirm the candidate protein expression levels and locations, respectively.
Results
Compared with the ERα+/+ group, the LPP and MUCP values of the ERα−/− group were significantly decreased. Additionally, we identified 11 differentially expressed proteins in the urethra of ERα−/− female mice; five proteins were down-regulated and six were up-regulated. The majority of the ERα knockout-modified proteins were involved in muscle development, contraction, and regulation, as well as immune response (amphoterin signaling and phagocytosis), proteolysis, and cell adhesion (platelet aggregation and integrin-mediated cell–matrix adhesion). IHC and Western blot confirmed the down-regulation of tropomyosin and up-regulation of myosin in urethra.
Conclusions
This is the first study to estimate protein expression changes in urethras from ERα−/− female mice. These changes could be related to the molecular mechanism of ERα in SUI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is defined as the involuntary leakage of urine under stress conditions such as coughing and sneezing [1, 2]. The effects of birth trauma, menopause, and aging may contribute to the development of SUI [3]. Estrogen exerts a variety of regulatory functions in the female urogenital system. Estrogen actions are mediated by estrogen receptors (ERs), encoded by two distinct genes, ERα and ERβ [4]. Estrogen has many roles in the reproduction and urinary systems, and the generation of the ERα and ERβ knockout mice has further illustrated its roles. It has been shown that ERα is more critical than ERβ in mediation of the estrogen actions necessary for maintenance of healthy somatic cell function [5]. In the female rodent, the infertility of the ERα knockout mouse is due in part to the insensitivity of the uterus to the mitogenic and different actions of estrogen [6]. In urogynecology, the efficacy of estrogen for SUI in postmenopausal women is still controversial [7]. Therefore, the specific roles of estrogen and ERα in SUI remained elusive.
Because of the limited availability of human tissue for study, animal models are an important adjunct in improving our understanding of SUI [8]. Vaginal distension and pudendal nerve transaction have been used for creation of SUI in rats, as evidenced by lowered leak point pressures (LPP) on urodynamic testing. The use of mice in various lines of translational research has made available transgenic and knockout technologies for conducting mechanistic studies of various target diseases [9]. The C57BL/6 mouse, for example, has been widely used for genetic manipulation in previous studies concerning urinary and pelvic disorders [10]. Interestingly, the decrease of ER in the pelvic floor tissues in pelvic organ prolapse (POP) patients may be closely related to the occurrence of SUI [3].
Proteomics approaches to identify and quantify the entire protein content of a tissue at a given time may provide insights into the mechanisms of diseases [11]. Our aim was to understand the molecular mechanism of ERα in SUI, and in this study using two-dimensional differential gel electrophoresis (2D DIGE) and liquid chromatography–mass spectrometry (LC–MS/MS) technology, we identified ERα pathway candidate proteins in urethra by comparing urethra tissues collected from ERα deficient and age-matched wild-type female mice.
Materials and methods
ERα knockout mice and genotyping
The ACTB-Cre/ERα knockout (ERα−/−) mice were generated via the Cre-loxP system by mating the floxed ERα homozygous female mice with ACTB-Cre transgenic male mice [12, 13]. After two generations of mating, wild-type (ERα+/+) heterozygote (ERα+/−) and knockout (ERα−/−) mice were developed. Genomic DNA was isolated from tail biopsies and used as a template for PCR genotyping. The primers used for ACTB-ERα mutant mice were as follows: P1, 5′-AGG CTT TGT CTC GCT TTC C-3′, P2, 5′-GAT CAT TCA GAG AGA CAA GAG GAA CC-3′; and Cre, sense, 5′-AGG TGT AGA GAA GGC ACT TAG C-3′; antisense, 5′-CTA ATC GCC ATC TTC CAG G-3′. The genotyping of ACTB-ERα mutant mice was determined by PCR using the P1, P2, and Cre primer mixture. The size of P1-P2 fragments for wild-type and knockout allele was 741 and 223 bp, respectively. The size of Cre fragments was 411 bp (Fig. 1) [12, 13].
Genotyping of different ERα mutant mice. Lane 1 ERα mice without ACTB-cre, Lane 2 ActB-cre transgenic mice (ActB-cre ERα+/+), the size of cre fragment was 411 bp, Lane 3 heterozygote ERα knockout mice (ActB-cre ERα+/−), in which the size of floxed ERα allele was reduced to 223 bp after the deletion of intervening DNA via ActB-cre recombinase, Lane 4 homozygote ERα knockout mice (ActB-cre ERα−/−), in which the size of both floxed ERα alleles was reduced to 223 bp by ActB-cre recombinase and ERα band was absent
The mice underwent suprapubic bladder tubing (SPT) placement [12, 13], and LPP and maximum urethral closure pressure (MUCP) were assessed in these mice under urethane (1 g/kg, i.p.) anesthesia (n = 6 for each group). After measurements, the animals were killed, and the urethras were removed for further analyses. Since the external urethral sphincter is mostly responsible to maintain the continence mechanism lies in the mid-urethra, the middle-one-third of urethras harvested from six rats for each group were used for proteomic analysis, immunohistochemistry (IHC), and Western blot. The tissue samples were divided into two parts, one part (n = 3) was frozen and homogenized for proteomic and Western blot analyses, and the other part (n = 3) was fixed with 4 % paraformaldehyde for further IHC analyses. All experimental protocols were approved by the Institutional Animal Care and Use Committee of China Medical University (Reference number: 101-201-N).
Suprapubic tube implantation
The surgical procedure was carried out under 1.5 % isoflurane anesthesia [12, 13]. An SPT (PE-10 tubing, Clay Adams, Parsippany, NJ, USA) was implanted in the bladder.
LPP measurement
Two days after implanting the bladder catheter, the LPP was assessed. The bladder catheter was connected to both a syringe pump and a pressure transducer. Pressure and force transducer signals were amplified and digitized for computer data collection at 10 samples per second (PowerLabs, AD Instruments, Bella Vista, Australia). The average bladder capacity of each mouse was determined after 3–5 voiding cycles. Subsequently, the LPP was measured [12, 13].
Urethral pressure profile (UPP)
UPP was assessed in these mice under urethane anesthesia. The bladder catheter was connected to a syringe pump with room temperature saline at 1 ml/h. The urethral catheter was connected to a pressure transducer. A withdrawal speed of 10 μm per minute was used. Pressure and force transducer signals were amplified and digitized for computer data collection at 10 samples per second. Three successive profiles were obtained in the supine position. The urethral closure pressure (P close) is the difference between the urethral pressure (P ure) and the bladder pressure (P ves): P close = P ure − P ves. Maximum urethral pressure and MUCP were determined from the UPP measurements taken [12, 13]. LPP and MUCP were performed by two independent examiners to avoid the possible operator-dependent bias.
Two-dimensional differential gel electrophoresis
Frozen pieces of urethra were homogenized. Protein was extracted, and the concentrations were determined [14]. Fifty micrograms of protein samples from vaginal tissue of ERα−/− mice was pooled and labeled with CyDye 3 (Cy 3) (400 pmol/μl) (GE Healthcare, Waukesha, WI, USA), those from wild-type mice were pooled and labeled with Cy 5, and internal standards were labeled by mixing of Cy 2 with 50 μg of protein samples (25 μg from treated mice plus 25 μg from control mice). The three labeled protein samples were mixed and denatured in 450 μl rehydration buffer, and the mixed sample was applied to a 3–10 24 cm IPG strip (GE Healthcare), followed with IEF as rehydration for 12 h, 500 V for 0.5 h, 1,000 V for 1 h, 8,000 V for 3 h, and 8,000 V for 24,000 Vhr. The two-dimensional electrophoresis was performed at 17 W until the bromophenol blue dye reached the bottom of the gel [14].
Protein spot analysis
The labeled gels were scanned using a Typhoon 9410 imager (GE Healthcare) with specific excitation and emission wavelengths. Difference in-gel analysis (DeCyder, v6.5, GE Healthcare) was used for intra-gel analysis. Biological variance analysis was used for inter-gel matching and statistical analysis [14].
In-gel digestion
The labeled gels were stained using silver staining for gel spots excision. The gel pieces were dehydrated with 100 % acetonitrile and rehydrated with 25 mM ammonium bicarbonate (pH 8.2) solution. After drying in a centrifugal concentrator, the gel pieces were rehydrated in 25 mM ammonium bicarbonate containing 1 ng/μl sequencing grade modified trypsin (Promega) for 1 h at 4 °C and then incubated at 37 °C overnight. After digestion, the tryptic peptides were extracted from the gel using 1 % FA in 50 % ACN [14].
Protein identification by LC–MS/MS
LC–MS/MS was performed using an ion trap mass spectrometer (HCTultra PTM Discovery, Bruker, Germany) coupled online with an Ultimate 3000 nanoLC system (Dionex). Peptide fragment mass spectra were acquired in data-dependent Auto-MS/MS mode with a scan range of 100–2,500 m/z, two averages, and five precursor ions selected from the MS scan range of 400–1,600 m/z. Peptide peaks were detected and deconvoluted automatically using Data Analysis 4.0 software (Bruker). Mass lists in the form of MASCOT Generic Files were created automatically and used as the input for MASCOT MS/MS ions searches of the NCBI database (release 20101220) using an in-house MASCOT 2.2.04 server. Search parameters were selected as taxonomy–mouse; enzyme–trypsin; fixed modifications–carbamidomethyl (C); variable modifications–oxidation (M); precursor peptide tolerance ±0.5 Da; MS/MS tolerance ±0.5 Da; significant threshold P < 0.05 [14].
Networks analysis using MetaCore
MetaCore (GeneGo, St. Joseph, MI, USA) was used to map the differentially expressed proteins into biological networks. Differentially expressed proteins were converted into gene symbols and uploaded into MetaCore for analysis. The biological process enrichment was analyzed based on Gene Ontology processes [14].
Immunohistochemistry (IHC) staining
The urethras were histologically examined with IHC staining for indicating the protein expression regions of the urethra. The sections were then incubated with the primary antibody derived from rabbit TPM3 and anti-myosin heavy chain antibodies (clone A4.1025, myosin, heavy polypeptide 1), 1:200 dilution, Millipore, MA, USA], followed by incubation with avidin–biotin–horseradish peroxidase complex, with 0.5 mg/ml of 3,3′-diaminobenzidine as chromogen (Vector Lab, USA).
Western blot analysis
Detection of proteins was performed with the use of the antibody derived from rabbit (anti-TPM3 and anti-myosin antibodies, Millipore, MA, USA). The intensity of each band was quantified using a densitometer (Molecular Dynamics, Sunnyvale, CA, USA) [15, 16].
Statistical analysis
Data are expressed as mean ± standard deviation (SD) of six individual mice. Student’s t test was used to compare two different groups of sample, and one-way analysis of variance (ANOVA) was used to compare data from more than two groups. P value <0.05 was considered statistically significant. All calculations were performed using the Statistical Package for Social Sciences (SPSS for Windows, SPSS Inc, Chicago, IL, USA).
Results
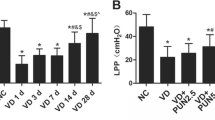
Decreased LPP and MUCP in ERα−/− mice
The ACTB-Cre/ERα knockout (ERα−/−) mice were generated via the Cre-loxP system by mating the floxed ERα homozygous female mice with ACTB-Cre transgenic male mice [12]. When female mice reach 8 weeks old, leak point pressures (LPP) and maximum urethral closure pressure (MUCP) were assessed in wild-type (ERα+/+), heterozygote (ERα+/−) and knockout (ERα−/−) female mice under urethane anesthesia.
Compared to ERα+/+ group, our data showed that LPP and MUCP values were slightly decreased in the ERα+/− heterozygote female group without statistical significance. By contrast, LPP and MUCP values were significantly decreased in the ERα−/− group compared with the ERα+/+ group (Fig. 2a, b).
LPP (a) and MUCP (b) value in the different groups. After SPT placement, the LPP and MUCP were assessed in mice under urethane anesthesia. LPP and MUCP were performed by two independent examiners to avoid the possible operator-dependent bias. Each bar represents the mean ± standard deviation of six individual mice. * P < 0.05 different from the value in the ERα+/+ group with one-way ANOVA
Protein expression profile from proteomic analysis
After the LPP and MUCP measurements, the urethras were removed for proteomic analysis using the 2D DIGE and LC–MS/MS technology. As there was no significant difference of LPP and MUCP measurements between ERα+/− and ERα+/+ group, we only compared the protein expression profile between ERα−/− and ERα+/+ group. We identified 11 urethra proteins differentially expressed with statistical significance in ERα+/+ and ERα−/− female mice. Additionally, five proteins were down-regulated and six were up-regulated (Table 1; Fig. 3). The majority of the ERα knockout-modified proteins were involved in muscle development, contraction, and regulation, as well as immune response (amphoterin signaling and phagocytosis), proteolysis, and cell adhesion (platelet aggregation and integrin-mediated cell–matrix adhesion) (Fig. 4a, b).
Biological network analysis (a) for differentially expressed proteins of urethra from ERα−/− female mice using MetaCore mapping tool (b). The network was generated using shortest path algorithm to map interaction between the proteins. The majority of the ERα knockout-modified proteins were involved in muscle development, contraction, and regulation, immune response (amphoterin signaling and phagocytosis), proteolysis, and cell adhesion (platelet aggregation and integrin-mediated cell–matrix adhesion)
Tropomyosin and myosin and expressions in the urethra of ERα−/− mice
We further focused on urethral contraction-related proteins including tropomyosin and myosin [17]. Since there are many different types (including various heavy and light chains) of tropomyosin and myosin, it is difficult to analyze all the protein subtypes, therefore, the most common commercially available antibodies, including anti-tropomyosin α-3 chain and anti-myosin heavy chain were chosen for the subsequent analyses. The urethras were histologically examined with IHC staining for indicating the protein expression regions of the urethra (Figs. 6a, 7a). Among these differentially expressed proteins of urethra, tropomyosin and myosin were chosen for further confirmation. From 2D DIGE (Fig. 5), and Western blot analyses, tropomyosin (Fig. 6b) in the urethra was significantly decreased in the ERα−/− group as compared with that in the ERα+/+ group. By contrast, myosin (Fig. 7b) expression in the urethra was significantly increased in the ERα−/− group.
Three-dimensional simulation figures of protein spots acquired gel images by 2D-DGE DeCyder 6.5 software. The labeled gels were scanned using a Typhoon 9410 imager with specific excitation and emission wavelengths. Difference in-gel analysis was used for intra-gel analysis. Biological variance analysis was used for inter-gel matching and statistical analysis. The data show spot identified as tropomyosin down-regulated in urethra of ERα−/− female mice
Alterations of tropomyosin expression in urethra as indicated by IHC staining (a) and Western blot (b) analyses. The urethra was histologically examined with IHC staining for indicating the protein expression regions of the urethra. The sections were then incubated with the primary antibody derived from rabbit [anti-tropomyosin α-3 chain (TPM3)]. Western blot analysis was performed with the use of the antibody. The intensity of each band was quantified using a densitometer. The values are calculated by fold and expressed as mean ± SD of at least three individual samples. *Significantly different from the value in the control group (P < 0.05) with Student’s t test
Alterations of myosin expression in urethra as indicated by IHC staining (a) and Western blot (b) analyses. The urethra was histologically examined with IHC staining for indicating the protein expression regions of the urethra. The sections were then incubated with the primary antibody derived from rabbit [anti-myosin heavy chain antibodies (clone A4.1025, myosin, heavy polypeptide 1)]. Western blot analysis was performed with the use of the antibody. The intensity of each band was quantified using a densitometer. The values are calculated by fold and expressed as mean ± SD of at least three individual samples. *Significantly different from the value in the control group (P < 0.05) with Student’s t test
Discussion
Estrogen actions mediated by ERs are known to modulate lower urinary tract (LUT) trophicity [18]. In the present study, no obvious structural differences were found between the wild-type and ERα−/− mice, but LPP and MUCP values were significantly decreased in the ERα−/− group compared with the ERα+/+ group, indicating an important role of ERα in SUI. Further explicit assessments of degree of inflammation and fibrosis may differ between these two groups.
This is the first study that estimates the changes in protein expression related to ERα in SUI. We found 11 differentially expressed proteins between ERα+/+ and ERα−/− female mice. The majority of the identified ERα knockout-modified proteins were involved in muscle development, contraction, and regulation, as well as immune response (amphoterin signaling and phagocytosis), proteolysis, and cell adhesion (platelet aggregation and integrin-mediated cell–matrix adhesion). We further focused on tropomyosin and myosin and explored their expressions by IHC and Western blot analyses.
Tropomyosin α-3 chain (TPM3), which was underexpressed in ERα−/− female mice by 1.44-fold, is a member of the tropomyosin family of actin-binding proteins involved in the contraction of striated and smooth muscles by controlling the binding of myosin heads to the actin filament [19]. Since tropomyosin is responsible for muscle contraction, this down-regulation of tropomyosin may be a mechanism of the loss of normal urethral function (lower LPP and MUCP values) in SUI.
Among 11 differentially expressed proteins between ERα+/+ and ERα−/− female mice, three of the proteins that exhibited overexpression in ERα−/− female mice were related to myosin, including myosin regulatory light chain 2, myosin light chain 3 and myosin light chain 1/3. Myosin proteins are composed of both heavy and light chains and are essential components of muscles. Myosin heavy chains help determine the speed of muscle contraction; in contrast, the role of myosin light chains (whose expression was 4.55-fold higher in ERα−/− female mice) is unclear. Since myosin is responsible for muscle contraction, this overexpression of myosin light chains may be a mechanism to counteract the loss of normal urethral function in SUI. This finding is consistent with previous work showing 4.4-fold overexpression of the myosin light chains in the pubococcygeus muscle of women with POP [20].
The expression of NADH dehydrogenase iron-sulfur protein 8 was significantly 6.09-fold higher in ERα−/− female mice than in ERα+/+ female mice. NADH dehydrogenase iron-sulfur protein 8 is a subunit of mitochondrial enzyme involved in the electron transfer process [21]. The expression of ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCH-L1) was significantly 2.25-fold higher in ERα−/− female mice than in wild-type female mice. UCH-L1 is a deubiquitinating enzyme that is responsible for making ubiquitin, which is required to target proteins for degradation by the ubiquitin–proteasome pathway [22].
Desmin, which was underexpressed in ERα−/− female mice by 1.76-fold, is a type III intermediate filament found near the Z line in sarcomeres [23]. Desmin down-regulation may induce muscle damage during contraction in the urethra [24], leading to lower LPP and MUCP values in ERα−/− female mice. Translationally controlled tumor protein (TCTP), which was underexpressed in ERα−/− female mice by 2.59-fold, is involved in a variety of cellular activities, including microtubule stabilization, calcium-binding activities, and apoptosis [25]. TCTP down-regulation may induce apoptosis. N(G),N(G)-dimethylarginine dimethylamino-hydrolase 2 (DDAH II), which was underexpressed in ERα−/− female mice by 1.58-fold, is important in removing methylarginines, generated by protein degradation, from accumulating and inhibiting the generation of nitric oxide [26]. DDAH II down-regulation may induce the generation of nitric oxide in the urethra, leading to lower LPP and MUCP values in ERα−/− female mice. Caprin-2, which was underexpressed in ERα−/− female mice by 5.62-fold, may regulate the transport and translation of mRNAs, modulating for instance the expression of proteins involved in synaptic plasticity in neurons [27].
Our study has certain limitations. First, the pelvic floor structure of the mouse, which is a quadruped and has a lax abdominal wall, is different from that of a human female; therefore, the results of this study need to be carefully applied to human subjects. Second, urodynamic studies were conducted under anesthesia; fortunately, none of our subjects manifested any evidence of bladder instability, implying that detrusor overactivity was not present, and giving credence to our interpretation of fluid expulsion in the absence of increased bladder pressure as evidence of SUI. Third, since too large profiles were found from proteomic study, we could not use Western blot to confirm all the expression levels of potential target proteins in urethras from mice. Fourth, the experimental conditions and the sample preparation techniques could have affected the detection of some proteins. For example, urethras harvested after LPP and MUCP measurements might cause the urethra fatigue and then alter the urethral function, thus possibly leading to biased results. By contrast, advanced analytic methods such as electromyography can be used to replace the LPP method [28].
Conclusions
The results suggest a role for ERα in SUI. This study is the first one to estimate protein expression changes in urethras from ERα+/+ and ERα−/− female mice. These changes could be related to the molecular mechanism of ERα functions in SUI.
References
Phe V, Nguyen K, Roupret M, Cardot V, Parra J, Chartier-Kastler E (2014) A systematic review of the treatment for female stress urinary incontinence by ACT(R) balloon placement (Uromedica, Irvine, CA, USA). World J Urol 32(2):495–505. doi:10.1007/s00345-013-1117-0
Bo K (2012) Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 30(4):437–443. doi:10.1007/s00345-011-0779-8
Zhu L, Lang J, Feng R, Chen J, Wong F (2004) Estrogen receptor in pelvic floor tissues in patients with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 15(5):340–343. doi:10.1007/s00192-004-1178-0
Shapiro LF, Freeman K (2014) The relationship between estrogen, estrogen receptors and periodontal disease in adult women: a review of the literature. N Y State Dent J 80(3):30–34
Curtis Hewitt S, Couse JF, Korach KS (2000) Estrogen receptor transcription and transactivation: estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res 2(5):345–352
Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O (1993) Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A 90(23):11162–11166
Hirai K, Tsuda H (2009) Estrogen and urinary incontinence. Int J Urol 16(1):45–48. doi:10.1111/j.1442-2042.2008.02164.x
Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF (2001) The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol 166(1):311–317
Ma WL, Jeng LB, Yeh CC, Chang C (2012) Androgen and androgen receptor signals jamming monocyte/macrophage functions in premalignant phase of livers. Biomedicine 2(4):155–159
Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36(2):178–182. doi:10.1038/ng1297
Hammack BN, Fung KY, Hunsucker SW, Duncan MW, Burgoon MP, Owens GP, Gilden DH (2004) Proteomic analysis of multiple sclerosis cerebrospinal fluid. Mult Scler 10(3):245–260
Chen M, Wolfe A, Wang X, Chang C, Yeh S, Radovick S (2009) Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem 321(1–2):145–153. doi:10.1007/s11010-008-9928-9
Chen M, Yeh CR, Chang HC, Vitkus S, Wen XQ, Bhowmick NA, Wolfe A, Yeh S (2012) Loss of epithelial oestrogen receptor alpha inhibits oestrogen-stimulated prostate proliferation and squamous metaplasia via in vivo tissue selective knockout models. J Pathol 226(1):17–27. doi:10.1002/path.2949
Chen HY, Chen CJ, Lin YN, Chen YH, Chen WC, Chen CM (2013) Proteomic analysis related to stress urinary incontinence following vaginal trauma in female mice. Eur J Obstet Gynecol Reprod Biol. doi:10.1016/j.ejogrb.2013.08.034
Liu PL, Tsai JR, Chiu CC, Hwang JJ, Chou SH, Wang CK, Wu SJ, Chen YL, Chen WC, Chen YH, Chong IW (2010) Decreased expression of thrombomodulin is correlated with tumor cell invasiveness and poor prognosis in nonsmall cell lung cancer. Mol Carcinog 49(10):874–881. doi:10.1002/mc.20663
Yang TL, Lin FY, Chen YH, Chiu JJ, Shiao MS, Tsai CS, Lin SJ, Chen YL (2011) Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. J Sci Food Agric 91(1):134–141. doi:10.1002/jsfa.4163
Chen HY, Lin YN, Chen YH, Chen WC (2012) Stress urinary incontinence following vaginal trauma involves remodeling of urethral connective tissue in female mice. Eur J Obstet Gynecol Reprod Biol 163(2):224–229. doi:10.1016/j.ejogrb.2012.04.012
Game X, Allard J, Escourrou G, Gourdy P, Tack I, Rischmann P, Arnal JF, Malavaud B (2008) Estradiol increases urethral tone through the local inhibition of neuronal nitric oxide synthase expression. Am J Physiol Regul Integr Comp Physiol 294(3):R851–R857. doi:10.1152/ajpregu.00467.2007
Nevzorov IA, Levitsky DI (2011) Tropomyosin: double helix from the protein world. Biochemistry (Mosc) 76(13):1507–1527. doi:10.1134/S0006297911130098
Athanasiou S, Lymberopoulos E, Kanellopoulou S, Rodolakis A, Vlachos G, Antsaklis A (2010) Proteomic analysis of pubocervical fascia in women with and without pelvic organ prolapse and urodynamic stress incontinence. Int Urogynecol J 21(11):1377–1384. doi:10.1007/s00192-010-1203-4
Procaccio V, Depetris D, Soularue P, Mattei MG, Lunardi J, Issartel JP (1997) cDNA sequence and chromosomal localization of the NDUFS8 human gene coding for the 23 kDa subunit of the mitochondrial complex I. Biochim Biophys Acta 1351(1–2):37–41
Chang C, Chang AY, Chan SH (2004) De novo synthesis of ubiquitin carboxyl-terminal hydrolase isozyme l1 in rostral ventrolateral medulla is crucial to survival during mevinphos intoxication. Shock 22(6):575–581
Lazarides E, Hubbard BD (1976) Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A 73(12):4344–4348
Scott RS, Li Z, Paulin D, Uvelius B, Small JV, Arner A (2008) Role of desmin in active force transmission and maintenance of structure during growth of urinary bladder. Am J Physiol Cell Physiol 295(2):C324–C331. doi:10.1152/ajpcell.90622.2007
Bommer UA, Thiele BJ (2004) The translationally controlled tumour protein (TCTP). Int J Biochem Cell Biol 36(3):379–385
Cooke JP (2004) Asymmetrical dimethylarginine: The Uber marker? Circulation 109(15):1813–1818. doi:10.1161/01.CIR.0000126823.07732.D5
Grill B, Wilson GM, Zhang KX, Wang B, Doyonnas R, Quadroni M, Schrader JW (2004) Activation/division of lymphocytes results in increased levels of cytoplasmic activation/proliferation-associated protein-1: prototype of a new family of proteins. J Immunol 172(4):2389–2400
Dissaranan C, Cruz MA, Kiedrowski MJ, Balog BM, Gill BC, Penn MS, Goldman HB, Damaser MS (2014) Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant 23(11):1395–1406. doi:10.3727/096368913X670921
Acknowledgments
We are grateful for the technical assistance of Miss Chi-Hsiang Wei. This work was supported in part by Taiwan Ministry of Science and Technology (NSC 103-2911-I-002-303, NSC101-2632-B039-001-MY3, NSC101-2314-B-039-018, and NSC102-2320-B-039-025), China Medical University Hospital (DMR-104-058), National Health Research Institutes (NHRI-EX102-10241BI), CMU under the Aim for Top University Plan of the Taiwan Ministry of Education, and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002 and MOHW104-TDU-B-212-113002).
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical standard
The procedures of the animal study received approval from the ethical committee of the Institutional Animal Care and Use Committee of China Medical University (Reference number: 101-201-N, Date: Feb. 3, 2012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y.-H. Chen and C.-J. Chen contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, YH., Chen, CJ., Lin, YN. et al. Proteomic analysis of urethral protein expression in an estrogen receptor α-deficient murine model of stress urinary incontinence. World J Urol 33, 1635–1643 (2015). https://doi.org/10.1007/s00345-014-1474-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1474-3