Abstract
Purpose
The aim of this study was to quantify the contributions of medial soft tissues to stability following cruciate-retaining (CR) or posterior-stabilised (PS) total knee arthroplasty (TKA).
Methods
Using a robotic system, eight cadaveric knees were subjected to ±90-N anterior–posterior force, ±5-Nm internal–external and ±8-Nm varus–valgus torques at various flexion angles. The knees were tested intact and then with CR and PS implants, and successive cuts of the deep and superficial medial collateral ligaments (dMCL, sMCL) and posteromedial capsule (PMC) quantified the percentage contributions of each structure to restraining the applied loads.
Results
In implanted knees, the sMCL restrained valgus rotation (62 % across flexion angles), anterior–posterior drawer (24 and 10 %, respectively) and internal–external rotation (22 and 37 %). Changing from CR TKA to PS TKA increased the load on the sMCL when resisting valgus loads. The dMCL restrained 11 % of external and 13 % of valgus rotations, and the PMC was significant at low flexion angles.
Conclusions
This work has shown that medial release in the varus knee should be minimised, as it may inadvertently result in a combined laxity pattern. There is increasing interest in preserving constitutional varus in TKA, and this work argues for preservation of the sMCL to afford the surgeon consistent restraint and maintain a balanced knee for the patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Instability arising from inadequate ligament restraint has been identified as the most common cause of short-term failure of total knee arthroplasty (TKA) [28]. Whilst there have been many studies examining the role of the medial passive soft tissue structures in the native and injured knee [8, 18, 35, 36, 40], there remains little information on the restraint that they provide in the presence of a TKA. In the case of primary TKA, which is most commonly performed using either a posterior cruciate-retaining (CR) or posterior-stabilised (PS) implant, the onus is on these soft tissues to help provide restraint, but the effect of the altered joint mechanics post-arthroplasty is not universally agreed upon [42]. In vivo and cadaveric studies have found conflicting differences between CR and PS in varus–valgus [19, 22], internal–external rotation [5, 47] and anterior drawer [23, 27]. Differences may be attributed to methodology, specimen/patient variability or even TKA designs between manufacturers.
The soft tissues on the medial aspect of the knee have been described as consisting of three distinct layers: the most superficial being a fascial layer, the second layer containing the superficial medial collateral ligament (sMCL) and the deepest layer containing the deep medial collateral ligament (dMCL) and the posteromedial capsule (PMC) [43]. The sMCL has a femoral attachment near the epicondyle [26] and inserts 60–80 mm distal to the joint line [37]. The dMCL, previously identified as the mid-third medial capsular ligament [43], lies deep to the posterior fibres of the sMCL as a two-part structure (meniscofemoral and meniscotibial) of the capsule [37]. Posterior to the dMCL, the PMC is formed of a large range of fibres attached to the femur around the base of the adductor tubercle and to the tibia just distal to the joint line posteromedially [37, 43].
During TKA surgery, it is general practice for ligaments and soft tissue to be balanced in order to align the knee in extension and flexion [11]. In the case of a varus knee, the medial structures may be overly tight and require judicious release to correct the deformity. However, there is a large discrepancy in suggested protocols between studies, with a lack of evidence to support them [20]. Delineation of the contribution of the medial soft tissues to functional constraint is imperative so as to avoid iatrogenic laxity [3]. Releasing the sMCL may correct varus deformity [45] and relax the lateral structures, but it may adversely affect stability in anterior drawer or internal–external rotation [8, 36]. Similarly, the dMCL may be damaged by tibial bone resection, particularly in a small knee [29], but the role of the dMCL post-arthroplasty has not been measured.
The objective of this study was to determine, for the first time, the contribution of different medial structures in stabilising the implanted knee and to identify whether the choice of implant affects this. Based on previous studies investigating the role of the medial structures in native knees, it was hypothesised that the sMCL would be an important restraint in valgus and internal–external rotation. Also, given that there is no clear trend in studies to suggest differences between CR and PS TKA, it was hypothesised that the contributions would be similar in both CR and PS knees.
Materials and methods
Following ethics approval, eight fresh-frozen human cadaver (four female and four male) knee specimens of median age 80 (range 59–96) were obtained from a tissue bank (five left-sided and three right-sided). None of the knees exhibited more than superficial articular surface changes, misalignment or fixed flexion. For each specimen, the tibia/fibula and femur were skeletonised 80 and 110 mm from the joint line, respectively; within these limits the skin, musculature and soft tissue structures were left intact. The head of the fibula was transfixed to the tibia in an anatomical position using a transcortical bone screw. Any excess proximal femoral bone and distal tibial and fibular bones were removed.
The femur and tibia were fixed in 60-mm-diameter cylindrical steel pots using polymethyl methacrylate bone cement. The tibia was aligned centrally in the bone pot using a jig with a pointer that located the centre of the tibia as between the tips of the tibial spines [2]. To access the tibial plateau, a midline incision was made to the skin and subcutaneous fat layer, followed by a medial parapatellar arthrotomy [41]. The arthrotomy was opened and resutured at each stage of the experiment. The femur was cemented into the bone pot whilst in full extension, and the posterior condylar axis was aligned parallel to the femoral fixture.
Robotic biomechanical testing system
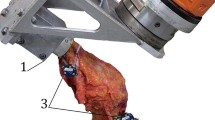
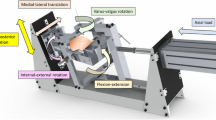
The cadaveric specimens were tested using a robotic knee joint biomechanical testing system, consisting of a six-degree-of-freedom (DOF) industrial robotic manipulator and robot controller (TX90 and CS8C; Stäubli Ltd, Zürich, Switzerland), and a six axis force/torque sensor (Omega 85; ATI Industrial Automation, Apex NC). The robotic manipulator had a payload of 200 N and repeatability of 0.03 mm in translation (manufacturer’s specification). The sensor had a force sensing range of 3800 N (resolution ±0.43 N) for the Z axis and 1900 N (resolution ±0.29 N) for the X and Y axes and torque sensing range of 80 Nm for Z, X and Y axes (Z resolution ±0.009 Nm and X–Y resolution ±0.013 Nm). The robotic system was capable of running in both force and position control. The femoral pot was fixed rigidly in a fixture on the base of the robot, and the tibial pot was attached to the force sensor connected to the end effector of the manipulator (Fig. 1).
Schematic diagram of the robotic knee joint testing system. The arrows detail the forces/torques applied to the knees during the testing protocol. AP anterior–posterior translation, VV varus–valgus rotation, and IE internal–external rotation. X, Y and Z are defined as the axes of the force/torque sensor
Testing protocol
The knee was manually flexed 20 times to avoid soft tissue hysteresis, and then in the robot the path of passive motion of the intact knee was found by applying a flexion rotation (with three repeats). During the flexion, the robotic system minimised forces and moments acting across the knee. The robotic system recorded the positions during the last passive flexion repeat, to determine the starting points for the loaded tests. To allow for variability to attain geometrical full extension in the implanted knees, the starting positions for the loaded tests were 4° (±3.8° standard deviation), then 19°, 49° and 79°. These flexion angles were nominally fixed by the robotic system and confirmed using high-resolution images and ImageJ software (Version 1.49 p, National Institutes of Health, Bethesda, MD).
At each flexion position, a ±90-N anterior–posterior (AP) force, a ±8-Nm varus–valgus (VV) torque and a ±5-Nm internal–external (IE) rotational torque were applied. In each situation, the robotic system minimised the loads in the secondary DOF to the primary applied force/torque. These loads were chosen as being comparable to other studies [13, 35, 36], with 90 N being a similar force to that applied by a KT 1000 arthrometer in AP drawer [10]. Each test was repeated three times.
After intact knee data collection, the knee was removed from the robot and a CR TKA (PFC Sigma; DePuy Synthes Joint Reconstruction, Leeds, UK) was implanted by a consultant orthopaedic surgeon using a medial parapatellar approach. The surgical technique used a standard combination of measured resection and gap balancing performed in full extension and 90° of flexion. The femur was referenced using an intramedullary guide rod set at 5° of valgus. The cutting block was placed against the distal femoral bone in neutral rotation with respect to the epicondylar axis, and a measured 9-mm resection was made from the least affected side of the distal femur. Femoral sizing was performed using an anterior down technique. On the tibial side, an intramedullary rod was used with a 3° posterior slope on the tibial block positioned with respect to the tibial anterior prominence. This corresponded to the centre of the tibial tuberosity in our knee specimens. Ten millimetre of bone was resected from the least affected, most superior proximal tibial surface. Gap balancing was confirmed using spacers to achieve a rectangular space both in full extension and flexion after bone resection but before chamfer femoral cuts. The tibial component was cemented to the bone, whilst the femoral component was implanted using a press-fit technique. It was known from prior work that press-fitting the femoral component gave secure fixation at the experimental loads [7]. No soft tissue releases were performed, and ‘tenting’ of the collateral ligaments was avoided by removing any osteophytes. A stable knee was taken as that which allowed for normal unaided unimpeded patellar tracking, did not exhibit either medial or lateral opening after implant trialling and was confirmed through a passive full range of movement from full extension. The knee joint with the CR TKA was then fixed back on the robot at the same angle of flexion as when the knee had been intact, but with other coordinates altered by the implant geometry. Loads of ±90 N AP, ±5 Nm IE and ±8 Nm VV were applied at the same angles of flexion as for the intact native knee.
The effect of a medial release with the CR TKA was tested by transecting in sequence the following structures: the dMCL, the sMCL and the PMC (Table 1). Fibres of the dMCL were cut just distal to the joint line, and remnants of the medial meniscus and its connected tissues were removed. The sMCL was released in two stages: firstly a ‘Whiteside’s release’ was performed using an osteotome passed deep to the anterior fibres and elevating them from their tibial attachment [45, 46]. The anterior fibres were easily differentiated in deep flexion because they were more taut than the posterior fibres. The second stage was a transection at the joint line across all the sMCL fibres. The PMC was cut across the fibres attached to the semi-membranosus tendon and across other connective tissues just distal to the joint line located posteromedially from the dMCL. These cuts were performed whilst the knee remained attached to the robot, so that any changes in the loads were caused solely by each of the transected structures, by the principle of superposition.
After each transection stage, the robot was utilised in position control to play back the exact kinematics of the CR TKA implanted stage. Thus, the reduction in restraining force/moment after each transection stage was attributed to the force/moment restraint which had been offered by the transected structure [38].
In four out of the eight knees, the CR TKA knee was taken out of the robot prior to the medial release stages and replaced with a PFC Sigma PS implant. The PS conversion retained the same tibial tray with a different polyethylene tibial inlay; the resection of the PCL required extra cuts into the femur to fit the PS femoral component with box feature, again cement free. The knee joint with the implanted PS TKA was then tested using the identical procedure as for the CR TKA, with the medial cuts performed with the knee remaining in the robotic fixtures.
Approval for this study (project code R13066) was given by the Imperial College Healthcare Tissue Bank under the Human Tissue Authority licence number 12275.
Statistical analysis
Mean peak forces/torques and translations/rotations from the three repeats were calculated using a custom MATLAB (MathWorks, Natick, MA) script. After each medial cut, the drop in force/torque required to repeat the kinematics was attributed to the restraint offered by the cut structure as a percentage of the original force/torque value. As the repeatability of the system was 0.03 mm, the output data were determined to one decimal place; however, the percentage results here are given to the nearest whole number.
Statistical analysis was performed in SPSS 22 (IBM SPSS Statistics, version 22, Armonk, NY). Two-way repeated-measures analysis of variance with pairwise comparisons with Bonferroni correction was performed to compare the force/torque contribution (dependent variable) to the medial structure cut at different flexion angles (independent variables) and to compare laxities (dependent) to the knee state (intact, CR TKA, PS TKA) at different flexion angles (independent), with significance level set at p < 0.05. A power analysis based on a prior study [40] indicated that when comparing between intact, CR and PS TKA laxities, four knees were needed to detect a clinically significant change of 4-mm translation and 8° rotation from standard deviations of 2 mm and 4°, respectively. A post hoc power analysis indicated that, when comparing the soft tissue contribution in the eight implanted knees with the standard deviations calculated, contributions of 9 % could be detected with 80 % power and 95 % confidence. Hence, a significant restraint/stabiliser at a given flexion angle was defined as having a mean resisting contribution greater than 10 % with p < 0.05.
To compare the contributions of the sMCL in CR and PS TKAs, a one-way analysis of variance was used with unpaired comparisons with Bonferroni correction. Power analysis indicated that with the standard deviations found, a difference in sMCL contribution of 18 % would be detected with 80 % power and 95 % confidence.
Results
Anterior–posterior translation
Under a 90-N anterior force, the CR knee was significantly more lax than the intact knee at 19° (p = 0.012), 49° (p < 0.001) and 79° (p = 0.002), whilst the PS knee was significantly more lax than the intact knee at 49° (p = 0.005) and 79° (p = 0.009). However, a significant difference was not found between the CR and PS knees at any flexion angle (Fig. 2). In all implanted knees, the sMCL was the greatest medial stabiliser to anterior translation (Fig. 3), with a percentage contribution ranging from 18 ± 12 % (mean ± standard deviation, p = 0.029) at 4° flexion, to 29 ± 11 % (p = 0.001) at 49°. Under a 90-N posterior force, both the CR and PS knees were significantly more lax than the intact knee at 4° (p = 0.034, 0.036), 19° (p = 0.007, <0.001) and 49° flexion (p = 0.037, 0.003), respectively. A significant difference was not found between the CR and PS knees at any flexion angle. On average, the sMCL contributed 10 % of the resistance to posterior drawer in implanted knees at all flexion angles (Fig. 3); however, this was only found to be significant at 4° (p = 0.03).
Anterior–posterior translation in response to a ±90-N anterior–posterior force. Error bars denote the standard deviation at each flexion angle. Asterisk indicates statistical significant translation compared with the intact state (p < 0.05). CR cruciate-retaining implant, PS posterior-stabilised implant
Percentage contributions of the deep and superficial medial collateral ligaments (dMCL and sMCL) and posteromedial capsule (PMC) in resisting 90-N anterior–posterior force in implanted knees, with 95 % CI. Asterisk indicates a statistically significant contribution greater than 10 % at the specified flexion angle (p < 0.05). For the sMCL, the contributions are further separated into cruciate-retaining (CR) and posterior-stabilised (PS) implants
Internal–external rotation
In response to a 5-Nm torque, no significant difference was found in internal rotation laxity between the intact, CR or PS knee at any flexion angle (Fig. 4). The dominant medial restraint to internal rotation in all implanted knees was the sMCL (Fig. 5), with contributions ranging from 17 ± 8 % at 4° (p = 0.004) to 25 ± 11 % at 49° (p = 0.003) flexion. When a 5-Nm external rotation torque was applied, no significant difference was found in rotational laxity between the intact, CR or PS knee at any flexion angle (Fig. 4). Again, the sMCL offered the greatest medial restraint to rotation in all implanted knees (Fig. 5), with contributions ranging from 33 ± 15 % (p = 0.003) at 4° to 39 ± 13 % (p < 0.001) at 49° flexion. The dMCL had a significant contribution to resisting external rotation of 11 ± 7 % at 19° flexion (p = 0.028).
Percentage contributions of the deep and superficial medial collateral ligaments (dMCL and sMCL) and posteromedial capsule (PMC) in resisting a 5-Nm internal–external moment in implanted knees, with 95 % CI. Asterisk indicates a statistically significant contribution greater than 10 % at the specified flexion angle (p < 0.05). For the sMCL, the contributions are further separated into cruciate-retaining (CR) and posterior-stabilised (PS) implants
Varus–valgus rotation
In response to an 8-Nm valgus torque, no significant difference in rotational laxity was found between the intact, CR or PS knee at any flexion angle (Fig. 6). In all implanted knees, the sMCL was the primary restraint to valgus rotation at all angles of flexion tested (Fig. 7), with nearly constant contributions from 59 ± 30 % (p < 0.001) at 4°, up to 65 ± 14 % (p < 0.001) at 19° flexion. ‘Whiteside’s release’ reduced the valgus restraint by 16 ± 13 % at 4° flexion (n.s.), to 21 ± 23 % at 79° flexion (n.s.); the change with knee flexion was not found to be significant. The PMC resisted 11 ± 7 % of the valgus torque at 4° (p = 0.028), which dropped substantially with increasing flexion, and the dMCL restrained 11 ± 6 % (p = 0.008) and 12 ± 7 % (p = 0.012) at 4° and 19°, respectively. Between the implants a trend was noted: with increasing flexion, there was a larger reliance on the sMCL to restrain the valgus torque in the PS than the CR implant, and this was found as significant at 19° (p = 0.034) and 49° flexion (p = 0.011).
Percentage contributions of the deep and superficial medial collateral ligaments (dMCL and sMCL), ‘Whiteside’s release’ of the anterior sMCL fibres, and posteromedial capsule (PMC) in resisting an 8-Nm valgus moment in implanted knees, with 95 % CI. Asterisk indicates a statistically significant contribution greater than 10 % at the specified flexion angle (p < 0.05). For the sMCL, the contributions are further separated into cruciate-retaining (CR) and posterior-stabilised (PS) implants, where ƚ indicates a statistically significant difference between the contribution in the CR and PS (p < 0.05)
In response to an 8-Nm varus torque, no significant difference was found in rotational laxity between the intact, CR or PS knee at any flexion angle (Fig. 6). None of the sectioned medial structures resisted the varus moment significantly.
Discussion
The most important finding of the study was that the sMCL is the primary medial ligamentous restraint in the implanted knee, demonstrating a consistent role in resisting valgus, internal–external rotations and anterior translation at all flexion angles examined. Another finding was that no significant difference in laxity was found between the CR and PS TKAs. When comparing the sMCL contributions between the implants, no evidence was found to suggest that the cam box mechanism of the PS TKA could improve stability of the implanted knee when faced with severe medial ligamentous deficiency; on the contrary, the loss of restraint by the PCL led to significantly raised restraining actions being imposed onto the sMCL after PS than CR TKA. Therefore, surgical release of the sMCL can result in gross laxity not compensated for by the other medial structures or the relatively unconstrained CR or PS implants. Attempts at correction of pre-existent varus through release of the sMCL may inadvertently result in a new combined laxity pattern.
Little work exists on how the contributions of the medial structures are affected by knee replacement [3]. Studies using a robotic system to test CR/PS TKA performance either have focussed on flexion arc comparisons [27, 33, 47] or have applied AP, IE and VV to implanted knees without investigating soft tissues [34]. Other papers that investigated the function of the MCL and PMC after TKA with surgical releases (such as in operative varus correction) were only able to compare increased laxity and were not able to determine the percentage contribution made by entire structures in restraining motion [39, 46]. It is mechanically equivalent to transect the ligaments as a subperiosteal release, but in this time-zero study, healing effects at the insertion points were not of interest.
Previous cadaveric studies on the sMCL in the intact knee have similar findings to the implanted knees in this study, so the mechanics of medial ligamentous stabilisation of the knee were largely preserved after TKA. The changes have been found include a significant contribution to resisting anterior drawer in an ACL-deficient knee, in internal–external rotation whilst in flexion and in valgus rotation at all flexion angles [24, 36, 44]. Along with the sMCL, it was found that the PMC acted as a restraint in valgus rotation when the knee was near full extension, and in valgus, internal and external rotations, the contribution of the PMC significantly decreased with increasing flexion. This concurs with the posterior fibres being stretched in extension and slackened with flexion [37]. In some specimens, it was difficult to identify the dMCL and its tibial attachments. This observation agrees with the study by Maes et al. [29], which found that on average 54 % of the dMCL tibial attachment area was resected in 33 cadaveric knees after a standard 9-mm TKA tibial cut. It was also evident that the dMCL attachment to the medial meniscus [37] was affected by removing the meniscus during implantation. Therefore, the data showing the role of the dMCL are likely to be at the lower bound of its contribution if some of the TKA procedures had caused damage. Previously, Griffith et al. [14] had reported that the dMCL is an important IE and valgus restraint in intact knees, although Robinson et al. [36] found no significant increases in IE laxity in intact knees when the dMCL was cut, and a significant increase in valgus laxity only if the sMCL had been cut previously.
This study found increased AP laxity with the CR and PS implants compared with the intact knee. This disagrees with the work by Saeki et al. [39], which only found an increase in AP laxity after TKA at 90° flexion. This may partially be explained by the lack of axial compression loading in the present study, which represented a clinical laxity test of the unloaded knee: here the knees were loaded in AP to ±90 N. Saeki et al. [17] applied a 45-N axial load and only a 35-N AP force. The concavities in the tibial plateaus of CR/PS implants locate the femoral condyles under axial joint compression and hence provide knee stability. Another explanation may be due to the unconstrained tests in the present study. With the robot being allowed to introduce secondary motion to minimise forces and moments in the other planes of motion, the absence of one or both of the cruciate ligaments in the implanted knees caused increased laxity. This study also found no significant difference in VV and IE laxity between the intact and implanted states. This was in agreement with Stoddard et al. [41], who applied 5-Nm IE and 3.5-Nm torques in addition to 400-N extensor loads during active flexion arcs and found no significant difference between intact and implanted state. Other cadaveric studies with loaded quadriceps and hamstrings, which applied VV and IE to the surgeon’s subjective endpoints rather than fixed moments as in this study, also found there to be similar rotatory kinematics between intact and implanted knees [12, 21].
When comparing the contributions of the sMCL between the implants, in most cases there were no large differences between the CR and the PS. However, a difference was observed in valgus rotation, when the PS implant had to rely more on the sMCL to offer restraint than the CR: without the PCL, which is a secondary restraint to valgus rotation [15], a larger load was imposed onto the sMCL. This finding supports a recent in vivo study with varus-matched pairs, which found that PS knees showed a greater medial gap increase then CR knees when extensive soft tissue release was required [25]. Matsumoto et al. [30] used an offset-type tensor to balance soft tissues with a stepwise medial release throughout a range of motion and reported that the PS-implanted knees increased joint centre gap in deep flexion compared with CR knees. Banks et al. [4] used fluoroscopy to compare the centre of rotations in CR and PS patients during a single-leg step up-and-down on a stair and found that whilst 63 % of CR had a lateral centre of rotation, 75 % of PS had a medial centre of rotation. This result would imply that during weight-bearing activities the CR would more likely tense the medial ligaments than in the PS (by having a larger moment arm from the centre of rotation). However, the effect of active muscles in vivo may account for difference in tibiofemoral movement found in vitro [5], and this study found that if the knee was exposed to a valgus moment and both implants were pivoted about the lateral condylar surface, the PS knee would require more sMCL restraint. Further studies should investigate the contribution of the PCL in the CR knee, which would be expected to be different than in a native knee due to the change in articular surface, tensioning disparities and potential partial resection caused by tibial plateau resection [32].
This cadaveric study has measured the contributions of each of the medial passive soft tissue restraints to tibiofemoral joint laxity in relatively normally aligned knees, whilst the surgeon in clinical practice may have to correct limb misalignment. In the varus knee, minimal medial release is required to achieve coronal plane symmetry and restore correct mechanical alignment such that the weight-bearing axis passes through the centre of the knee [6]. Over release of any medial (contracted or otherwise) structures risks defunctioning the knee and creating an instability pattern. The ‘Whiteside’s release’ of the anterior fibres caused a 18 % loss of valgus restraint on average across flexion angles, equivalent to losing approximately a third of the restraining action of the sMCL. However, that may be regained by healing post-surgery, as hypothesised in a recent study reporting good results with subperiosteal release of the MCL in PS knees 6 months and 1 year post-operatively [9]. In valgus alignment, which is more likely to load the MCL during gait, only the anterior portion of the deep medial collateral ligament should be elevated from the tibial bone and only to a depth sufficient to allow for safe tibial bone resection. Such measured release is akin to measured bone resection performed as a standard operating technique. Further studies would be required to ascertain the increased laxity changes from stepwise release of each of the structures. Instrumented implants [16] could be used in vitro to find whether CR and PS implants produced different centre of pressures and rotations when affected by step change release of the medial structures.
Limitations of the cadaveric study include being conducted at time-zero and therefore investigation of long-term stability of the implants and ligaments was not possible. However, since ligament balancing is conducted per operatively, the study gave an accurate representation of how the medial soft tissues act to restrain knee laxity at the end of this process. This experiment did not add axial joint compression or simulate muscle tensions (both of which would increase the stability of the implanted knees), but simulated a clinical evaluation of joint laxity. An example of muscle loading would be the semimembranosus tendon, to which the PMC has several connections [26], although it may be suggested that due to the orientation of the tendon it is unlikely to provide any restraint itself [1]. Release of the pes anserinus tendons [31] was not assessed because this study investigated the passive mechanics of the ligamentous tissue on the medial side of the knee.
Conclusion
This work has, for the first time, measured the relative contributions of the medial soft tissue structures to stability of the implanted primary TKA. There is increasing interest in preserving constitutional varus and minimising medial release and this work argues for preservation of the sMCL to afford the surgeon consistent restraint and a balanced knee for the patient, with both CR and PS TKAs. The cam box of the PS TKA did not augment valgus stability; rather, the loss of the PCL caused greater loads to fall onto the MCL than in a CR TKA.
References
Amis AA, Bull AMJ, Gupte CM, Hijazi I, Race A, Robinson JR (2003) Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc 11:271–281
Amis AA, Scammell BE (1993) Biomechanics of intra-articular and extra-articular reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br 75:812–817
Athwal KK, Hunt NC, Davies AJ, Deehan DJ, Amis AA (2014) Clinical biomechanics of instability related to total knee arthroplasty. Clin Biomech 29:119–128
Banks SA, Hodge WA (2004) Implant design affects knee arthroplasty kinematics during stair-stepping. Clin Orthop Relat Res 426:187–193
Banks SA, Markovich GD, Hodge WA (1997) In vivo kinematics of cruciate-retaining and -substituting knee arthroplasties. J Arthroplasty 12:297–304
Bellemans J (2011) Neutral mechanical alignment: a requirement for successful TKA: opposes. Orthopedics 34:507–509
Bull AMJ, Kessler O, Alam M, Amis AA (2008) Changes in knee kinematics reflect the articular geometry after arthroplasty. Clin Orthop Relat Res 466:2491–2499
Butler DL, Noyes FR, Grood ES (1980) Ligamentous restraints to anterior-posterior drawer in the human knee: biomechanical study. J Bone Joint Surg Am 62:259–270
Cho W-S, Byun S-E, Lee S-J, Yoon J (2015) Laxity after complete release of the medial collateral ligament in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 23:1816–1823
Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R (1985) Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am 67A:720–726
Fujimoto E, Sasashige Y, Masuda Y, Hisatome T, Eguchi A, Masuda T, Sawa M, Nagata Y (2013) Significant effect of the posterior tibial slope and medial/lateral ligament balance on knee flexion in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21:2704–2712
Ghosh KM, Blain AP, Longstaff L, Rushton S, Amis AA, Deehan DJ (2014) Can we define envelope of laxity during navigated knee arthroplasty? Knee Surg Sports Traumatol Arthrosc 22:1736–1743
Gollehon DL, Torzilli PA, Warren RF (1987) The role of the posterolateral and cruciate ligaments in the stability of the human knee: a biomechanical study. J Bone Joint Surg Am 69A:233–242
Griffith CJ, LaPrade RF, Johansen S, Armitage B, Wijdicks C, Engebretsen L (2009) Medial knee injury part 1, static function of the individual components of the main medial knee structures. Am J Sports Med 37:1762–1770
Grood ES, Noyes FR, Butler DL, Suntay WJ (1981) Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am 63:1257–1269
Gustke KA (2014) Soft-tissue and alignment correction: the use of smart trials in total knee replacement. Bone Joint J 96B:78–83
Haider H, Walker PS (2005) Measurements of constraint of total knee replacement. J Biomech 38:341–348
Haimes JL, Wroble RR, Grood ES, Noyes FR (1994) Role of the medial structures in the intact and anterior cruciate ligament-deficient knee: limits of motion in the human knee. Am J Sports Med 22:402–409
Hino K, Ishimaru M, Iseki Y, Watanabe S, Onishi Y, Miura H (2013) Mid-flexion laxity is greater after posterior-stabilised total knee replacement than with cruciate-retaining procedures: a computer navigation study. Bone Joint J 95B:493–497
Hunt NC, Ghosh KM, Athwal KK, Longstaff LM, Amis AA, Deehan DJ (2014) Lack of evidence to support present medial release methods in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22:3100–3112
Hunt NC, Ghosh KM, Blain AP, Athwal KK, Rushton SP, Amis AA, Longstaff LM, Deehan DJ (2014) How does laxity after single radius total knee arthroplasty compare with the native knee? J Orthop Res 32:1208–1213
Hunt NC, Ghosh KM, Blain AP, Rushton SP, Longstaff LM, Deehan DJ (2015) No statistically significant kinematic difference found between a cruciate-retaining and posterior-stabilised Triathlon knee arthroplasty: a laboratory study involving eight cadavers examining soft-tissue laxity. Bone Joint J 97B:642–648
Ishii Y, Matsuda Y, Ishii R, Sakata S, Omori G (2005) Sagittal laxity in vivo after total knee arthroplasty. Arch Orthop Trauma Surg 125:249–253
Kanamori A, Sakane M, Zeminski J, Rudy TW, Woo SL (2000) In-situ force in the medial and lateral structures of intact and ACL-deficient knees. J Orthop Sci 5:567–571
Kim S-M, Jang S-W, Seo J-G, Lee S-S, Moon Y-W (2015) Comparison of cruciate retaining and PCL sacrificing TKA with respect to medial and lateral gap differences in varus knees after medial release. J Arthroplasty 30:26–30
LaPrade RE, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L (2007) The anatomy of the medial part of the knee. J Bone Joint Surg Am 89A:2000–2010
Li G, Zayontz S, Most E, Otterberg E, Sabbag K, Rubash HE (2001) Cruciate-retaining and cruciate-substituting total knee arthroplasty: an in vitro comparison of the kinematics under muscle loads. J Arthroplasty 16:150–156
Lombardi AV Jr, Berend KR, Adams JB (2014) Why knee replacements fail in 2013—patient, surgeon, or implant? Bone Joint J 96-B(11 Suppl A: Current concepts in koint replacement):101–104
Maes M, Luyckx T, Bellemans J (2014) Does a conservative tibial cut in conventional total knee arthroplasty violate the deep medial collateral ligament? Knee Surg Sports Traumatol Arthrosc 22:2735–2739
Matsumoto T, Kubo S, Muratsu H, Matsushita T, Ishida K, Kawakami Y, Oka S, Matsuzaki T, Kuroda Y, Nishida K, Akisue T, Kuroda R, Kurosaka M (2013) Different pattern in gap balancing between the cruciate-retaining and posterior-stabilized total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21:2338–2345
Matsumoto T, Muratsu H, Kubo S, Matsushita T, Kurosaka M, Kuroda R (2011) The influence of preoperative deformity on intraoperative soft tissue balance in posterior-stabilized total knee arthroplasty. J Arthroplasty 26:1291–1298
Matziolis G, Mehlhorn S, Schattat N, Diederichs G, Hube R, Perka C, Matziolis D (2012) How much of the PCL is really preserved during the tibial cut? Knee Surg Sports Traumatol Arthrosc 20:1083–1086
Most E, Zayontz S, Li GA, Otterberg E, Sabbag K, Rubash HE (2003) Femoral rollback after cruciate-retaining and stabilizing total knee arthroplasty. Clin Orthop Relat Res 410:101–113
Mueller JK, Wentorf FA, Moore RE (2014) Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc 22:3003–3011
Petersen W, Loerch S, Schanz S, Raschke M, Zantop T (2008) The role of the posterior oblique ligament in controlling posterior tibial translation in the posterior cruciate ligament-deficient knee. Am J Sports Med 36:495–501
Robinson JR, Bull AMJ, Thomas RRD, Amis AA (2006) The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med 34:1815–1823
Robinson JR, Sanchez-Ballester J, Bull AMJ, Thomas RDM, Amis AA (2004) The posteromedial corner revisited: an anatomical description of the passive restraining structures of the medial aspect of the human knee. J Bone Joint Surg Br 86B:674–681
Rudy TW, Livesay GA, Woo SLY, Fu FH (1996) A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech 29:1357–1360
Saeki K, Mihalko WM, Patel V, Conway J, Naito A, Thrum H, Vandenneuker H, Whiteside LA (2001) Stability after medial collateral ligament release in total knee arthroplasty. Clin Orthop Relat Res 392:184–189
Sakane M, Livesay GA, Fox RJ, Rudy TW, Runco TJ, Woo SLY (1999) Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc 7:93–97
Stoddard JE, Deehan DJ, Bull AMJ, McCaskie AW, Amis AA (2013) The kinematics and stability of single-radius versus multi-radius femoral components related to Mid-range instability after TKA. J Orthop Res 31:53–58
Verra WC, van den Boom LGH, Jacobs W, Clement DJ, Wymenga AAB, Nelissen RGHH (2013) Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev 10:CD004803
Warren LF, Marshall JL (1979) Supporting structures and layers on the medial side of the knee: anatomical analysis. J Bone Joint Surg Am 61:56–62
Warren LF, Marshall JL, Girgis F (1974) Prime static stabilizer of medial side of knee. J Bone Joint Surg Am A 56:665–674
Whiteside LA (2002) Soft tissue balancing: the knee. J Arthroplasty 17:23–27
Whiteside LA, Saeki K, Mihalko WM (2000) Functional medial ligament balancing in total knee arthroplasty. Clin Orthop Relat Res 380:45–57
Wünschel M, Leasure JM, Dalheimer P, Kraft N, Wülker N, Müller O (2013) Differences in knee joint kinematics and forces after posterior cruciate retaining and stabilized total knee arthroplasty. Knee 20:416–421
Acknowledgments
This study was supported through educational grants from Newcastle Healthcare Charities. Surgical instruments and implants were supplied by DePuy Synthes Joint Reconstruction. The robotic test system was supported by the Wellcome Trust and EPSRC Centre of excellence for application of technology to osteoarthritis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Athwal, K.K., Daou, H.E., Kittl, C. et al. The superficial medial collateral ligament is the primary medial restraint to knee laxity after cruciate-retaining or posterior-stabilised total knee arthroplasty: effects of implant type and partial release. Knee Surg Sports Traumatol Arthrosc 24, 2646–2655 (2016). https://doi.org/10.1007/s00167-015-3796-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3796-0