Abstract
Purpose
The restoration of the labrum complex and the influence on secondary osteoarthritis after arthroscopic Bankart repair on magnetic resonance imaging (MRI) remain unclear.
Methods
Twenty-one patients were retrospectively followed after unilateral primary arthroscopic Bankart repair with knot-tying suture anchors (8.8 ± 2.5 years after surgery, age 25.3 ± 6.3 years). Bilateral structural MRI was performed to assess labrum–glenoid restoration by measurements of the labrum slope angle, height index, and labrum interior morphology according to the Randelli classification. Osteoarthritic status was bilaterally assessed by a modified assessment based on the Samilson–Prieto classification.
Results
MRI assessment revealed full labrum–glenoid complex restoration with equivalent parameters for anterior slope angle (mean ± SD: 21.3° ± 2.6° after Bankart repair vs. 21.9° ± 2.6° control) and height index (2.34 ± 0.4 vs. 2.44 ± 0.4), as well as the inferior slope angle (23.1° ± 2.9° vs. 23.3° ± 2.1°) and height index (2.21 ± 0.3 vs. 2.21 ± 0.3) (all n.s.). The labrum morphology showed only for the anterior labrum significant alterations (1.4 ± 0.9 vs. 0.6 ± 0.7, p < 0.05), the inferior labrum occurred similarly (1.3 ± 0.8 vs. 0.8 ± 0.5, n.s.). Osteoarthritic changes were significantly increased after Bankart repair compared to the uninjured shoulder (4.8 ± 5.1 mm vs. 2.5 ± 1.0 mm; p < 0.05), with a significant correlation of osteoarthritis status between both shoulders (p < 0.05). Scores generally decreased after Bankart repair (constant 84.6 ± 9.5 vs. 94.5 ± 4.9 control, p < 0.05; Rowe 84.5 ± 6.5 vs. 96.2 ± 4.2, p < 0.05; Walch–Duplay 82.4 ± 7.0 vs. 94.3 ± 4.0, p < 0.05) with a strong correlation with osteoarthritis status (p < 0.05).
Conclusions
Arthroscopic Bankart repair enabled good clinical outcomes and complete quantitative labrum restoration parameters. Next to several well-known parameters, secondary osteoarthritis after arthroscopic Bankart repair significantly correlated with osteoarthritic status of the uninjured contralateral shoulder but was not influenced by quantitative labrum restoration. The recommendation for arthroscopic Bankart repair should be based on clinical parameters and not on prevention of secondary osteoarthritis.
Study design
Case series.
Level of evidence
IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arthroscopic stabilization of Bankart lesions using suture anchors demonstrates comparable results to open stabilization, with advantages in functional outcomes and treatments of concomitant pathology [2, 5, 10, 12, 15, 16, 20, 24]. A single dislocation can damage these structures and thus increase the risk of osteoarthritis [11, 27]. Multiple dislocations progressively damage the anteroinferior labrum ligament complex and glenoid morphology [9, 19, 29]. Greis et al. [8] demonstrated that labral and bony defects of the anteroinferior quadrant increase glenohumeral pressure by up to 300%. Ruckstuhl et al. confirmed these findings in a meta-analysis; hereafter, chronic humeroglenoidal overload after Bankart repair was one of the key factors for secondary osteoarthritis [26].
Secondary osteoarthritis after Bankart repair is well documented in the long term and has been found in as many as 83% of open stabilization study patients [23]. The number of anchors, age at time of dislocation and at surgery, and intraoperative status of the labrum were identified as risk factors for the development of secondary glenohumeral osteoarthritis [7, 21]. A meta-analysis of 26 studies showed glenohumeral arthropathy in 33 and 39% of patients undergoing open and arthroscopic shoulder stabilization [10]. In a long-term study, Plath et al. [21] found mild, moderate, and severe osteoarthritis in 41, 16, and 12% of shoulders after arthroscopic stabilization, respectively. These findings are based on conventional radiologic assessments, but the literature is lacking with regard to structural analysis of labrum restoration and the status of osteoarthritis on long-term MRI assessments. The finding of Greis et al. [8] and Ruckstuhl et al. [26] allow us to hypothesize that insufficient labrum restoration after Bankart repair leads to increased glenohumeral peak pressure with consecutive secondary osteoarthritic changes.
The aim of the present study was to investigate the structural integrity of the anterior and inferior labrum with regard to slope, height, and interior morphology using a standardized MRI protocol. An additional aim of the present study was to analyze glenohumeral osteoarthritis status and its correlation to labrum restoration status, compared to the uninjured contralateral shoulder. In addition, it was measured whether osteoarthritic changes impact clinical status, functional scores, and scores specific for instability.
Materials and methods
All consecutive patients between 1998 and 2005 with an initial posttraumatic unilateral glenohumeral joint dislocation and arthroscopic Bankart repair were retrospectively analyzed according to intraoperative inclusion criteria: age at surgery 18–45 years, primary arthroscopic shoulder stabilization with suture anchors after unilateral traumatic anteroinferior instability, no lesions in the rotator cuff, no preoperative arthropathy, no instability of the superior labrum (SLAP lesion > 1°), no pulley lesion, no osteoarthritis, preoperative neurological deficits, long-term steroid therapy, and tumors. The postoperative inclusion criteria were: compliance to the rehabilitation protocol, minimum follow-up period of 5 years, no macrotrauma of both shoulders. The study was conducted by the member of the research commitee (T.S.) of the German-speaking Association for Arthroscopy (AGA).
Arthroscopic procedure and rehabilitation
Surgery was performed by the same surgeon in the lateral decubitus position with the arm in 45° abduction as in previously described 3-portal techniques [28, 30]. After an adequate 180° mobilization, the anterior capsule–labrum–ligament (CLL) complex anchors (3.0 mm × 11 mm Fastak™; Poly-L-Lactide-Acid; Arthrex®) were single loaded (Ethibond/FW™) and placed through the anteroinferior portals according to the anchor-first-principle of knot-tying anchor systems. The knots were set anteromedial to the labrum to increase repositioning of the CLL tissue onto the anteroinferior glenoid and to avoid contact to the humeral cartilage.
According to the rehabilitation protocol, the shoulder was placed in an arm sling immediately after surgery for 4 weeks. Physiologic range of motion was allowed from month 4 with no load and from month 7 with physiologic load.
Clinical assessment
A complete examination was performed on both shoulders and data on external rotation in 0° and 90° abduction was collected. Three scores were measured. The Constant–Murley score was used to determine the general status of the shoulder [6]. Scores specific for instability include the Rowe score [25] and the Walch–Duplay score [33]. The Rowe score exists in a number of versions; for this study, the “stability-specific” Rowe score from 1978 was used [25]. Shoulder pain and general function of the shoulder were recorded using a visual analogue scale (VAS: 1 = strong pain, no function to 15 = no pain, good function). All examinations were performed by the same surgeon, who was not the operating surgeon.
Radiologic assessment
All patients underwent standardized magnetic resonance imaging (MRI; 1.5 T Siemens Magnetom Avanto/Numaris 4; Siemens Healthcare Diagnostics, Eschborn, Germany) using a specialized shoulder coil (Shoulder array: Siemens Healthcare Diagnostics). MRI was performed for all patients for both shoulders. The following standardized sequences were prepared for the coronal and transaxial slices: STIR-T2 (image size 256 × 256 mm2; thickness 4 mm); T1 (image size 576 × 576 mm2; thickness 3.5 mm); Dual Turbo Spin Echo (image size 512 × 512 mm2; thickness 3.0 mm); PDW-TSE-SPI (image size 512 × 512 mm2; thickness 4.0 mm), and PDW EXP (image size 512 × 512 mm2; thickness 3.0 mm). The coronal and transaxial planes were set orthogonal to the specific joint space with orientation at the maximal anterior–posterior perspective and cranial–caudal glenoidal rim heights. Labrum assessments were performed at these slices with maximal osseous glenoid rim heights. The analysis of linear and angular measurements was performed according to published protocols [3, 32, 34]. All analyses were performed by the same two non-operating orthopedic scientists under the advice of an independent radiologist. All assessments of analyzed parameters were measured by the same scientists blinded to patients’ data and the assessments were performed twice with a time interval of 3 months to calculate the correlation coefficients. For the data analysis, the means of both MRI measurements were used.
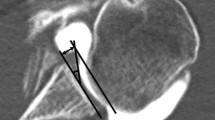
The assessment of the osteophyte was performed in T1-weighted MRI sequences in the coronal plane with the biceps anchor in the most prominent position (Fig. 1, P1, P4). A circle was placed into the humeral head with a tangent at the 12 o’clock position and at the 3 o’clock and 9 o’clock positions for the right and left shoulders, respectively (Fig. 1, P2, P5). The size of the osteophyte was measured from the tangent of the circle perpendicular to the highest point of the osteophyte (Fig. 1, P3, P6). Occurrence of inferior humeral and/or glenoid osteophyte was assessed using MRI. The grade of osteoarthritis was evaluated based on the Samilson–Prieto classification (Tables 3, 4). Labrum interior morphology was measured on the same T2-weighted sequence and graduated from 0° to III° according to descriptive graduation, as published by Randelli et al. (Table 7). Separated for the anterior and inferior portion, the labral height (LH) and glenoid height (GH) were measured as the maximum distance (in millimeters) to the lowest portion of the glenoid cavity (Table 8). The labrum glenoid height index (LGHI) was measured as the quotient of the labral height to the glenoid height for the anterior and inferior area. Analogue, the anterior and inferior labrum glenoid slopes (labrum glenoid slope = LGS) were defined as the angle between the tangent at the lowest point of the glenoid cavity and the tip of the maximum labral height (Table 8). These parameters were measured in transaxial PDW EXP-weighted images for the anterior capsulolabral complex and in coronal T2-weighted images for the inferior area. MRI assessments were performed according to the established protocol of Yoo et al. [34]. The labrum MRI assessments were performed according to the established and validated protocol of Yoo et al. [34], which was applied in several comparable protocols [31, 32].
All data collection and randomizing procedures were approved by our institutional ethics committee (FF 71/2009). All subjects provided their informed consent prior to participating in this study.
Statistical analysis
The mean, standard deviation, and Pearson correlation were calculated using Microsoft Excel 2016. The non-parametric Wilcoxon test for paired data and Chi-squared test were used to determine statistically significant differences in scores, slope, height index, and morphology, which were calculated using WINSTAT for Microsoft Excel version 2012.1.0.96. The level of statistical significance was set at an alpha level of p < 0.05.
Our own pilot assessments for the present study protocol showed that the restored anterior and inferior labrum recorded for the height (aLH 6.2 ± 1.3 mm; iLH 7.3 ± 1.1) and for the slope (aLGS 24.8 ± 3.4 mm; iLGS 25.4 ± 2.6), compared to the situation of complete labrum dislocation (Bankart lesion) with a glenoid height (aGH 2.2 ± 0.7 mm; iGH 3.0 ± 0.7) and for the glenoid slope (aGS 6.1 ± 1.1 mm; iLGS 6.2 ± 1.0). These data of the pilot assessments confirmed the data of Yoo et al. [34]. For measurement accuracy, the test–retest reliabilities for the labrum height and slope were measured. The intraclass correlation coefficients (ICC) were for the labrum height and slope > 0.97. The coefficient of variations (CV) were calculated as follows: (standard deviation)/(mean value). The CV for the MRI assessments was 0.05.
For measurement accuracy of the present data analysis, the test–retest reliability was calculated. The ICC for the MRI labrum slope assessments was 0.91 and the CV was 0.03. For the MRI labrum height assessments the ICC was 0.91 and the CV was 0.05 and for the osteophyte assessments the ICC was 0.99 and the CV was 0.03.
The power analysis for labrum assessment, using GPower 3.1.9.2, two groups t test revealed for the labrum height and slope assessments a sample size of 8 and accepted alpha error of 0.05 with a power of 0.95.
Results
Between 1998 and 2005, 62 patients met intraoperative inclusion criteria. Thirty-seven of 62 patients (59.7%) were contactable and were interviewed. Twenty-one of the 37 contacted patients (56.8%) met postoperative inclusion criteria and agreed to clinical and radiologic postoperative assessment, including bilateral MRI and physical examination with a clinical score system over a mean follow-up period of 8.8 years. Sixteen of 37 (43.2%) patients were excluded because of reinjury (6.3%), redislocation (31.3%), and other reasons (62.4%). Preoperatively, 18 of the 21 patients in this study had multiple dislocations, with an average of 3.8 ± 2.0 (range 2–8) dislocations per patient. All demographic data of these patients are shown in Table 1.
Clinical outcomes
In general, arthroscopic Bankart repair is associated with good long-term clinical outcomes, but significant impairment compared to the uninjured control side. The functional Constant score has good and excellent results for the ipsilateral and contralateral shoulders. The instability specific Walch–Duplay score and Rowe score revealed good outcomes data with significant deficits compared to the excellent contralateral results. The analysis of VA pain scores revealed significantly increased pain after arthroscopic Bankart repair compared to the contralateral side. Analogous to these findings, the VAS for function was on average significantly decreased. Nineteen of 21 patients reported a lower VAS for function compared to the control shoulder. The treated shoulder had an average deficit in deep external rotation of 19° (SD 7.4°), and in high external rotation of 12.9° (SD 8.0°) compared to the healthy shoulder. All clinical outcomes data are shown in Table 2.
Radiologic outcomes
There was a significant difference between the ipsilateral and the contralateral shoulders with regard to osteoarthritis, based on the Samilson–Prieto classification (p < 0.05, Table 3). After Bankart repair, only 14.3% showed no osteoarthritic changes and 42.9% no or mild osteoarthritis, whereas the control side had no osteoarthritic changes in 47.6% and no or mild osteoarthritic changes in 85.7%. One patient who showed the lowest values of the group for all scores and for both VAS scales had an arthritic degeneration grade 4. A correlation was seen for the grades of osteoarthritis according to Samilson–Prieto and the VASfunction, VASpain (Table 4). Interestingly, we detected a strong relationship between both shoulders with regard to glenoidal arthropathy (p < 0.05). All applied scores for function and stability and external rotation showed a significant correlation to osteoarthritis status (Tables 5, 6). No correlation was seen between osteoarthritis status and age at surgery, osteoarthritis status, or number of preoperative dislocations.
The descriptive MRI assessment of labrum interior morphology, according to the Randelli graduation, showed significantly increased intralabral changes for the anterior labrum portion (mean ± SD ipsilateral 1.4 ± 0.9 vs. contralateral 0.6 ± 0.7, p = 0.007), whereas the status of the inferior labrum showed nonsignificant changes (mean ± SD ipsilateral 1.3 ± 0.8 vs. contralateral 0.8 ± 0.5, p = 0.06). No correlation was seen between status of osteochondral changes, number of preoperative dislocations, or labrum morphology (Table 7).
The quantitative labrum assessments detected that labrum slope and height were restored without significant side-to-side differences to healthy controls (Table 8). There were no correlations between anterior and inferior slope angle, anterior and inferior GLHI, or number of preoperative dislocations.
Discussion
The most important result of the present analysis was that the grade of osteoarthritis correlated with the grade of osteoarthritis on the uninjured contralateral side, whereas labrum restoration and labrum morphology had no influence on the grade of osteoarthritic changes after arthroscopic Bankart repair. These findings lead us to the assumption that there are individual predispositions and factors for osteoarthritic progression. These findings refute our initial hypothesis and previous convictions of recent publications, that adequate labrum restoration improves the glenohumeral congruence and consecutively reduces glenohumeral peak pressure to decrease the prevalence of secondary glenohumeral osteoarthritic changes.
Previous labrum assessment studies revealed that knot-tying and knotless anchor systems enabled similarly adequate restoration of the labrum complex and, respectively, the glenohumeral concavity [31, 34]. The restored congruence of the glenohumeral labrum complex was supposed to reduce glenohumeral peak pressure [8]. The present data show that the labrum complex remains completely restored at the anterior and inferior portion for the glenoidal slope angle and the glenoid–labrum height index, even in the long term. Comparing absolute data for labrum restoration after Bankart repair in the short term of Yoo et al. [34] and Stein et al. [31], the present labrum assessment showed similar parameters for slope, height, and morphology. Only the interior morphology of the anterior labrum, which is the main area for anchor and suture material placement in Bankart repair, showed a significant structural alteration compared to the control labrum. It can be hypothesized that the quantitatively restored labrum remains biomechanically and qualitatively inferior, with inadequate reduction of the glenohumeral pressure. These conclusions confirm the findings of Greis et al., who revealed significantly increased rates of glenohumeral peak pressures after sequential loss of the anterior labrum [8].
The instability arthropathy after traumatic shoulder dislocation was described and graded by Samilson and Prieto [27], and a number of other authors [10, 11, 21, 23]. Whereas Marx et al. detected a 10- to 20-fold increase in glenohumeral arthritis after the initial glenohumeral dislocation [17], Hovelius et al. demonstrated that, after nonsurgical therapy, osteoarthritic changes occurred in 29% mild, in 9% moderate and in 17% severe [11] cases. The absolute osteoarthritis incidence after Bankart repair is high and remains strongly variant in the literature, between 21.8% [7], 68% [13], 69% [21], 80% [22], and 76% (Table 3). These values should be always analyzed in relationship to osteoarthritis incidence at the uninjured shoulder (38%, Table 3) and after nonsurgical treatment of the unstable shoulder of 56% [11]. The present subanalysis in Table 4 revealed that MRI-assessed osteoarthritis occurred in the majority of mild and moderate cases. These data are in accordance with that of Plath et al., who revealed in conventional radiologic assessments that osteoarthritis after Bankart repair was mild in 41%, moderate in 16%, and severe in only 12% [21], comparable to nonsurgical treatment with moderate or severe osteoarthritic changes [11]. The authors concluded that insufficient glenohumeral concavity would display a prearthritic factor for secondary instability induced osteoarthritis of the shoulder [8, 11, 21, 26]. The present data allow us to assume that restoration of the labrum has no key role for reduction of osteoarthritic progression after shoulder dislocation.
Next to initial shoulder dislocation mechanism [17] and the joint incongruence [31, 34], there are several risk factors which lead to progressive instability and osteoarthritis: higher age at primary dislocation and surgery, long interval between initial dislocation and surgical stabilization, high number of dislocation mechanisms, glenohumeral malposition, persisting deficit of the external rotation, implant associated factors (> 3 anchors and implant overlapping), and alcoholism [4, 7, 11, 18].
For the clinical relevance of the present data and comparing the present osteoarthritic progression after Bankart repair to the contralateral uninjured shoulder, there was an expected, significantly increased incidence of osteoarthritis after shoulder dislocation followed by surgical shoulder stabilization. In only 33.3% of cases the same grade was found, in 38.1% one grade higher was found, and in 28.6% two or more grades higher were found. There was a significant relationship between both shoulders with regard to osteoarthritis status (p = 0.004). In addition to current findings in the literature, our data led us to assume that there is an individual predisposition for progression of osteoarthritic changes, which also affects the grade of secondary instability arthritis. The present data revealed no relationship between the grade of osteoarthritis and number of preoperative dislocations, which is heterogenous in the current literature [4, 14, 17, 21, 23, 27]. The present clinical assessment revealed persisting deficits for all scores and VAS after arthroscopic Bankart repair compared to uninjured controls (Table 2). In the subanalysis of clinical status, patients with increased osteoarthritic status revealed a worse clinical outcome (Table 4). These findings confirmed those of Hovelius and Saeboe, who described the negative influence of progressive osteoarthritis on clinical status [11]. Plath et al. [21] and Allain et al. [1] concluded that slight glenohumeral osteoarthritis does not appear to influence functional status, whereas strong and severe osteoarthritis generates notable clinical deficits [11]. Regarding the influence of an external rotation deficit on osteoarthritis, the meta-analysis of Papalia et al. [18] confirmed our findings (Table 2) and revealed, after analysis of 14 studies, significantly negative effects with an average loss of external rotation of 13.8° and 16.4° in 0° and in 90° abduction. In this regard, Hovelius and Saeboe detected that restriction of external rotation after 10 years did not influence evolution of arthropathy after 25 years [11]. From our data and the conclusion of the current literature, there remains a lack of knowledge if loss of external rotation follows progressive osteoarthritis or if the postoperative persistent limitation triggers anterior osteoarthritic changes (capsulorrhaphy arthropathy).
There are a number of limitations in this study. First, there was no comparable preoperative MRI assessment, which would have given information about preoperative status. Second, a direct contrast medium MRI arthrography would provide information about labrum morphology, including incomplete healing and persistent ruptures; the contrast medium application was restricted by our institutional ethics committee, and the majority of patients would refuse this invasive application.
Conclusions
The long-term labrum assessment after arthroscopic Bankart repair showed a complete quantitative restoration for labrum slope and height; only the anterior labrum had interior labrum morphology alterations. Adequate restoration of the labrum complex appears not to influence the incidence of the secondary long-term osteoarthritis. Next to several well-known parameters, the incidence of secondary osteoarthritis after arthroscopic Bankart repair shows a significant correlation between osteoarthritic status of the uninjured contralateral shoulder.
References
Allain J, Goutallier D, Glorion C (1998) Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am 80(6):841–852
Bottoni LCR, Smith MEL, Berkowitz MMJ, Towle CRB, Moore CJH (2006) Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med 34(11):1730–1737
Burkhart SS, Debeer JF, Tehrany AM, Parten PM (2002) Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy 18(5):488–491
Buscayret F, Edwards TB, Szabo´ I, Adeleine P, Coudane H, Walch G (2004) Glenohumeral arthritis in anterior instability before and after surgical treatment: incidence and contributing factors. Am J Sports Med 32(5):1165–1172
Castagna A, Garofalo R, Conti M, Flanagin B (2015) Arthroscopic Bankart repair: have we finally reached a gold standard? Knee Surg Sports Traumatol Arthrosc 24(2):398–405
Constant CR, Murley AH (1987) A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 214:160–164
Franceschi F, Papalia R, Del Buono A, Vasta S, Maffulli N, Denaro V (2011) Glenohumeral osteoarthritis after arthroscopic Bankart repair for anterior instability. Am J Sports Med 39(8):1653–1659
Greis PE, Scuderi MG, Mohr A, Bachus KN, Burks RT (2002) Glenohumeral articular contact areas and pressures following labral and osseous injury to the anteroinferior quadrant of the glenoid. J Shoulder Elbow Surg 11(5):442–451
Habermeyer P, Gleyze P, Rickert M (1999) Evolution of lesions of the labrum-ligament complex in posttraumatic anterior shoulder instability: a prospective study. J Shoulder Elbow Surg 8(1):66–74
Harris JD, Gupta AK, Mall NA, Abrams GD, McCormick FM, Cole BJ, Bach BR Jr, Romeo AA, Verma NN (2013) Long-term outcomes after Bankart shoulder stabilization. Arthroscopy 29(5):920–933
Hovelius L, Saeboe M (2009) Arthropathy after primary anterior shoulder dislocation—223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg 18(3):339–347
Imhoff AB, Ansah P, Tischer T, Reiter C, Bartl C, Hench M, Spang JT, Vogt S (2010) Arthroscopic repair of anteriorinferior glenohumeral instability using a portal at the 5:30-o’clock position: analysis of the effects of age, fixation method, and concomitant shoulder injury on surgical outcomes. Am J Sports Med 38(9):1795–1803
Kavaja L, Pajarinen J, Sinisaari I, Savolainen V, Björkenheim JM, Haapamäki V, Paavola M (2012) Arthritis of glenohumeral joint after arthroscopic Bankart repair: a long-term follow-up of 13 years. J Shoulder Elbow Surg 21(3):350–355
König DP, Rutt J, Treml O, Kausch T, Hackenbroch MH (1993) Osteoarthritis following the Putti-Platt operation. Arch Orthop Trauma Surg 115:231–232
Marquardt B, Witt KA, Gotze C, Liem D, Steinbeck J, Potzl W (2006) Longterm results of arthroscopic Bankart repair with a bioabsorbable tack. Am J Sports Med 34(12):1906–1910
Martetschläger F, Imhoff AB (2014) Shoulder dislocation in athletes: current therapy concepts. Orthopäde 43(3):236–243
Marx RG, McCarty EC, Montemurno TD, Altchek DW, Craig EV, Warren RF (2002) Development of arthritis following dislocation of the shoulder: a case-control study. J Shoulder Elbow Surg 1(1):1–5
Papalia R, Osti L, Del Buono A (2010) Glenohumeral arthropathy following stabilization for recurrent instability. Br Med Bull 96:75–92
Park JY, Lee SJ, Lhee SH, Oh JH (2012) Change in labrum height after arthroscopic Bankart repair: correlation with preoperative tissue quality and clinical outcome. J Shoulder Elbow Surg 21:1712–1720
Petrera M, Patella V, Patella S, Theodoropoulos J (2010) A meta-analysis of open versus arthroscopic Bankart repair using suture anchors. Knee Surg Sports Traumatol Arthrosc 18(12):1742–1747
Plath JE, Aboalata M, Seppel G, Juretzko J, Waldt S, Vogt S, Imhoff AB (2015) Prevalence of and risk factors for dislocation arthropathy: radiological long-term outcome of arthroscopic Bankart repair in 100 shoulders at an average 13-year follow-up. Am J Sports Med 43:1084–1090
Privitera DM, Bisson LJ, Marzo JM (2012) Minimum 10-year follow-up of arthroscopic intra-articular Bankart repair using bioabsorbable tacks. Am J Sports Med 40(1):100–107
Rachbauer F, Ogon M, Wimmer C, Sterzinger W, Huter B (2000) Glenohumeral osteoarthritis after the Eden-Hybbinette procedure. Clin Orthop Relat Res 373:135–140
Rhee YG, Lim CT, Cho NS (2007) Muscle strength after anterior shoulder stabilization: arthroscopic versus open Bankart repair. Am J Sports Med 35(11):1859–1864
Rowe CR, Patel D, Southmayd WW (1978) The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am 60(1):1–16
Ruckstuhl H, de Bruin ED, Stussi E, Vanwanseele B (2008) Posttraumatic glenohumeral cartilage lesions: a systematic review. BMC Musculoskelet Disord 9:107
Samilson RL, Prieto V (1983) Dislocation arthropathy of the shoulder. J Bone Joint Surg Am 65(4):456–460
Scheibel M, Nikulka C, Dick A, Schroeder RJ, Popp AG, Haas NP (2007) Structural integrity and clinical function of the subscapularis musculotendinous unit after arthroscopic and open shoulder stabilization. Am J Sports Med 35:1153–1161
Spatschil A, Landsiedl F, Anderl W, Imhoff A, Seiler H, Vassilev I, Klein W, Boszotta H, Hoffmann F, Rupp S (2006) Posttraumatic anterior-inferior instability of the shoulder: arthroscopic findings and clinical correlations. Arch Orthop Trauma Surg 126(4):217–222
Stein T, Buckup J, Efe T, von Eisenhart-Rothe R, Hoffmann R, Zimmermann E, Welsch F (2015) Structural and clinical integrity of the rotator cuff in athletes after arthroscopic Bankart repair using the three-portal technique. Arch Orthop Trauma Surg 135:369–382
Stein T, Buckup J, Mehling AP, Hoffmann R, Efe T, von Eisenhart-Rothe R, Welsch F (2014) Restoration of joint congruency and the glenoidal labrum after arthroscopic revision Bankart repair: a MRI match-paired analysis comparing primary Bankart repair and the uninjured labrum. Arch Orthop Trauma Surg 134:1121–1134
Stein T, Mehling AP, Reck C, Buckup J, Efe T, Hoffmann R, Jäger A, Welsch F (2011) MRI assessment of the structural labrum integrity after Bankart repair using knotless bioanchors. Knee Surg Sports Traumatol Arthrosc 19(10):1771–1779
Walch G (1987). Walch-Duplay rating sheet for anterior instability of the shoulder. SESEC/ESSE, Paris
Yoo JC, Lee YS, Tae SK, Park JH, Park JW, Ha HC (2008) Magnetic resonance imaging appearance of a repaired capsulolabral complex after arthroscopic bankart repair. Am J Sports Med 36(12):2310–2316
Funding
This study was funded by the Department of Sporttraumatology—Knee- and Shoulder-Surgery, Berufsgenossenschaftliche Unfallklinik Frankfurt am Main, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Bock J, Buckup J, Reinig Y, Zimmermann E, Colcuc C, Hoffmann R and Welsch F declared no conflicts of interest. Stein T has received payments for instructions at human cadaver courses.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards and the vote of the ethical committee. All data collection and randomizing procedures were approved by the institutional ethics committee 2009.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Bock, J., Buckup, J., Reinig, Y. et al. The arthroscopic Bankart repair procedure enables complete quantitative labrum restoration in long-term assessments. Knee Surg Sports Traumatol Arthrosc 26, 3788–3796 (2018). https://doi.org/10.1007/s00167-018-4922-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-4922-6