Abstract
Purpose
The aim of this two-centre RCT was to compare pre- and post-operative radiological, clinical and functional outcomes between patient-specific instrumentation (PSI) and conventional instrumented (CI) unicompartmental knee arthroplasty (UKA). It was hypothesised that both alignment methods would have comparable post-operative radiological, clinical and functional outcomes.
Methods
One hundred and twenty patients were included, and randomly allocated to the PSI or the CI group. Outcome measures were peri-operative outcomes (operation time, length of hospital stay and intra-operative changes of implant size) and post-operative radiological outcomes including the alignment of the tibial and femoral component in the sagittal and frontal plane and the hip–knee–ankle-axis (HKA-axis), rate of adverse events (AEs) and patient-reported outcome measures (PROMs) pre-operatively and at 3, 12 and 24 months post-operatively.
Results
There was a statistically significant difference (p < 0.05) in alignment of the femoral component in the frontal plane in favour of the CI method. No statistically significant differences were found for the peri-operative data or in the functional outcome at 2-year follow-up. In the PSI group, the approved implant size of the femoral component was correct in 98.2% of the cases and the tibial component was correct in 60.7% of the cases. There was a comparable rate of AEs: 5.1% in the CI and 5.4% in the PSI group.
Conclusion
The PSI method did not show an advantage over CI in regard of positioning of the components, nor did it show an improvement in clinical or functional outcome. We conclude that the possible advantages of PSI do not outweigh the costs of the MRI scan and the manufacturing of the PSI.
Level of evidence
Randomised controlled trial, level I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unicompartmental knee arthroplasty (UKA) has been used in the treatment of unicompartmental knee osteoarthritis for several decades. Advantages of UKA over total knee arthroplasty (TKA) include faster return to work and a lower rate of adverse events (AEs) [12, 35]. Furthermore, a recent study also showed UKA is more cost-effective than TKA in patients with medial compartment osteoarthritis [3]. Large cohorts have demonstrated excellent long-term survival rates [1, 33]. However, the joint registries have demonstrated a lower survival rate compared to TKA [28]. As the survival of UKA is influenced by the alignment of the implants, the surgical technique can still be improved [18]. Achieving the correct alignment in UKA is technically demanding and associated with a significant learning curve [30].
Deviations of more than 3 degrees from a perpendicular cut to the tibial mechanical axis are associated with decreased survival of the prosthesis, which would suggest a low tolerance for tibial component malpositioning [10, 18, 30, 36]. An instrumentation method that has now been available for 10 years is the patient-specific instrumentation (PSI). With this instrumentation method, it is theoretically possible to accurately plan the position and size of the implants, prior to surgery, based on magnetic resonance imaging (MRI) or computed tomography (CT) [33]. Another possible advantage of the PSI is a shorter operation time and reduced blood loss, as already described in the use of PSI in TKA, but research on this topic in UKA is still inconclusive [8, 9, 11]. Retrospective research of the present study group showed no advantage in alignment or in functional outcome in comparison to conventional instrumentation [27]. As correct component positioning is critical to the success and survival of UKA, technologies to improve alignment accuracy may, therefore, greatly benefit UKA and need to be thoroughly investigated. This is the first independent multi-centre RCT, assessing the radiological and functional outcome, as well as the rate of AEs of PSI in UKA in a study population of 120 patients. It was hypothesised that both alignment methods would have comparable clinical results, post-operative radiological and functional outcomes.
Materials and methods
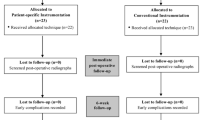
Between April 2015 and July 2017, 120 patients were prospectively included in two different teaching hospitals, the Amphia Medical Centre (Breda, the Netherlands) and the Zuyderland Medical Centre (Sittard-Geleen, the Netherlands). Inclusion criteria were: a painful and disabled knee joint resulting from single medial osteoarthritis with at least Kellgren–Lawrence grade II on the radiograph [22]; age above 18 years; body mass index (BMI) below 35 kg/m2; an informed consent read, understood and signed by the patient; willingness to follow instructions and return to the outpatient clinic for follow-up. The following characteristics were considered as exclusion criteria: patients with osteoarthritis of more than only the medial knee compartment; patients with rheumatoid arthritis; a failure of previous joint replacement; major knee surgery (e.g. an open reduction and internal fixation of a tibial plateau fracture) except for arthroscopic meniscectomy; those who have or had an active infection in the knee or an active infection elsewhere in the body which could spread to the implant site; patients with metal implants such as plates and screws or a joint prosthesis near the knee joint (MRI scan not possible); individuals who were not eligible for MRI for other reasons (e.g. presence of a pacemaker, an implantable cardioverter-defibrillator) and those with anterior or posterior cruciate ligament insufficiency.
Two patients in the intervention group withdrew from the study. Two patients who were randomised to the intervention group were operated on using conventional instrumentation. One because the quality of the MRI scan was not suitable for the manufacturing of PSI. The other patient received an implantable cardioverter-defibrillator (ICD) after inclusion, and was, therefore, not suitable for MRI. These patients were included in the CI group (intention to treat) to avoid the effects of crossover and dropout—this is acceptable for the analysis of controlled clinical trials [32]. In the CI group, three patients received a TKA instead of a UKA. One patient received a TKA because of an anterior cruciate ligament (ACL) tear, another patient because of peri-operatively observed grade IV lateral chondropathy and the third patient because there was a mismatch in the flexion–extension gap.
An informed consent was signed by all patients who were enrolled in this study. In both Medical Centres, patients were treated by one surgeon (RG and NK). Both surgeons are considered high volume surgeons in UKA, with more than 40 cases per year [2]. Patients were randomly allocated to one of the two treatment groups; the PSI group (intervention group) or the CI group (control group), with the use of a computer-based random number generator (www.randomizer.org). Patients had an equal opportunity of being assigned to one of both groups. A total of 120 cases was included, independent of numbers per centre. Forty-seven patients were treated in the Amphia medical centre, whilst the remaining 73 patients were treated in the Zuyderland medical centre. Patients were not informed on the type of group allocation. The examiner, an orthopaedic surgery resident, was blinded to the treatment allocation of the patients in both centres. The surgeon was not blinded for group allocation. For blinding purposes, an MRI was done in all included patients in both the intervention and control group, according to the PSI (Signature™, Zimmer-Biomet Inc., Warsaw, IN) scanning protocol. This protocol includes low-resolution scans of the hip and ankle, and a high-resolution scan of the knee [37]. The MRI scan was performed at least 6 weeks prior to the day of surgery. In case the patient was treated in the intervention group, the PSI system (Signature™, Zimmer-Biomet Inc., Warsaw, IN) was used. In case the patient was treated in the control group, the CI (Microplasty®, Zimmer-Biomet Inc., Bridgend, UK) was used. All patients were operated with the mobile-bearing Oxford® Partial Knee Phase III (Zimmer-Biomet Inc., Bridgend, UK) [6] and use of a tourniquet.
If the patient was allocated to the intervention group, a digital plan of the proposed per-operative positioning of the prosthesis was made available by a technician, based on the MRI scan. The surgeon approved every default setting and made adjustments in implant component size if necessary. After the approval of the default plan by the surgeon, PSI was manufactured (Materialise, Leuven, Belgium). The default component position was set at 0 degrees to the mechanical axis for both the femur and tibia in the frontal plane, 10 degrees flexion in relation to the anatomical axis for the femoral component in the sagittal plane and 4 degrees posterior slope in relation to the mechanical axis for the tibial component in the sagittal plane [21, 37].
Pre- and post-surgery, a standard anterior–posterior (AP), lateral radiograph and a long-leg standing radiograph were obtained. Six weeks post-operatively a fluoroscopically checked radiograph (radiograph centred to obtain a true AP view of the tibial component) was obtained. The fluoroscopically checked radiographs and long-leg standing radiographs at 6 weeks were used to determine the alignment and placement of the prosthesis (Figs. 1, 2), according to the guidelines for radiologic evaluation of the Oxford group [6]. In the frontal plane, the position of the femoral component was determined as the angle between the mechanical axis of the femur and the lateral side of the component. The tibial position in the frontal plane was determined as the angle between the mechanical axis of the tibia and the tibial component. In the sagittal plane, the femoral position was determined as the angle between the anatomical femoral axis and the central post of the component, and the tibial position as the angle between the posterior tibial cortex and the undersurface of the tibial component. The software used for measuring could measure within 0.1°. Deviations into varus, flexion and posterior slope are positive values, whereas deviations into valgus, extension and anterior slope are negative values. Outliers were described as out of the safe zone as described by the phase III Oxford Partial Knee manual, and can be found in Table 2 [6]. The long-leg standing X-rays were used to determine the hip–knee–ankle-axis (HKA-axis). This radiographic evaluation was performed twice by two independent examiners (AL and MK). The time between two readouts varied between 2 and 8 weeks. The intra- and inter-class correlation coefficients (ICCs) were calculated.
Radiologic evaluation of the components in the frontal plane. a Angle between the mechanical axis and the femoral component (varus/valgus). b Angle between the mechanical axis of the tibia and the undersurface of the tibial component (varus/valgus) [27]
Radiologic evaluation of the components in the lateral plane. c Angle between the anatomical femoral axis and the post of the component (flexion/extension). d Angle between the posterior tibial cortex and undersurface of the tibial component (posterior slope). [27]
Peri-operative data, including operative time and length of hospital stay were recorded.
The number of pre-operative changes in component size compared to the default plan was recorded. The number of peri-operative adjustments in component size compared to the surgeons’ pre-operative plan was also recorded. These changes were divided in 3 categories: identical size, deviation of 1 size, deviations of more than 1 size and whether up- or downsized.
Pre-operatively and at any follow-up appointment at 3, 12 and 24 months, patients were asked to complete four PROMs prior to consultation. The Oxford Knee Score (OKS) is a 12-item patient-reported outcome measure that assesses pain, function activities of daily living and knee-stability after knee arthroplasty and scores 12–60; 12 being the best outcome [17]. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a self-administered questionnaire for people with osteoarthritis of the knee and hip, divided into three subscales: pain, stiffness and functional limitation. It scores from 0 to 100; 100 being the best outcome [5]. The EuroQuol-5D Visual Analogue Scale (EQ-5D-VAS) assesses overall health status using a visual analogue scale from 0 to 100; 100 being the best outcome and comprises 5 dimensions; mobility, self-care, usual activities, pain/discomfort and anxiety/depression [15]. The Numeric Rating Scale for pain (NRS-pain) scores 0–10; 10 being the most pain the patient can imagine [25]. The Forgotten Joint Score (FJS) was completed at 24-month follow-up [34]. The FJS assesses the patients’ ability to forget their artificial joint in daily life. It consists of 12 questions and the score ranges from 0 to 100; 100 being the best outcome [34].
AEs were registered during the follow-up period of 2 years and were retrieved from the digital patient files.
This study was approved by the ethical committee (Zuyd Heerlen, the Netherlands; IRBNr. 12T92) and registered online at the Dutch Trial Register (www.trialregister.nl, Nr. NTR4278).
Statistical analysis
All statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, Illinois). This study was powered on two primary endpoints. One being the difference in Oxford Knee Score (OKS) at 2-year follow-up. The other being the difference in percentage of outliers in alignment in the frontal position of the femoral and tibial component at 6 weeks post-operative, as measured on fluoroscopically checked X-rays. Based on the former (OKS), 110 patients should be included [26, 29]. For the radiological outcome, 22 patients would suffice to generate enough power for the alignment endpoint. In anticipation of possible loss-to-follow-up, 120 patients were included in this study. The Shapiro–Wilk test showed that the data were normally distributed. Chi-square test was used to examine differences in proportions. Student’s t tests were performed on significant interactions. Intra- and inter-class correlation coefficients (ICCs) with 95% confidence intervals were calculated to check for intra- and inter-observer reliability (ICC 0.5–0.75 is considered to be moderate, ICC 0.75–0.9 is considered to be good and ICC ≥ 0.9 is considered to be excellent) [23]. A threshold for all statistical comparisons of p value ≤ 0.05 was considered to be statistically significant. Data are presented as means with standard deviations (SD), or frequencies (%).
Results
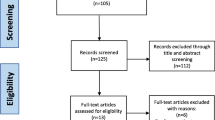
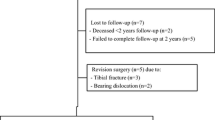
Figure 3 shows a diagram of the number of patients enrolled and analysed in the study during the 2-year follow-up period.
Baseline and operative data can be found in Table 1.
The inter-class coefficient between both investigators for X-ray measurements was 0.668 for the A-angle, 0.898 for the C-angle, 0.700 for the B-angle and 0.733 for the D-angle. The mean pre-operative HKA-axis in the PSI group was 6.0 (2.8) and in the CI group 6.2 (3.1) (p = n.s.). The mean post-operative HKA-axis was 5.2 (3.0) in the PSI group and 5.1 (3.2) in the CI group (p = n.s.).
Alignment of the femoral and tibial components and outliers can be found in Table 2.
Table 3 shows the number and percentage of identical sized in approved templates (surgeon) and agreement between the surgeon and technician.
There were no statically significant differences in the outcome of the PROMs between both the groups. PROMs are summarised in Table 4.
There were no statistically significant differences in the total rate of AEs (p < 0.05) between both groups. The AEs and their percentages in the total population can be found in Table 5.
Discussion
The most important finding of this study was a statistically significant higher accuracy (according to the safe zones as described in the phase III Oxford Partial Knee Manual [6]) of the femoral component positioning on the post-operative X-rays in the frontal plane using CI compared to PSI while peri-operative data (OR time and length of hospital stay), functional outcome (PROMs) and rate of AEs were comparable. The femoral component showed fewer outliers than the tibial component, which is in line with prior research [24]. The mean varus/valgus angle was statistically differently closer to the desired angle of zero degrees according to the phase III Oxford Partial Knee Manual [6] in of CI UKA. However, as there is only one outlier in the PSI group, the clinical relevance is probably rather low. There is no clinical evidence showing that the safe zones for the femoral component as prescribed by the Oxford Guidelines would be too large as there is for the tibial component [10, 18, 36]. The radiological outcome in the frontal plane of the tibial component showed the largest difference in the number of outliers in favour of the PSI group according to the phase III Oxford Partial Knee Manual [6]. This difference did not reach significance. This study showed no difference in posterior slope in favour of CI like a study by Alvand et al. did [1]. Modifications to prevent deep tibial cuts were made to the PSI algorithm in the meantime, which can be an explanation for this [1]. The alignment of the tibial component is associated with prosthesis survival as malpositioning increases the risk of component migration and loosening [10, 18, 19, 36]. The Oxford Guidelines, describe rather large ‘safe zones’, whilst some research suggests as little as 3° deviation in the frontal plane is correlated with decreased prosthesis survival [10, 18, 36]. Due to radiation exposure, a standard post-operative CT-scan was not performed. Since there is only limited literature available on this particular subject, efforts should be made in the future to investigate this as a recent study by Kamenaga et al. showed an important role of tibial component rotation in short-term clinical outcome [20]. The mean post-operative HKA-axis has a slight degree of varus, which in line with previously recommended range for improving survivorship [4].
In this study, no statistically significant differences could be observed in OR time, which is in line with prior research on this topic [27]. A possible explanation for this is that the Microplasty instrumentation is already highly efficient.
Several other studies showed that the accuracy in predicting the implanted femoral component size was higher than the tibial component size. [1, 4, 16, 27, 33]. In the present study, the tibial component was downsized in the majority of cases. A possible explanation for this is that attempts not to injure the anterior cruciate ligament (ACL) could result in unexpected changes in the tibial tray surface and therefore changes in component size [13]. If the pre-operative plan would consider the position of the ACL, this could theoretically improve the accuracy. As MRI scanning is used in the pre-operative plan, this might be a possibility. Another reason for the downsizing of the tibial component is most likely that after the removal of osteophytes, the tibial component can be downsized. In our experience, the size of these osteophytes is difficult to determine on the pre-operative MRI scan and to avoid suboptimal bone coverage the planning software has a tendency to overestimate the size of the tibial component.
A previous study by this research group showed that the planning, approved by the surgeon, was more accurate than the default planning [27]. In this study, the difference between default and approved planned is less outspoken. In general, further improvements to the accuracy of predicting the tibial component size are necessary to reduce hospital stock, and therefore, costs to improve operating room efficacy and fine-tune hospital stock [31]. The present findings open an interesting discussion on the cost-effectiveness of PSI in UKA. Only one study mentions an estimated rise in costs of €1200 per patient when using PSI [33].
The functional outcomes were comparable between both groups. However, they show a ceiling effect after 3 months post-surgery. This is a well-known limitation of certain PROMs [14] and opens an interesting discussion about the future of performance-based measurements to assess the functional outcome after arthroplasty [7].
The rate of AEs was comparable between both groups and the nature of the AEs was comparable to current literature. Chronic persisting knee pain is a known complication of UKA and literature suggests it is the cause of 8% of early failures. Bearing dislocation is another well-known complication of UKA. Literature reports an incidence between 2 and 3% [12]. The previous study of this research group reported a high rate of tibial fractures, probably caused by the transition from cemented to uncemented UKA. The present study supports this assumption as there was only one tibial plateau fracture [27].
There are several limitations to this study. First, the post-operative alignment was assessed on radiographs and not on CT-scan as routine CT-scan post-surgery is not common practice at the participating centres due to radiation exposure. Therefore, the rotational angle could not be determined. Furthermore, a part of the post-operative radiographs was suboptimal, adequate measurement could not be performed on these X-rays. The time between two readouts varied between 2 and 8 weeks, and therefore, memory confinement could not be ruled out. Secondly, all patients were operated by two surgeons who have extensive experience with UKA and PSI. Therefore, no statements can be made about the outcome of PSI in UKA when used by surgeons with less experience in UKA surgery. Third, as this study has a follow-up of 2 years, no conclusions can be drawn yet about the long-term follow-up.
Conclusions
The PSI method did not show an advantage over CI in regard of positioning of the components, nor did it show an improvement in clinical or functional outcome. We conclude that the possible advantages of PSI do not outweigh the costs of the MRI scan and the manufacturing of the PSI.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- AE:
-
Adverse event
- AP:
-
Anterior–posterior
- BMI:
-
Body mass index
- CI:
-
Conventional instrumented
- CT:
-
Computed tomography
- EQ-5D-VAS:
-
EuroQuol-5D Visual Analogue Scale
- FJS:
-
Forgotten Joint Score
- HKA-axis:
-
Hip–knee–ankle-axis
- ICD:
-
Implantable cardioverter-defibrillator
- MRI:
-
Magnetic resonance imaging
- NRS-pain:
-
Numeric Rating Scale for pain
- OKS:
-
Oxford Knee Score
- PROMs:
-
Patient-reported outcome measures
- PSI:
-
Patient-specific instrumentation
- RCT:
-
Randomised controlled trials
- SD:
-
Standard deviation
- TKA:
-
Total knee arthroplasty
- UKA:
-
Unicompartmental knee arthroplasty
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
References
Alvand A, Khan T, Jenkins C, Rees JL, Jackson WF, Dodd CAF, Murray DW, Price AJ (2018) The impact of patient-specific instrumentation on unicompartmental knee arthroplasty: a prospective randomised controlled study. Knee Surg Relat Res 26:1662–1670
Badawy M, Espehaug B, Indrekvam K, Havelin L, Furnes O (2014) Higher revision risk for unicompartmental knee arthroplasty in low-volume hospitals: data from 5791 cases in the Norwegian Arthroplasty Register. Acta Orthop 85(4):342–347
Beard DJ, Davies LJ, Cook JA, MacLennan G, Price A, Kent S, Hudson J, Carr A, Leal J, Campbell H, Fitzpatrick R, Arden N, Murray D, Campbell MK (2019) The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5 year outcomes of a rondomised controlled trial. Lancet 394(10200):746–756
Bell SW, Stoddard J, Bennett C, London NJ (2014) Accuracy and early outcomes in medial unicompartmental knee arthroplasty performed using patient specific instrumentation. Knee 21(S1):S33–S36
Bellamy N, Burchanan WW, Goldsmith CH et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Biomet. Oxford partial knee—microplasty instrumentation: surgical technique (2013). http://www.zimmerbiomet.com/content/dam/zimmer-biomet/medical-professionals/000-surgical-techniques/knee/oxford-partial-knee-microplasty-instrumentation-surgical-technique.pdf. Accessed 15 Oct 2020
Bolink SAAN, Grimm B, Heyligers IC (2015) Patient-reported outcome measures versus inertial performance-based outcome measures: a prospective study in patients undergoing primary total knee arthroplasty. Knee 22(6):618–623
Boonen B, Schotanus MG, Kort NP (2012) Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop 83:387–393
Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C (2013) A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J 95-B(3):354–359
Chatellard R, Sauleau V, Colmar M, Robert H, Raynaud G, Brilhault J (2013) Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res 99(4 SUPPL):S219–S225
Chotanaphuti T, Wangwittayakul V, Khuangsirikul S, Foojareonyos T (2014) The accuracy of component alignment in custom cutting blocks compared with conventional total knee arthroplasty instrumentation: prospective control trial. Knee 21(1):185–188
Crawford DA, Berend KR, Thienpont E (2020) Unicompartmental knee arthroplasty: US and global perspective. Orthop Clin North Am 51(2):147–159
Dao Trong ML, Diezi C, Goerres G, Helmy N (2014) Improved positioning of the tibial component in unicompartmental knee arthroplasty with patient-specific cutting blocks. Knee Surg Sports Traumatol Arthrosc 23:1993–1998
Eckhard L, Munir S, Wood D, Talbot S, Brighton R, Walter B, Baré J (2020) The ceiling effects of patient reported outcome measures for total knee arthroplasty. Orthop Trauma Surg Res. https://doi.org/10.1016/j.otsr.2020.102758
EuroQuol Group (1990) EuroQuol—a new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208
Flury A, Hasler J, Dimitriou D, Antoniadis A, Finsterwald M, Helmy N (2019) Midterm clinical and radiographic outcomes of 115 consecutive patient-specific unicompartmental knee arthroplasties. Knee 26(4):889–896
Haverkamp D, Breugem SJ, Sierevelt IN et al (2005) Translation and validation of the Dutch version of the Oxford 12-item knee questionnaire for knee arthroplasty. Acta Orthop 76:347–352
Jones GG, Logishetty K, Clarke S, Collins R, Jaere M, Harris S, Cobb JP (2018) Do patient-specific instruments (PSI) for UKA allow non-expert surgeons to achieve the same saw cut accuracy as expert surgeons? Arch OrthopTrauma Surg 138:1601–1608
Kalache H, Müller JH, Saffarini M, Gancel E (2020) Patient-specific instrumentation does not improve tibial component coronal alignment for medial UKA compared to conventional instrumentation. J Exp Orthop 7:42
Kamenaga T, Hiranaka T, Kikuchi K, Hida Y, Fuijshiro T, Okamoto K (2018) Influence of tibial component rotation on short-term clinical outcomes in Oxford mobile-bearing unicompartmental knee arthroplasty. Knee 25(6):1222–1230
Kerens B, Leenders AM, Schotanus MGM, Boonen B, Tuinebreijer WE, Emans PJ, Jong B, Kort NP (2017) Patient-specific instrumentation in Oxford unicompartmental knee arthroplasty is reliable and accurate except for the tibial rotation. Knee Surg Sports Traumatol Arthrosc 26(6):1823–1830
Kohn MD, Sassoon AA, Fernando ND (2016) Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop 474(8):1886–1893
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Kort NP, Bemelmans YFL, Schotanus MGM (2017) Outpatient surgery for unicompartmental knee arthroplasty is effective and safe. Knee Surg Sports Traumatol Arthrosc 25(9):2659–2667
McCormack HM, Horne DJ, Sheather S (1988) Clinical applications of visual analogue scales: a critical review. Psychol Med 18:1007–1101
Murray DW, Fitzpatrick RK, Pandit H, Beard DJ, Carr AJ, Dawson J (2007) The use of the Oxford hip and knee scores. J Bone Joint Surg Br 89(8):1010–1014
Leenders AM, Schotanus MGM, Wind RJP, Borghans RAP, Kort NP (2018) A high rate of tibial plateau fractures after early experience with patient-specific instrumentation for unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Artrosc 26(11):3491–3498
National Joint Registry for England, Wales and Northern Ireland (2016) 13th annual report
Pandit H, Jenkins C, Beard DJ, Gallagher J, Price AJ, Dodd CAF, Goodfellow JW, Murray DW (2009) Cementless Oxford unicompartmental knee replacement shows reduced radiolucencz at one year. J Bone Surg Br 91(2):185–189
Sanz-Ruiz P, Matas-Diez JA, Carbo-Laso E, Perez-Mañanes R, Vaquero-Martín J (2019) Patient-specific instrument can improve functional and radiographic results during learning curve for Oxford unicompartmental knee arthroplasty. J Knee Surg 32(2):180–185
Schotanus MGM, Schoenmakers DAL, Sollie R, Kort NP (2017) Patient-specific instruments for total knee arthroplasty can accurately predict the component size as used peroperative. Knee Surg Sports Traumatol Arthrosc 25(12):3844–3848
Schotanus MGM, Sollie R, van Haaren EH, Hendrickx RPM, Jansen EJP, Kort NP (2016) A radiological analysis of the difference between MRI- and CT-based patient-specific matched guides for total knee arthroplasty from the same manufacturer. Bone Joint J 98-B(6):786–792
Seeber GH, Kolbow K, Maus U, Kluge A, Lazovic D (2016) Medial unicompartmental knee arthroplasty using patient-specific instrumentation—accuracy of preoperative planning, time saving and cost efficiency. Z Orthop Unfall 154:287–293
Shadid MB, Vinken NS, Marting LN, Wolterbeek N (2016) The Dutch version of the Forgotten Joint Score: test-retesting reliability and validation. Acta Orthop Belg 82:112–118
Wilson HA, Middleton R, Abram SGF, Smith S, Alvand A, Jackson WF, Bottomley N, Hopewell S, Price AJ (2019) Patient relevant outcomes of unicompartmental versus total knee replacement: systematic review and meta-analysis. BMJ 364:1352
Zhang Q, Zhang Q, Guo W et al (2014) The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LCCUSUM). J Orthop Surg Res 9:81
Zimmer-biomet and materialise. SurgiCase knee planner: software user manual. https://www.zimmerbiomet.com/content/dam/zimmer-biomet/drive/User%20Manual%20SurgiCase%20Knee%20Planner.pdf Accessed 15.10.2020
Acknowledgements
The authors would like to thank Ms Margreet Boevé, from the Amphia Hospital for her assistance in the data gathering, Ms Madelon Knijf for her collaboration on the radiologic evaluation and Ms Sharmila Thompson-Venkatesan, MD, and Mr Jan Truijen, MD, who served as external readers, and for their comments that greatly improved the manuscript.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
AL gathered the data, wrote the initial draft of the manuscript and managed the study. MS designed the study and analysed the data, and revised the manuscript. JM analysed the data and revised the manuscript. NK, RG and BK conceived the study and revised the manuscript. KK and BB were involved in the drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
NK is a paid consultant for BodyCad, Bioventus, Stryker, Supportho Medical and Zimmer-Biomet. RG is a paid consultant for Zimmer-Biomet. The research foundation of the Amphia Hospital (RG and KK) receives funding from Zimmer-Biomet, Stryker and Mathys.
Ethical approval
This study was approved by the Independent Review Board (IRB, Zuyd Heerlen, the Netherlands; IRBNr. 12T92) and registered online at the Dutch Trial Register (www.trialregister.nl, Nr. NTR4278).
Informed consent
An informed consent was signed by all patients who were enrolled in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leenders, A.M., Kort, N.P., Koenraadt, K.L.M. et al. Patient-specific instruments do not show advantage over conventional instruments in unicompartmental knee arthroplasty at 2 year follow-up: a prospective, two-centre, randomised, double-blind, controlled trial. Knee Surg Sports Traumatol Arthrosc 30, 918–927 (2022). https://doi.org/10.1007/s00167-021-06471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06471-5