Abstract
Purpose
The purpose of the present study was to determine how the medial structures and ACL contribute to restraining anteromedial instability of the knee.
Methods
Twenty-eight paired, fresh-frozen human cadaveric knees were tested in a six-degree of freedom robotic setup. After sequentially cutting the dMCL, sMCL, POL and ACL in four different cutting orders, the following simulated clinical laxity tests were applied at 0°, 30°, 60° and 90° of knee flexion: 4 Nm external tibial rotation (ER), 4 Nm internal tibial rotation (IR), 8 Nm valgus rotation (VR) and anteromedial rotation (AMR)—combined 89 N anterior tibial translation and 4 Nm ER. Knee kinematics were recorded in the intact state and after each cut using an optical tracking system. Differences in medial compartment translation (AMT) and tibial rotation (AMR, ER, IR, VR) from the intact state were then analyzed.
Results
The sMCL was the most important restraint to AMR, ER and VR at all flexion angles. Release of the proximal tibial attachment of the sMCL caused no significant increase in laxity if the distal sMCL attachment remained intact. The dMCL was a minor restraint to AMT and ER. The POL controlled IR and was a minor restraint to AMT and ER near extension. The ACL contributed with the sMCL in restraining AMT and was a secondary restraint to ER and VR in the MCL deficient knee.
Conclusion
The sMCL appears to be the most important restraint to anteromedial instability; the dMCL and POL play more minor roles. Based on the present data a new classification of anteromedial instability is proposed, which may support clinical examination and treatment decision. In higher grades of anteromedial instability an injury to the sMCL should be suspected and addressed if treated surgically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Injury to the anterior cruciate ligament (ACL) and capsuloligamentous structures on the medial side of the knee occurs frequently [22, 42]. Persistent laxity of the medial structures can result in increased load in the ACL [4, 24, 26] and following ACL reconstruction, residual medial laxity may ultimately result in early ACL graft failure [1, 8, 39, 44].
Several studies have examined the roles of the superficial medial collateral ligament (sMCL), deep MCL (dMCL) and posterior oblique ligament (POL) in restraining external, internal and valgus rotations of the knee [9, 10, 18, 31, 33, 40]. However, studies on how the ACL and the ligamentous structures of the medial aspect of the knee interact to restrain tibio-femoral laxity, such as “antero-medial rotatory instability” (AMRI), remain scarce [11, 31, 43]. Slocum and Larson [37] reported that rupture of the middle third of the medial capsular ligament, now generally described as the dMCL, was the basic lesion permitting AMRI that was further increased following successive rupture of the sMCL and ACL. Recently, an isolated reefing of the posteromedial capsule has been reported to control AMRI [30]. This surgical approach is based on the findings by Hughston et al. [12,13,14] who highlighted that the POL is the key structure to restore medial stability.
For clinical examination and further treatment it is important to understand the damage that has occurred in an injured knee and its effect on the tibio-femoral laxity. The anteromedial drawer test (applying an anterior drawer force to the tibia with the foot held in external rotation) has been proposed for the detection of AMRI [7, 28, 37, 42, 43]. However, the absence of a biomechanical validation [36] and lack of consensus in the literature as to its primary and secondary restraints [7, 11, 13, 28, 37, 42, 43] has meant that the interpretation of examination findings remains challenging.
The purpose of this study was to not only evaluate the individual contributions of the medial ligamentous structures and ACL to restraining simple, uniplanar rotations (external, internal and valgus rotation), but also to restraining a combined external rotation and anterior translation force, simulating an anteromedial drawer test. It was hypothesized that the sMCL is the most important restraint to anteromedial instability.
Materials and methods
Specimen preparation
Thirty paired cadaveric knees were dissected and tested under permission of the “Gesetz über das Leichen-, Bestattungs- und Friedhofswesen (Bestattungsgesetz) des Landes Schleswig–Holstein vom 04.02.2005, Abschnitt II, §9 (Leichenöffnung, anatomisch)”. Two specimens were used to develop the sectioning and robotic testing protocol. The remaining 28 paired specimens (10 female, 4 male) with a median age of 81 years (range 60–103 years) were stored at − 20 °C and thawed at room temperature for 24 h prior to testing. The femur and tibia were cut 200 mm from the joint line and prepared by removing the skin and subcutaneous fat, while leaving the remaining muscles and fascia intact. A modified transpatellar approach [27] was made to access the intercondylar notch to allow sectioning of the ACL and to evaluate the knee for any exclusion critera, including signs of prior surgery, severe osteoarthritic changes, or ligamentous injuries. The patella was predrilled, from medial to lateral, with a 2.7-mm diameter drill, before it was split longitudinally in the midline, with a fine oscillating saw. The split was completed with a scalpel and extended distally into the patellar tendon. To improve visualization of the ACL, the patellar fat pad was partially resected. The patella was fixed again with two 4.0-mm cancellous screws. Previous studies have shown that the transpatellar approach and re-fixation does not change patellofemoral kinematics and length-change patterns of peripatellar retinacula [27].
The cut ends of the femur and tibia were then secured in aluminum tubes using polymethyl methacrylate bone cement. The tibia was centralized in the tube to standardize rotational effects, with the tube axis in line with the condylar eminence at the center of the tibial plateau [31].
Testing setup
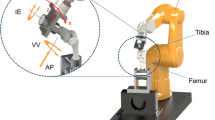
The biomechanical testing platform (Fig. 1), which has previously been described [16], was a 6° of freedom (DOF) industrial robot (6DOF; KR 125; KUKA Robotics, 86165 Augsburg, Germany) and a force/moment sensor (UFS; FTI Theta 1500–240; Schunk, Lauffen, Germany). The robot had a repeatability of ± 0.2 mm and maximum load capacity of 2450 N. The UFS`s resolution was 0.25 N and 0.05 Nm for forces and torques, respectively.
An optical tracking setup (Optotrak Certus Motion Capture, Northern Digital, Ontario, Canada) with an accuracy of up to 0.1 mm and resolution of 0.01 mm was used to track knee kinematics via rigid bodies fixed to the tibia and femur using custom made nails. Six tibial (medial/lateral tibial shaft, and medial/lateral/anterior/posterior tibial plateau) and four femoral landmarks (medial/lateral femoral shaft, and medial/lateral femoral epicondyle) were digitized using a digitizing probe to define the knee coordinate system. Visual 3D (C-Motion Inc, Germantown, Maryland) created a segment coordinate system of the tibia using a Cardan sequence x–y–z. The digitized landmarks served to create a static and moving knee model at each tested flexion angle. This allowed 6° of freedom kinematics to be computed in terms of rotations and translations in respect to the static tibia model at the knee’s neutral starting position. The difference in translation (mm) or rotation (degree) between the static and moving model at the end of each simulated laxity test was calculated.
Biomechanical testing
Each knee specimen was flexed and extended ten times to minimize tissue hysteresis before it was mounted upside-down into the robot. The tibia was secured to the manipulator of the robot, while the femur was fixed into the stationary base unit. To find the knee´s neutral starting position, the forces (< 1 N) and moments (< 0.5 Nm) were minimized at full extension and the passive flexion–extension path was determined for each knee from 0° to 90° in 1° increments by the robotic system. The position of the knee at each flexion angle was recorded.
Tibiofemoral joint laxity for each testing state was measured with simulated knee laxity tests applied to the knee at 0°, 30°, 60° and 90° of flexion. The motion path of the simulated laxity tests in the intact state was recorded. After each resection/cut was performed, simulated laxity tests were repeated three times for each cutting state and the mean data of each test taken. External rotation (ER) and internal rotation (IR) laxity was measured in response to the application of a 4 Nm rotation torque. Valgus laxity was measured in response to an 8 Nm valgus rotation torque (VR). To simulate an anteromedial drawer test, a coupled anterior tibial drawer force (89 N) and ER torque (4 Nm) was applied simultaneously [16]. To clarify motion of the medial tibial plateau in response to this test, we distinguished between anteromedial translation (AMT), defined as the maximum anterior translation of the midpoint of the medial tibial plateau, and anteromedial rotation (AMR), defined as the maximum ER occurring around the longitudinal axis of the tibia [16, 43]. The midpoint of the medial tibial plateau was determined as 25% of the distance between the most medial digitized point and the most lateral digitized point on the tibial plateau.
Tissue resection and cutting order
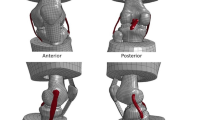
Following analysis of the knee in its intact state, the medial structures and ACL were cut sequentially whilst the knee remained mounted in the robotic testing rig (Fig. 2).
Medial aspect of a right knee: the femur extends proximally to the right, and the tibia extends distally to the left with the patella pointing toward the superior left corner. Shown after removal of the fascial layer, leaving the sMCL and POL exposed. The dMCL (1) was cut transversely at the midsubstance of its meniscofemoral and meniscotibial divisions deep to the sMCL. The approach was performed via a longitudinal incision of the anteromedial fascia anterior to the sMCL as previously shown by Robinson et al. [33]. The proximal (2a) and distal (2b) tibial attachments of the sMCL were identified and divided alternately as previously described by Griffith et al. [9]. The POL (3) was completely dissected including the superficial, central and capsular arm. (4) The ACL was resected via a transpatellar approach [27]
Four protocols (Fig. 3) were used to allow the identification of the individual roles these structures have. Each knee of a pair was allocated to a testing protocol in which the medial structures were cut first and subsequently the ACL (n = 14) or to a protocol in which the ACL was divided prior to the medial structures (n = 14). The data from one knee was lost due to a failed connection in the robotic setup, leaving 5 knees for analysis in protocol 3.
The medial structures: dMCL (deep medial collateral ligament), sMCL (superficial medial collateral ligament) and POL (posterior oblique ligament) and the ACL were divided according to four cutting protocols. prox/dist the proximal and distal tibial attachment of the sMCL were cut in an alternating order, n number of specimens
In all protocols the dMCL was sectioned before cutting the sMCL. Previous studies have shown that the shorter, weaker fibres of the dMCL [32, 41] suffer a greater tensile strain (extension as a proportion of original length) than do the longer sMCL fibres for the same bone-to-bone separation, causing the dMCL to fail at significantly less elongation than the sMCL. Thus, consistent with this differential strain behavior [2], isolated rupture of the dMCL may occur clinicaly whilst the sMCL remains intact but not vice versa [13, 31, 32]. The dMCL was approached via a longitudinal incision of the anteromedial fascia anterior to the sMCL as previously shown by Robinson et al. [33]. The dMCL is clearly devided from the sMCL at the joint line, which allowed an isolated cut of the meniscofemoral and meniscotibial divisions of the dMCL [18, 33].
The proximal and distal tibial attachments of the sMCL were identified and divided alternately, either proximal attachment or distal attachment first (Figs. 2, 3). Tibio-femoral laxity testing was undertaken in each state to determine the contribution of each attachment to stability, before completely resecting the sMCL at both femoral and tibial attachments [18]. The proximal tibial attachment to soft tissue around the anterior arm of the semimembranosus tendon [18] was divided by carefully introducing a scalpel between the longitudinal fibers of the sMCL and the dMCL at the level of the joint line. The distal tibial attachment was divided by sharp dissection of the sMCL fibres as they attached to bone.
The POL was carefully dissected from its attachment to the medial meniscus before it was completely resected from its femoral and tibial attachments. Its anterior margin was defined as the posterior border of the sMCL and included the superficial, central and capsular arm (Fig. 2). The posterior margin of the POL was defined at the posterior border of the capsular arm where it blended with the soft tissues over the medial gastrocnemius tendon [14, 18, 33].
The ACL was resected through the previously established transpatellar approach, while the knee remained mounted in the robotic testing rig at 90° of flexion [27]. The patella was re-fixed prior to testing.
Statistical analysis
The kinematic data for each tested state, load and flexion angle were analyzed using a two-factor repeated-measures analysis of variance (ANOVA) and post-hoc Bonferroni corrections for multiple comparisons. The two independent factors were the state of the knee joint with its respective cutting condition (5 states) and the knee flexion angle (4 angles) within the same specimen. The dependent variables were the resulting angles (ER/AMR, IR, VR) and medial tibial translation distances (AMT). One-tailed paired Student t tests were used to analyze the specific effect of cutting the proximal and distal division of the sMCL in group 1 and 2. This was performed using SPSS version 24 (IBM Corp.), with significance level set to 0.05.
A power analysis (G*Power 3.1; α = 0.5) was conducted based on prior work on medial knee laxity, which found statistically significant results with less than six knee specimens [31]. An expected moderate/high effect size (f = 0.5/f = 0.6) determined a sample size minimum of five specimens in each cutting protocol with power of 0.80.
Results
Detailed results are available as supplemental material including a comprehensive table (Appendix 1).
Laxity with anteromedial drawer testing—coupled anterior tibial drawer (89 N) and ER torque (4 Nm)
Anteromedial translation (AMT)
With the ACL intact (Fig. 4a) cutting the dMCL at 0°, 30° and 60°of flexion produced a small but significant increase in AMT (p < 0.05). Further cutting of the sMCL caused a larger increase in AMT at all angles of knee flexion (p < 0.05). This effect was seen with releasing the distal rather than proximal tibial attachment of the sMCL. Cutting of the proximal sMCL tibial attachment in the dMCL deficient knee produced no significant increase in AMT (0.1 mm ± 0.1 mm at 0° flexion to 0.2 mm ± 0.1 mm at 90° flexion) when the distal attachment remained intact.
Anteromedial translation, i.e., anterior translation of the medial tibial plateau, during a combined 89 N anterior tibial load (ATT) and 4 Nm external rotation torque (ER) with the knee intact, and the knee sectioned according to group 1 (a) and group 4 (b). Statistically significant differences compared to the previous states are indicated (*p < 0.05, **p < 0.01, ***p < 0.001). Error bars indicate the standard error of the mean
In ACL deficient knees (Fig. 4b), cutting the dMCL produced small increases in AMT at all flexion angles (p < 0.05). A larger increase in AMT was seen with further sectioning of the sMCL (p < 0.05). Subsequent devision of the POL yielded a further increase in AMT predominantly near extension (p < 0.05).
Anteromedial rotation (AMR)
AMR in response to a combined anterior tibial drawer force and ER torque is shown in Fig. 5 With the ACL intact (Fig. 5a), cutting the dMCL produced a small (< 2°) increase in AMR at 0° and 30° flexion with no effect found at higher flexion angles. With the knee in extension, further cutting of the sMCL and then the POL produced small increases in AMR. At higher flexion angles, cutting the sMCL increased AMR at 30°, 60° and 90° of flexion (p < 0.01). This increase was observed after cutting the distal rather than the proximal sMCL tibial attachment. Cutting the proximal tibial sMCL attachment in the dMCL deficient knee produced no significant effect on AMR at any flexion angle (0.1° ± 0.2° at 0° flexion to 0.3° ± 0.2° at 90° flexion).
Anteromedial rotation, i.e., tibial external rotation, during a combined 89 N anterior tibial load (ATT) and 4 Nm external rotation torque (ER) with the knee intact, and the knee sectioned according to group 1 (a) and group 4 (b). Statistically significant differences compared to the previous states are indicated (*p < 0.05, ** p < 0.01). Error bars indicate the standard error of the mean
At 0° and 30°, isolated cutting of the ACL (Fig. 5b) decreased AMR (p < 0.05) compared to the intact state. Further release of the POL and dMCL produced no significant effect at any angle of knee flexion. However, further release of the sMCL produced a large increase in AMR (p < 0.05).
Laxity with uniplanar rotations
External rotation
Tibial ER increased from the intact state following cutting the dMCL at all flexion angles (p < 0.05) (Fig. 6a). A larger increase in laxity was noted with subsequent cutting of the sMCL (p < 0.01). Sectioning of the distal tibial sMCL attachment significantly increased ER from 1.5° ± 0.5° at 0° flexion to 4.7° ± 1.7° at 90° flexion (p < 0.05). Whereas cutting the proximal tibial attachment of the sMCL in the dMCL deficient knee caused no significant increase in ER (0.2° ± 0.1° at 0° flexion to 0.2° ± 0.2° at 90° flexion). Further results are sown in Fig. 6b and in the supplemental material.
Knee angulation during a 4 Nm external rotation torque (ER) with the knee intact, and the knee sectioned according to group 1 (a) and group 4 (b). Statistically significant differences compared to the previous states are indicated (*p < 0.05, **p < 0.01). Error bars indicate the standard error of the mean
Internal rotation
Cutting the dMCL resulted in a small increase in IR laxity from the intact state at 30°, 60° and 90° (p < 0.05) (Fig. 7a). Further cutting of the sMCL caused a further small increase in IR at all angles of knee flexion (p < 0.05). A marked increase was then noted with further sectioning of the POL at 0° and 30° (p < 0.01). Further results are sown in Fig. 7b and in the supplemental material.
Knee angulation during a 4 Nm internal rotation torque (ER) with the knee intact, and the knee sectioned according to group 1 (a) and group 2 (b). Statistically significant differences compared to the previous states are indicated (*p < 0.05, **p < 0.01, ***p < 0.001). Error bars indicate the standard error of the mean
Valgus rotation
Cutting the dMCL in intact knees produced no effect on valgus laxity at any angle of knee flexion (Fig. 8a). Further cutting of the sMCL increased valgus rotation at 30°, 60° and 90° (p < 0.05). This effect occurred as a result of sectioning the distal tibial sMCL attachment. Sectioning of the proximal tibial sMCL attachment caused less than 0.3 ± 0.1° increase in VR at any flexion angle (p < 0.05 at 0° and 60°) if the distal tibial sMCL attachment remained intact. Cutting the distal tibial attachment first caused an increase in valgus laxity ranging from 1.3 ± 0.5° at 0° to 3.5 ± 1.4° at 60° (p < 0.05). Further cutting of the POL produced increased valgus laxity at 0°, 30° and 60° (p < 0.05). Final sectioning of the ACL produced a further large, statistically significant effect on valgus laxity at all angles of flexion (p < 0.05).
Knee angulation during a 8 Nm valgus rotation torque (ER) with the knee intact, and the knee sectioned according to group 1 (a) and group 4 (b). Statistically significant differences compared to the previous states are indicated (*p < 0.05, **p < 0.01). Error bars indicate the standard error of the mean
In the ACL deficient knee, cutting the POL produced no effect on valgus laxity (Fig. 8b). A dramatic increase in valgus laxity occurred at all angles of knee flexion with final sectioning of the sMCL (p < 0.01).
Discussion
The main finding of this study was that the sMCL is the most important medial capsuloligamentous structure restraining anteromedial rotatory instability of the knee. Sectioning the sMCL always had a larger effect on combined anterior translation of the medial plateau (AMT) and external tibial rotation (AMR) than sectioning the POL or dMCL. In agreement with the previous literature the sMCL was also the primary medial restraint to uniplanar valgus rotation and tibial ER [9,10,11, 31].
It is well understood that isolated ACL rupture shifts the rotational axis of the tibia medially allowing the lateral tibial plateau to subluxate anteriorly [3, 21, 25]. This is typically coupled with an increase in IR laxity near extension as shown by the present data and others [11, 17, 21, 25]. Conversely, concomitant injury to the ACL and medial capsuloligamentous structures moves the axis of rotation laterally, such that knee pivots about the lateral capsuloligamentous structures [37]. This allows the medial tibial plateau to subluxate anteriorly accompanied when a uniplanar ER torque is applied to the tibia [11, 37, 40, 43]. The present data showed marked increases in AMR and AMT in response to a combined external rotation torque and anterior drawer force with the sMCL being the key restraining structure.
In the current study cutting the proximal tibial attachment of the sMCL produced no significant effect on AMT, AMR, ER or VR, whilst the distal tibial insertion remained intact. This is contrary to the findings of Griffith et al. [9], who reported that the proximal insertion of the sMCL is the primary valgus stabilizer. However, this finding is most likely due to the cutting order used in their study: the distal insertion was always cut first. A subsequent study by Griffith et al. [10] used buckle transducers to measure force in the MCL and found significant higher loads on the distal division of the sMCL in response to a valgus torque.
Haimes et al. [11] also reported the motion limits measured in human cadaveric knees after sectioning the sMCL, POL and ACL while the dMCL was left intact. The authors found that cutting the sMCL increased uniplanar external rotation laxity independent of whether the ACL was intact or sectioned. This is contrary to Slocum and Larson [37], who observed that rupture of the medial capsular ligament was the basic lesion permitting abnormal external rotation of the tibia. Kennedy and Fowler [15] also noted that the dMCL failed before the sMCL with abnormal valgus and/or external rotation injury, yet Warren et al. [40] found that cutting the dMCL in an otherwise intact knee had a "minimum effect on restraining rotation between the femur and the tibia". The present findings were similar with isolated sectioning of the dMCL resulting in only small increase in ER (< 2° at all angles tested). Much larger increases in ER laxity were seen after additional cutting the sMCL. The findings that cutting the dMCL appears to have little effect on rotatory laxity but that the structure ruptures with excessive external rotation are not counter intuitive and are consistent with previous understanding of differential strain behavior [2]: the shorter, weaker fibers of the dMCL [32, 41] fail at less elongation than the sMCL, resulting in isolated dMCL rupture for the same bone to bone displacement [13, 31, 32]. In some cases isolated injury to the dMCL cause persistant symptoms and may require surgical repair as suggested by Narvani et al. [29]. In fact, the deep portion of the MCL is not addressed in current anatomic reconstruction techniques [7, 8, 19, 20].
Wijdicks et al. [43] measured knee kinematics in response to a simulated anteromedial drawer test and also found the sMCL to be an important restrain. However, they sectioned only the sMCL whilst leaving the dMCL, POL and ACL intact. In comparison to the current study the authors found less anterior tibial translation, and higher AMR and ER, reporting 11.9 ± 3.2° AMR and 3.7 ± 1.9 AMT compared to 7.2 ± 1.7° AMR and 6.0 ± 1.8 mm AMT following sectioning the sMCL at 90° in the present data. These differences may be the result of the experimental setup: in the present study a lower rotational torque was applied (4 Nm versus 5 Nm), which may explain the reduced rotation values.
Although this may limit comparison with previous studies, repeated application of coupled anterior tibial drawer force and 5 Nm ER torques caused specimen failure during pilot testing. Therefore, reduced rotational torques of 4 Nm were utilized. This is consistent with other recent biomechanical studies [16]. The higher values of translation found in the present study are likely to be due to the fact that translation of the medial tibial plateau was measured whilst Wijdicks et al. [43] reported overall tibial translation. Additionally the dMCL was intact in their study.
Injury to the POL occurs when the knee is towards extension as the posteromedial capsule tightens [14, 33, 37] and is usually associated with injuries to the MCL or cruciate ligaments [23, 35]. In the present study, cutting the POL caused no increase in AMR as long as the sMCL remained intact. Therfore, the POL acted as a secondary restraint to anteromedial instability, contrary to earlier clinical observations that described repair of the POL as the key element in controlling AMRI [13, 14, 28]. However, according to the present data the POL was a major restraint to IR and a secondary restraint to VR near extension, which has been shown to provide load sharing to the ACL during the pivot-shift examination [34].
Combining the results of the present study with those of previous findings [5, 9, 11, 31, 43] the following for clinical examination of anteromedial instability is proposed (Table 1).
-
1.
The anteromedial drawer test (anterior drawer with the foot rotated externally) performed at 30° to 90° of flexion shows increased AMT (> 5 mm) and AMR (> 5°) when the dMCL and sMCL are deficient. An isolated medial injury to the dMCL allows a small increase in AMT (< 2 mm) and is most obvious in the ACL deficient knee (AMT > 5 mm).
-
2.
The external rotation dial test performed at 30° to 90° of flexion allows increased ER (> 5°) when the dMCL and sMCL are deficient. An isolated medial injury to the dMCL shows a small increase in ER (< 2°). The POL and ACL act as secondary restraints to ER.
-
3.
The internal rotation dial test performed in full extension allows increased IR (> 5°) when the POL and a concomitant structure (dMCL, sMCL, ACL) are insufficient.
-
4.
The valgus stress test performed at 30° of flexion shows increased valgus instability (> 3°) when the dMCL and sMCL are deficient. Concomitant insufficiency of the POL or ACL leads to further increase of laxity (> 5°) at 0° and 30° of flexion. Massive valgus laxity (> 10° at 0° of flexion) occurs when all the medial capsuloligamentous structures and ACL are insufficient.
Minor anteromedial instability, without any valgus instability, can only be observed with an isolated injury of the dMCL that was most obvious with a concomitant ACL lesion. Higher grade instabilities always involved a valgus instability. According to these findings an easy to use anteromedial grading system (Table 1) would be: grade 1: “isolated” AMT (< 10 mm) without valgus instability; grade 2: “moderate” AMT (< 10 mm) with valgus instability; grade 3: “severe” AMT (> 10 mm) with valgus instability.
Some inherent limitations in this study should be noted. The current results relate only to the passive stabilizing structures on the medial aspect of the knee. Recently, the semimembranosus muscle has been confirmed to be a major active restraint to anteromedial instability, especially in absence of the MCL and POL at higher flexion angles [16]. The posterior horn of the medial meniscus is another important structure acting as a “wheel brake” against anterior subluxation of the medial tibial plateau [28]. Thus medial meniscectomy or an isolated posteromedial ramp lesion, may have a role in anteromedial instability [6, 13, 28, 37, 38]. Furthermore, the present study's results were derived by cutting the medial structures and the ACL and may not reflect the findings that might be seen with partial ligament ruptures. Thus, the grading proposed may be applicable when structures are completely ruptured and further clinically validation is needed.
The present study used matched-pairs of specimens to help eliminate bias between specimens when testing the effect of cutting the medial structures in the ACL intact and ACL deficient states [43]. However, care must be taken when interpreting results from defined sequential cutting sequences. The conditions where each of the three individual medial structures acted alone or in combination with each of the others were not created; however, previous studies have reported that the dMCL fails before the sMCL [9, 11, 31, 43] and thus it was reasonable not to test an isolated cut of the sMCL in the dMCL intact knee. In addition, the effect of isolated dissection of the sMCL on simulated clinical laxity tests has been reported extensively [9, 11, 31, 43]. Another limitation might be that the analysis of the effect of cutting the proximal and distal tibial attachments of the sMCL was based on fewer specimens due to the alternating cutting sequence. However, the current results are consistent with previous studies that have shown higher loads at the distal attachment with valgus loading of the knee [9].
Conclusion
This study demonstrates the individual contributions of the medial capsuloligamentous structures and ACL in controlling anteromedial instability. The sMCL was found to be the key medial restraint to valgus rotation as well as anteromedial translation and external tibial rotation consitituting pathological laxity in anteromedial instability.
References
Ahn JH, Lee SH (2016) Risk factors for knee instability after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 24:2936–2942
Amis A (1985) Biomechanics of ligaments. In: Jenkins D (ed) Ligament injuries and their treatment. Chapman and Hall, London, pp 3–28
Amis AA, Bull AMJ, Lie DTT (2005) Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Oper Tech Orthop 15:29–35
Battaglia MJ, Lenhoff MW, Ehteshami JR, Lyman S, Provencher MT, Wickiewicz TL, Warren RF (2009) Medial collateral ligament injuries and subsequent load on the anterior cruciate ligament. Am J Sports Med 37:305–311
Cavaignac E, Carpentier K, Pailhé R, Luyckx T, Bellemans J (2015) The role of the deep medial collateral ligament in controlling rotational stability of the knee. Knee Surg Sports Traumatol Arthrosc 23:3101–3107
DePhillipo NN, Moatshe G, Brady A, Chahla J, Aman ZS, Dornan GJ, Nakama GY, Engebretsen L, LaPrade RF (2018) Effect of meniscocapsular and meniscotibial lesions in ACL-deficient and ACL-reconstructed knees: a biomechanical study. Am J Sports Med 46:2422–2431
Engebretsen L, Lind M (2015) Anteromedial rotatory laxity. Knee Surg Sports Traumatol Arthrosc 23:2797–2804
Funchal LFZ, Astur DC, Ortiz R, Cohen M (2019) The presence of the arthroscopic “floating meniscus” sign as an indicator for surgical intervention in patients with combined anterior cruciate ligament and grade II medial collateral ligament injury. Arthroscopy 35:930–937
Griffith CJ, LaPrade RF, Johansen S, Armitage B, Wijdicks C, Engebretsen L (2009) Medial knee injury: Part 1, static function of the individual components of the main medial knee structures. Am J Sport Med 37:1762–1770
Griffith CJ, Wijdicks CA, LaPrade RF, Armitage BM, Johansen S, Engebretsen L (2009) Force measurements on the posterior oblique ligament and superficial medial collateral ligament proximal and distal divisions to applied loads. Am J Sport Med 37:140–148
Haimes JL, Wroble RR, Grood ES, Noyes FR (1994) Role of the medial structures in the intact and anterior cruciate ligament-deficient knee. Am J Sports Med 22:402–409
Hughston JC, Andrews JR, Cross MJ, Moschi A (1976) Classification of knee ligament instabilities. Part I. The medical compartment and cruciate ligaments. J Bone Jt Surg Am 58:159–172
Hughston JC, Barrett GR (1983) Acute anteromedial rotatory instability. Long-term results of surgical repair. J Bone Jt Surg Am 65:145–153
Hughston JC, Eilers AF (1973) The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Jt Surg Am 55:923–940
Kennedy JC, Fowler PJ (1971) Medial and anterior instability of the knee. An anatomical and clinical study using stress machines. J Bone Jt Surg Am 53:1257–1270
Kittl C, Becker DK, Raschke MJ, Müller M, Wierer G, Domnick C, Glasbrenner J, Michel P, Herbort M (2019) Dynamic restraints of the medial side of the knee: the semimembranosus corner revisited. Am J Sports Med 47:863–869
Kittl C, El-Daou H, Athwal KK, Gupte CM, Weiler A, Williams A, Amis AA (2016) The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med 44:345–354
LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L (2007) The anatomy of the medial part of the knee. J Bone Jt Surg Am 89:2000–2010
Lee DW, Kim JG (2019) Anatomic medial complex reconstruction in serious medial knee instability results in excellent mid-term outcomes. Knee Surg Sports Traumatol Arthrosc 28:725–732
Lind M, Jacobsen K, Nielsen T (2019) Medial collateral ligament (MCL) reconstruction results in improved medial stability: results from the Danish knee ligament reconstruction registry (DKRR). Knee Surg Sports Traumatol Arthrosc 28:881–887
Lipke JM, Janecki CJ, Nelson CL, McLeod P, Thompson C, Thompson J, Haynes DW (1981) The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Jt Surg Am 63:954–960
Lundblad M, Hägglund M, Thomeé C, Hamrin Senorski E, Ekstrand J, Karlsson J, Waldén M (2019) Medial collateral ligament injuries of the knee in male professional football players: a prospective three-season study of 130 cases from the UEFA Elite Club Injury Study. Knee Surg Sports Traumatol Arthrosc 27:3692–3698
Lundquist RB, Matcuk GR, Schein AJ, Skalski MR, White EA, Forrester DM, Gottsegen CJ, Patel DB (2015) Posteromedial corner of the knee: the neglected corner. RadioGraphics 35:1123–1137
Mancini EJ, Kohen R, Esquivel AO, Cracchiolo AM, Lemos SE (2017) Comparison of ACL strain in the MCL-deficient and MCL-reconstructed knee during simulated landing in a cadaveric model. Am J Sports Med 45:1090–1094
Mannel H, Marin F, Claes L, Dürselen L (2004) Anterior cruciate ligament rupture translates the axes of motion within the knee. Clin Biomech 19:130–135
Mehl JT, Kia C, Murphy M, Obopilwe E, Cote M, Imhoff FB, Imhoff AB, Arciero RA, Beitzel K, Otto A (2019) Posteromedial ligament repair of the knee with suture tape augmentation: a biomechanical study. Am J Sports Med 47:2952–2959
Merican AM, Ghosh KM, Deehan DJ, Amis AA (2009) The transpatellar approach for the knee in the laboratory. J Orthop Res 27:330–334
Müller W (1983) The knee: form, function, and ligament reconstruction. Springer, Berlin Heidelberg New York, pp 68–77
Narvani A, Mahmud T, Lavelle J, Williams A (2010) Injury to the proximal deep medial collateral ligament: a problematical subgroup of injuries. J Bone Jt Surg Br 92:949–953
Offerhaus C, Balke M, Arner JW, Musahl V, Höher J (2018) Reefing of the posteromedial capsule in anteromedial rotatory instability. Arthrosc Tech 7:e547–e551
Robinson JR, Bull AM, Thomas RR, Amis AA (2006) The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sport Med 34:1815–1823
Robinson JR, Bull AMJ, Amis AA (2005) Structural properties of the medial collateral ligament complex of the human knee. J Biomech 38:1067–1074
Robinson JR, Sanchez-Ballester J, Bull AMJ, de Thomas WMR, Amis AA (2004) The posteromedial corner revisited. J Bone Jt Surg Br 86(674):681
Schafer KA, Tucker S, Griffith T, Sheikh S, Wickiewicz TL, Nawabi DH, Imhauser CW, Pearle AD (2016) Distribution of force in the medial collateral ligament complex during simulated clinical tests of knee stability. Am J Sports Med 44:1203–1208
Sims WF, Jacobson KE (2004) The posteromedial corner of the knee. Am J Sports Med 32:337–345
Sirisena D, Papi E, Tillett E (2017) Clinical assessment of antero-medial rotational knee laxity: a systematic review. Knee Surg Sports Traumatol Arthrosc 25:1068–1077
Slocum DB, Larson RL (1968) Rotatory instability of the knee. Its pathogenesis and a clinical test to demonstrate its presence. J Bone Jt Surg Am 50:211–225
Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA (2016) Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med 44:400–408
Svantesson E, Hamrin Senorski E, Alentorn-Geli E, Westin O, Sundemo D, Grassi A, Čustović S, Samuelsson K (2018) Increased risk of ACL revision with non-surgical treatment of a concomitant medial collateral ligament injury: a study on 19,457 patients from the Swedish National Knee Ligament Registry. Knee Surg Sports Traumatol Arthrosc 27:2450–2459
Warren LF, Marshall JL, Girgis F (1974) The prime static stabilizer of the medial side of the knee. J Bone Jt Surg 56:665–674
Wijdicks CA, Ewart DT, Nuckley DJ, Johansen S, Engebretsen L, Laprade RF (2010) Structural properties of the primary medial knee ligaments. Am J Sports Med 38:1638–1646
Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF (2010) Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Jt Surg Am 92:1266–1280
Wijdicks CA, Michalski MP, Rasmussen MT, Goldsmith MT, Kennedy NI, Lind M, Engebretsen L, LaPrade RF (2013) Superficial medial collateral ligament anatomic augmented repair versus anatomic reconstruction: an in vitro biomechanical analysis. Am J Sport Med 41:2858–2866
Zaffagnini S, Bignozzi S, Martelli S, Lopomo N, Marcacci M (2007) Does ACL reconstruction restore knee stability in combined lesions? Clin Orthop Relat Res 454:95–99
Acknowledgements
The authors wish to acknowledge the assistance of Lisa Lechner, PhD, and Marcus Mueller, MS, in the data management and engineering.
Funding
The funding has been received from Deutsche Kniegesellschaft with Grant No. DKG, Paracelsus Medizinische Privatuniversität with Grant No. Clinical Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.R. has received study grants from Smith and Nephew, Stryker and Nexclips; has received institutional funding from Smith and Nephew, Corin and Stryker; and is a paid consultant for British Standards Institute, Newclip Technics, and Smith and Nephew. A.W. has received royalties from Karl Storz and Linvatec; has received speaker fees from DJO/ORMED; and is a paid consultant for Karl Storz and Medacta. C.F. has received royalties from Karl Storz and Medacta; has received speaker fees from Karl Storz and Medacta; and is a paid consultant for Medacta. M.H. has received royalties from Medacta; has received speaker fees from Conmed Linvatec, Mathys, and Medacta; and is a paid consultant for Medacta and Conmed Linvatec.
Ethical approval
Ethics approval for this laboratory study was granted following the local law of the province of Schleswig-Holstein (Bestattungsgesetz Abschnitt II, § 9).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wierer, G., Milinkovic, D., Robinson, J.R. et al. The superficial medial collateral ligament is the major restraint to anteromedial instability of the knee. Knee Surg Sports Traumatol Arthrosc 29, 405–416 (2021). https://doi.org/10.1007/s00167-020-05947-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-05947-0