Abstract
Purpose
Meniscal changes in the lateral knee compartment after medial-opening high tibial osteotomy (HTO) with valgic overcorrection have not been investigated clinically with magnetic resonance imaging (MRI). The hypothesis of this study was that HTO with valgic overcorrection affects the lateral compartment due to the change in the bearing axis and the shift in the pressure load from the medial to the lateral compartment. This should lead to an accelerated degeneration of the lateral compartment.
Methods
Twenty-four patients (mean age, 45.7 ± 7.6 years) were prospectively and longitudinally monitored with MRI after medial-opening high tibial osteotomy. The degeneration of the meniscus was assessed at the anterior horn, pars intermedia, and posterior horn using the Stoller classification. The morphological changes were also assessed at the anterior horn, pars intermedia, and posterior horn using the relative vertical and transverse diameters of the examined part of the meniscus, according to the ratio maximal meniscal diameter/maximal tibial width. These parameters were analyzed preoperatively and at follow-up (mean 5.3 ± 1.5 years after surgery).
Results
During the follow-up period, there were no significant morphological changes in either the anterior horn, pars intermedia, or posterior horn. Nor were there any correlations between the morphological parameters, bodyweight, and the follow-up period. Despite these findings, an MRI evaluation showed progressive degeneration in every part of the meniscus according to the Stoller classification, and this was significant at the anterior horn (p < 0.01), pars intermedia (p = 0.021), and posterior horn (p < 0.01).
Conclusion
High tibial osteotomy did not lead to morphological changes in the external meniscus over a follow-up period of 5.3 ± 1.5 years. However, progressive degeneration of the meniscus was observed in the imaging data. Further research is required to confirm the observed degeneration and to evaluate the consequences of the observed degeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Malalignment promotes development and progression of unicompartmental gonarthrosis [9] due to an abnormal bearing axis in malalignment and an increased load transfer in the affected compartment [2, 13, 19]. Furthermore, a recent study suggested a link between meniscal damage and the development and progress of osteoarthritis [10, 26], not only for mechanical reasons but even arising from systemic effects [1].
High tibial osteotomy (HTO) has been used since the 1960s to treat medial gonarthrosis associated with varus malalignment with good pain reduction, functional and radiological outcomes [5, 6, 12, 24, 29, 30].
Experimental studies have shown that changing the bearing axis with HTO relieves the pressure on the affected compartment [1].
Despite this, only a few studies have investigated the effects of axis correction with valgic overcorrection on the lateral compartment. Parker et al. [18] performed a clinical trial on ten patients treated with HTO for medial gonarthrosis. Changes in the cartilage surface were investigated with MRI in a 2-year follow-up. No significant differences were detected in the lateral compartment, meniscus was not examined.
In a study by Madry et al. [17], structural changes in the cartilage and meniscus in the lateral compartment after HTO were examined in a sheep model. No significant changes in the lateral compartment were found. In an arthroscopic study by Spahn et al. [25], no changes in the cartilage in the lateral compartment were observed after HTO during a follow-up period of 1.5 years.
The hypothesis of this study was that HTO with valgic overcorrection affects the lateral compartment due to the change in the bearing axis and the shift in the pressure load from the medial to the lateral compartment.
Therefore, the purpose of this prospective study was to detect forced degeneration and morphological changes of the external meniscus, associated with HTO, using an established MRI examination method.
Materials and methods
For this prospective follow-up study, all consecutive patients with a diagnosis of primary medial gonarthrosis with varus malalignment who underwent medial-opening HTO over a 5.3 ± 1.5-year period at a special trauma clinic in Frankfurt am Main, Germany, were included in this study. Twenty-four patients met the pre- and postoperative inclusion criteria and were followed throughout the follow-up period. Preoperative inclusion criteria were the presence of primary medial gonarthrosis and varus malalignment, no degeneration of the lateral meniscus greater Stoller grade 2, no significant comorbidities, no previous surgery on the lateral compartment, no ligament instability and no smoking. Postoperative inclusion criteria were no further surgery on the lateral compartment during follow-up period and no need for revision surgery during the follow-up period. The number of patients undergoing MRI pre- and postoperative was 24 (4 female, 20 male), the age at surgery was 45.7 ± 7.6 years, the age at follow-up was 51.3 ± 7.7 and with this the follow-up period was 5.3 ± 1.5 years.
Operative procedure and postoperative treatment
All the patients were treated with medial open-wedge osteotomy, and fixation was performed with a plate fixator. Surgery was performed by three senior surgeons who were blinded to the study. All the patients were realigned to a correction point of 62.5% (Fujisawa point) [11] which is a well-established procedure reported in a great number of scientific works [4, 16, 22]. Because this procedure is commonly used, the results of this study would be better comparable and could gain a greater impact. An intraoperative X-ray with the use of a grid board confirmed the correct conversation. The postoperative treatment of all patients included a 6-week relief phase, followed by a 6-week loading phase. Preoperative and postoperative MRI images were compared to assess the effects of HTO on the lateral meniscus.
MRI imaging, assessment and evaluation
All the patients underwent preoperative MRI scans. Standardized follow-up MRI scans were made of all patients, with the leg in neutral flexion. A 1.5 T Philips Gyroscan MRI Scanner (Philips Healthcare, Hamburg, Germany) with a knee coil was used. The following sequences were recorded on coronal, transverse, and sagittal views: proton density turbo spin echo fast suppression [320 × 320; thickness 3 mm; repeat time (TR) 3,00 ms; echo time (TE) 37 ms]; T1 (384 × 384; thickness 3 mm; TR 717 ms; TE 10 ms); T2 (512 × 512; thickness 3 mm; TR 460 ms; TE 15 ms) and short tau inversion recovery (STIR) (512 × 512; thickness 3 mm; TR 3200 ms; TE 49 ms). The interslice gap was 1.5 mm for all sequences. The MRI images were analyzed on a Mac-based Osirix imaging software system (version 8.0.2, Bernex, Switzerland) by one reader who had a background in sports traumatology. Confirmation was provided by a senior surgeon in sports traumatology. These readers were blinded to the results of the clinical assessment. The clinical assessment was performed by one student with an orthopedic background who was also blinded to the radiological assessment results. To comparatively evaluate the MRI images, the external meniscus was divided into three parts: the anterior horn, pars intermedia, and posterior horn.
The extent of degeneration of the external meniscus and its possible progression were investigated with the classification system described and established by Stoller et al. [27]. According to Stoller, grade 0 indicates a normal finding, grade 1 corresponds to punctate elevated signals with no connection to the meniscal surface, grade 2 indicates a linear signal elevation with no contact with the articular meniscal surface, and grade 3 indicates a linear signal elevation with at least one point of contact with the meniscal surface.
The thickness and width of individual parts of the meniscus were also measured as the parameters of morphological change, and were compared using the methods of Samoto et al. [23] and Ahn et al. [3], modified according to Lee et al. [14]. This procedure was chosen to determine whether the overcorrection during HTO, followed by a shift in the pressure load to the lateral compartment, led to a morphological change in the meniscus. The rationale behind this was that the meniscal tissue has a systemic influence on osteoarthritis [8], so changes in the tissue, such as a reduction in thickness, could lead to forced osteoarthritis. For this purpose, the absolute thickness and width of the pars intermedia and the anterior and posterior horns were measured at their midpoints.
The parameter ‘absolute meniscal width’ was defined as the narrowest point of the external meniscus in coronal view. The parameters ‘absolute meniscal width of the anterior horn’ and ‘absolute meniscal width of the posterior horn’ were recorded in sagittal view, at the point at which the external meniscus was thickest.
To obtain comparable relative values, the relative meniscal width was calculated as a percentage of the maximum tibial width (the maximum extent of the tibia in coronal view, measured from the corticalis to corticalis).
We used a similar approach to estimate the maximum thickness of the anterior horn and posterior horn. To obtain the relative meniscal widths at the anterior horn and posterior horn, the corresponding relative values were calculated as percentages of the maximum meniscal diameter in the sagittal plane.
To determine the absolute meniscal thickness of the pars intermedia, we used a coronal image. In this image, the thickness of the outer meniscus was measured at the point of its maximum vertical extent. Correspondingly, the anterior meniscal thicknesses at the anterior and posterior horns were also determined in the same images.
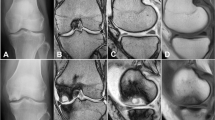
The relative meniscal thickness and width at the pars intermedia was calculated as a percentage of the maximum tibial width, and the relative meniscal thicknesses and widths at the anterior and posterior horns as percentages of the maximum diameter of the meniscus (Fig. 1).
Measurement of the morphological parameters of the lateral meniscus on MRI. A coronal MR image was used to define the relative meniscal thickness and width at the pars intermedia. a Tibial width (mm); b absolute meniscal thickness at the pars intermedia (mm); c absolute meniscal width at the pars intermedia (mm). Relative meniscal thickness at the pars intermedia (%) = B/A × 100; relative meniscal width at the pars intermedia (%) = C/A × 100. A sagittal image was used to define the relative meniscal thickness and the width at the anterior and posterior horns. d Maximal meniscal diameter (mm); e absolute meniscal thickness at the anterior horn (mm); f absolute meniscal thickness at the posterior horn (mm); g absolute meniscal width at the anterior horn (mm); H: absolute meniscal width at the posterior horn (mm). Relative meniscal thickness at the anterior horn (%) = E/D × 100; relative meniscal thickness at the posterior horn (%) = F/D × 100; relative meniscal width at the anterior horn (%) = G/D × 100; relative meniscal width at the posterior horn (%) = H/D × 100
Clinical assessment
For the clinical assessment, pre- and postoperative Lysholm scores [28] were used. This score consists of eight items and was tested with a questionnaire completed by the patients. In addition, the time of the patient’s return to sports was assessed.
IRB approval
The local Ethics Committee approved all the procedures performed and all the subjects gave their informed consent to the procedures (Ethic Committee of “Landesärztekammer Hessen, Frankfurt am Main”, ID number FF 81/2014).
Statistical analysis
All statistical analyses were performed and the corresponding graphics were created with the SPSS® Statistics 23.0 package (IBM®, Armonk, Westchester County, New York USA).
The significance level for all data was p < 0.05. The Wilcoxon rank test was used as a nonparametric test to analyze the changes in the relative thickness and width of the meniscus. As explained by Rosenthal [21], the standardized effect-strength measure of the individual parameters was then recorded to see their influence on the results. A preliminary test was performed to evaluate the changes in the Stoller classification. The influence of the factor weight and follow-up time on the parameters examined was determined with linear regression.
For power analysis, we calculated the effect size. For α = 0.05 and β = 0.20, we got an effect size of 0.47 which means an effect size between medium (0.30) and large (0.50).
Results
Morphological changes of the lateral meniscus
In Table 1, the mean values and standard deviations are given for the relative meniscal thickness and width at the pars intermedia, anterior horn, and posterior horn according to the examination time (preoperative and follow-up).
No significant changes in the relative meniscal thickness or in the area of the pars intermedia or the anterior or posterior horns (see bar charts in Figs. 2, 3, 4) were found.
Therefore, only small effect intensities were observed for the parameters evaluated here. In a regression analysis, no significant correlations were established between these parameters and bodyweight or the follow-up period (in years).
Degeneration of the lateral meniscus
23 patients were included in the evaluation of degeneration according to the Stoller classification. One follow-up MRI could not be accurately assessed because the image quality was poor.
According to the Stoller classification, we also found significant degeneration in the region of the anterior horn (p < 0.01) as in the pars intermedia (p = 0.021) and the posterior horn (p < 0.01). For details, see Tables 2, 3 and 4.
Clinical assessment
There was a significant improvement in the Lysholm score (p > 0.016), increasing from 54.9 ± 15.9 points preoperatively to 68.5 ± 21.2 points postoperatively.
Furthermore, the proportions of patients who returned to sport were: about 40% for swimming, 10% for cycling or jogging on smooth ground, 5.6% for jogging on rough ground, 3.3% for tennis and basketball, and 3.3% for soccer.
Discussion
The most important finding of this study was that there were no significant changes in the relative thickness or the relative width of the external meniscus attributable to an increase in the pressure load in the lateral compartment after HTO. Furthermore, a progress in degeneration of the lateral meniscus was observed. These results were independent of the bodyweight of the patient. We also detected no influence of the follow-up time on the results, thus longer use did not appear to cause a change in the meniscal thickness or width. This is consistent with the study of Madry et al. [17], whose animal experiments showed no structural changes in histology after either standard osteotomy or osteotomy with valgic overcorrection.
In a large number of subjects, progression in the sense of the Stoller classification, up to manifest tears, was observed according to the imaging data. On the cellular level, Madry et al. [17] also detected a reduction in the number of cells in the region of the external meniscus in the group of patients treated with overcorrection, whereas the group with a neutral axis showed no such change. MR-tomography-detected degeneration may be the long-term result of this loss of cells because the follow-up period was longer in this study. This is particularly important in view of the high correlation between meniscal damage and cartilage damage [15]. The meniscus protects the underlying articular cartilage, and if it is cracked, the incidence of cartilage damage increases significantly [10]. Spahn et al. [26] showed that degenerative meniscal damage is associated with degenerative cartilage damage and that cartilage damage is always associated with isolated degenerative meniscal damage. Furthermore, Englund et al. [8] showed that damage to the meniscal tissue has an systemic effect on the development of osteoarthritis.
It was possible to determine whether the degeneration observed in this study was truly caused by the after-effects of HTO or whether it was a normal sign of aging. Englund et al. [7] examined 991 random subjects, aged from 50 to 90 years, with meniscal damage. They defined ‘damage’ as an elevated signal intensity in meniscal tissue associated with any surface of the meniscus, as in Stoller grades 2 and 3. The prevalence of meniscal damage in the lateral meniscus ranged from 9% (men) to 11% (women). Compared with the findings of the present study, it seems plausible that the reported degeneration was not merely caused by aging because the rate of damage classified Stoller grade 2 or 3 in a younger population was 34.8% when averaged over all parts of the meniscus.
In the present study, the assessed clinical outcomes improved significantly. A longer follow-up will be necessary to determine whether degeneration progresses further, which may alter the clinical data.
Based on these findings, it has been shown that with a change in the bearing axis and the resulting increased load transmission and pressure load on the lateral knee compartment, there is an increase in degeneration in the area of the external meniscus according to the imaging data.
The study had limitations that mainly resulted from the low number of cases included, even though the statistical analysis was robust. To determine if degeneration is caused by HTO, it would also be useful to include a healthy control group, with no axis deviation, for comparison. To confirm the reported degeneration, it would be useful to perform preoperative MRI on all patients with the same protocol as used for postoperative MRI, so differences in the water content of the meniscal tissue at both time points could be compared. It would also be useful include a second round of arthroscopy in the follow-up period to confirm the degeneration observed in the imaging data. During this procedure, it would also be possible to perform a biopsy to confirm the results histologically. Arthro-computed tomography is another procedure that could be used for validation [20]. In the setting of this study, both second-look arthroscopy and arthro-computed tomography were not possible for ethical reasons. It would be also interesting to examine the changes in the cartilage in the lateral compartment after HTO, which was not possible in this work because we lacked the special MRI sequences and procedures during the preoperative MRIs (for example, dGEMRIC). In the follow-up examination, we could only take full-leg weight-bearing radiographs on symptomatic patients for ethical reasons. Therefore, the potential loss of correction was not addressed. To draw a definitive conclusion about the consequences of meniscal degeneration, a larger and longer follow-up study is required.
Beside this, it has been shown that it is important for the clinical routine to record meniscal damage, respectively, degeneration carefully, before setting the indication for HTO.
Conclusion
The imaging data presented in this study demonstrate that although there are no morphological changes of the lateral meniscus after HTO, structural changes, with significant progressive degeneration, occur in the lateral meniscus. These findings seem to be independent of the bodyweight of the patient and the follow-up period.
Therefore, this study should introduce a discussion of whether standard valgic overcorrection during HTO [22] should be abandoned in favor of a neutral axis correction. The results presented above show that further investigation of this issue is necessary. Further work is necessary to better assess the consequences of the degeneration observed in this study.
References
Agneskirchner JD, Hurschler C, Stukenborg-Colsman C, Imhoff AB, Lobenhoffer P (2004) Effect of high tibial flexion osteotomy on cartilage pressure and joint kinematics: a biomechanical study in human cadaveric knees. Winner of the AGA-DonJoy Award 2004. Arch Orthop Trauma Surg 124:575–584
Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P (2007) The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy 23:852–861
Ahn JH, Shim JS, Hwang CH, Oh WH (2001) Discoid lateral meniscus in children: clinical manifestations and morphology. J Pediatr Orthop 21:812–816
Amis AA (2013) Biomechanics of high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 21:197–205
Becker R, Hirschmann M (2017) The pertinent question in treatment of unicompartmental osteoarthritis of the knee: high tibial osteotomy or unicondylar knee arthroplasty or total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:637–638
Coventry MB (2001) Osteotomy of the upper portion of the tibia for degenerative arthritis of the knee: a preliminary report. JBJS 83:1426
Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M et al (2008) Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 359:1108–1115
Englund M, Haugen IK, Guermazi A, Roemer FW, Niu J, Neogi T et al (2016) Evidence that meniscus damage may be a component of osteoarthritis: the Framingham study. Osteoarthr Cartil 24:270–273
Felson DT, Niu J, Gross KD, Englund M, Sharma L, Cooke TDV et al (2013) Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the multicenter osteoarthritis study and the osteoarthritis initiative. Arthritis Rheum 65:355–362
Fine KM, Glasgow SG, Torg JS (1995) Tibial chondral fissures associated with the lateral meniscus. Arthroscopy 11:292–295
Fujisawa Y, Masuhara K, Shiomi S (1979) The effect of high tibial osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop Clin N Am 10:585–608
Hantes ME, Natsaridis P, Koutalos AA, Ono Y, Doxariotis N, Malizos KN (2017) Satisfactory functional and radiological outcomes can be expected in young patients under 45 years old after open wedge high tibial osteotomy in a long-term follow-up. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4816-z
Heller MO, Matziolis G, König C, Taylor WR, Hinterwimmer S, Graichen H et al (2007) Musculosceletal biomechanic of the knee joint, in German. Orthopade 36:628–634
Lee C-R, Bin S-I, Kim J-M, Kim N-K (2016) Magnetic Resonance imaging findings in symptomatic patients after arthroscopic partial meniscectomy for torn discoid lateral meniscus. Arthroscopy 32:2366–2372
Lewandrowski K-U, Müller J, Schollmeier G (1997) Concomitant meniscal and articular cartilage lesions in the femorotibial joint. Am J Sports Med 25:486–494
Lobenhoffer P (2014) Importance of osteotomy around to the knee for medial gonarthritis. Indications, technique and results. Orthopade 43:425–431
Madry H, Ziegler R, Pape D, Cucchiarini M (2014) Structural changes in the lateral tibiofemoral compartment after high tibial osteotomy. Orthopade 43:958–965
Parker DA, Beatty KT, Giuffre B, Scholes CJ, Coolican MRJ (2011) Articular cartilage changes in patients with osteoarthritis after osteotomy. Am J Sports Med 39:1039–1045
Parker DA, Viskontas DG (2007) Osteotomy for the early varus arthritic knee. Sports Med Arthrosc 15:3–14
Pujol N, Panarella L, Selmi TAS, Neyret P, Fithian D, Beaufils P (2008) Meniscal healing after meniscal repair: A CT arthrography assessment. Am J Sports Med 36:1489–1495
Rosenthal R (1991) Meta-analytic procedures for social research. Sage Publications, Thousand Oaks, p 155
Sabzevari S, Ebrahimpour A, Roudi MK, Kachooei AR (2016) High tibial osteotomy: a systematic review and current concept. Arch Bone Jt Surg 4:204–212
Samoto N, Kozuma M, Tokuhisa T, Kobayashi K (2002) Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging 20:59–64
Schuster P, Gesslein M, Schlumberger M, Mayer P, Mayr R, Oremek D et al (2018) Ten-year results of medial open-wedge high tibial osteotomy and chondral resurfacing in severe medial osteoarthritis and varus malalignment. Am J Sports Med 46:1362–1370
Spahn G, Klinger HM, Harth P, Hofmann GO (2012) Cartilage regeneration after high tibial osteotomy. Results of an arthroscopic study. Z Orthop Unfall 150:272–279
Spahn G, Plettenberg H, Hoffmann M, Klemm H-T, Brochhausen-Delius C, Hofmann GO (2017) The frequency of cartilage lesions in non-injured knees with symptomatic meniscus tears: results from an arthroscopic and NIR- (near-infrared) spectroscopic investigation. Arch Orthop Trauma Surg 137:837–844
Stoller DW, Martin C, Crues JV, Kaplan L, Mink JH (1987) Meniscal tears: pathologic correlation with MR imaging. Radiology 163:731–735
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
van Outeren MV, Waarsing JH, Brouwer RW, Verhaar JAN, Reijman M, Bierma-Zeinstra SMA (2017) Is a high tibial osteotomy (HTO) superior to non-surgical treatment in patients with varus malaligned medial knee osteoarthritis (OA)? A propensity matched study using 2 randomized controlled trial (RCT) datasets. Osteoarthr Cartil 25:1988–1993
W-Dahl A, Toksvig-Larsen S, Lindstrand A (2017) Ten-year results of physical activity after high tibial osteotomy in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 25:902–909
Acknowledgements
The authors thank the assistance of Dr. Johannes Herrmann in statistical guidance. The authors appreciate the support of performing the MR imaging of all medical technical assistants of the Department for Diagnostic and Interventional Radiology of our hospital, and our Department assistant, Brigitte Mack, for assistance in collecting the data.
Funding
The Magnetic Resonance Imaging was partially financed by third-party funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The local Ethics Committee approved all the procedures performed (Ethic Committee of “Landesärztekammer Hessen, Frankfurt am Main”, ID number FF 81/2014).
Informed consent
All the subjects gave their informed consent to the procedures.
Rights and permissions
About this article
Cite this article
Bick, F., Iffland, Y., Zimmermann, E. et al. The medial open-wegde osteotomy generates progressive intrameniscal integrity changes in the lateral knee compartment: a prospective MR-assessment after valgic osteotomy in the varus gonarthritic knee. Knee Surg Sports Traumatol Arthrosc 27, 1339–1346 (2019). https://doi.org/10.1007/s00167-018-5073-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5073-5